Arylazolyl(azinyl)thioacetanilides: Part 19†: Discovery of Novel Substituted Imidazo[4,5-b]pyridin-2-ylthioacetanilides as Potent HIV NNRTIs Via a Structure-based Bioisosterism Approach
Abstract
With the continuation of our unremitting efforts toward the discovery of potent HIV-1 NNRTIs, a series of novel imidazo[4,5-b]pyridin-2-ylthioacetanilides were designed, synthesized, and evaluated for their antiviral activities through combining bioisosteric replacement and structure-based drug design. Almost all of the title compounds displayed moderate to good activities against wild-type (wt) HIV-1 strain with EC50 values ranging from 0.059 to 1.41 μm in a cell-based antiviral assay. Thereinto, compounds 12 and 13 were the most active two analogues possessing an EC50 value of 0.059 and 0.073 μm against wt HIV-1, respectively, which was much more effective than the control drug nevirapine (EC50 = 0.26 μm) and comparable to delavirdine (EC50 = 0.038 μm). In addition, one selected compound showed a remarkable reverse transcriptase inhibitory activity compared to nevirapine and etravirine. In the end of this manuscript, preliminary structure–activity relationships (SARs) and molecular modeling studies were detailedly discussed, which may provide valuable insights for further optimization.
The reverse transcriptase (RT) of human immunodeficiency virus type 1 (HIV-1) is presently a primary therapeutic target for the exploration of highly effective anti-HIV drugs due to its crucial function during replication process 1. According to the different mechanisms of action, two kinds of inhibitors targeting RT have been studied intensively and applied to clinic extensively; especially, non-nucleoside RT inhibitors (known as NNRTIs), as key components of highly active antiretroviral therapy (HAART), have played an inherent and definitive role in first-line drug regimens with five drugs approved by the US FDA 2-7. However, drug resistance due to rapid RT mutations and serious side-effects has inevitably emerged after long-term clinical use, which greatly hampered the effectiveness of NNRTIs 8-10. As a consequence, developing additional NNRTIs with innovative structures and broad-spectrum antimutant activities is still a highly significative and imperative mission for further study on the anti-HIV drugs 11-16.
During the medicinal chemistry campaigns on seeking novel NNRTIs, substantial efforts from many laboratories (including ours) were devoted to chemical and biological studies of arylazolyl(azinyl)thioacetanilides (AATAs) because of their interesting structural skeleton and high potency against wild-type (wt) HIV-1 as well as resistant strains 17-29. In particular, two representative 1,2,4-triazolythioacetanilide derivatives, VRX-480733 and RDEA-806 (in phase IIa clinical trials, 2008), were once chosen as candidates for further clinical studies 22 (Figure 1A).
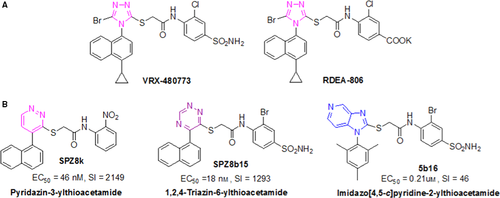
Based on our initial exploration on chemical space of this scaffold, there is a large body of evidence that different central heterocycle portion of AATAs makes distinct electronic and conformational contributions to the scaffold function of orienting other pharmacophore elements into the proper geometry 23-29. The replacement of the five-membered azoles moiety by an array of six-membered or fused heterocyclic bioisosteres (such as pyrazine, pyridazine 1,2,4-triazine, pyrimidine and imidazopyridine) led to the identification of additional promising AATAs with potent anti-HIV activities, such as SPZ8k, SPZ8b15, and 5b16 23-35 (Figure 1B).
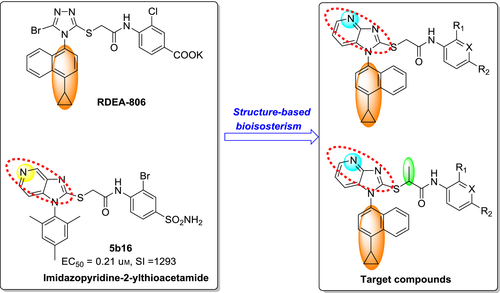
Specific to our investigation of imidazo[4,5-c]pyridinylthioacetanilides (represented by 5b16), a preliminary conclusion has been obtained that the N atom position in the imidazopyridine core has a significant effect on the antiviral activity and a suitable position of the N atom was probably beneficial for improving the binding affinity. Motivated by this hypothesis and in order to further explore the chemically diverse space and to elucidate the structure–activity relationships (SARs) of AATAs, a novel series of imidazo[4,5-b]pyridine derivatives were rationally designed through combining bioisosteric replacement and structure-based drug design. Notably, in the newly designed analogues, the 1-cyclopropylnaphthalene motif of REDA806 was maintained to keep the potential interactions, while different substituents, varying in size and electronic nature, were introduced in the phenyl ring of the anilide moiety to further investigate potential interactions. Moreover, a methyl-substituted acetamide linker (propanamide) was also employed to probe SARs in this region (Figure 2). Herein, the synthesis, anti-HIV activities, preliminary SARs, and molecular modeling studies of novel substituted imidazo[4,5-b]pyridin-2-ylthioacetanilides will be discussed in detail.
Experimental Section
Synthetic procedures and analytical data
Mass spectrometry was performed on an API 4000 triple-quadrupole mass spectrometer (Applied Biosystems/MDS Sciex, Concord, ON, Canada). 1H-NMR and 13C-NMR spectra were recorded on a Bruker AV-400 spectrometer (Bruker BioSpin, Jinan, Switzerland), using solvents as indicated (DMSO-d6). Chemical shifts were reported in δ values (ppm) with tetramethylsilane as the internal reference, and J values were reported in hertz (Hz). Melting points (mp) were determined on a micromelting point apparatus (Tian Jin Analytical Instrument Factory, Nankai, Tianjin, China). Flash column chromatography was performed on columns packed with silica gel 60 (200–300 mesh) (Qingdao waves silica gel desiccant co., Ltd, Qingdao, China). Thin-layer chromatography was performed on precoated HUANGHAI® HSGF254, 0.15- to 0.2-mm TLC plates (Yantai Jiangyou Silica Gel Development Co., Ltd., Yantai, Shandong, China).
General procedure for the synthesis of 2-nitropyridin-3-yl trifluoromethane-sulfonate (2)
Trifluoromethanesulfonic anhydride (0.35 g, 0.14 mmol) was added dropwise under stirring to a solution of 2-nitropyridin-3-ol (1) (0.10 g, 0.71 mmol) and triethylamine (Et3N) (0.11 g, 1.07 mmol) in anhydrous CH2Cl2 (10 mL) at 0 °C, and the mixture was stirred for 3 h. Then, water (50 mL) was added and extracted with CH2Cl2 (2 × 10 mL). Combined organic phase was washed with saturated brine (2 × 10 mL), dried over anhydrous Na2SO4, filtered, and concentrated to give the corresponding crude product (dark purple oil). Yield: 94.9%. ESI-MS: m/z 271.3 (M-1), C6H3F3N2O5S [271.97].
General procedure for the synthesis of 4-cyclopropylnaphthalen-1-amine (4)
A solution of 4-bromonaphthalen-1-amine (3) (0.50 g, 2.25 mmol), cyclopropylboronic acid (0.21 g, 2.47 mmol), Pd(PPh3)4 (0.26 g, 0.23 mmol), K3PO4 (1.67 g, 7.87 mmol) in PhMe/H2O (25/1, 26 mL) was stirred at 90 °C for 6 h under N2. After cooling, insoluble substance was filtered out and the solvent was evaporated under reduced pressure. Then, water (50 mL) was added and extracted with EtOAc (2 × 10 mL). Combined organic phase was washed with brine, dried over anhydrous Na2SO4, filtered, and concentrated to obtain compound 4 as dark purple oil. Yield: 95.3%. ESI-MS: m/z 184.3 (M + 1), C13H13N [183.1].
General procedure for the synthesis of N-(4-cyclopropylnaphthalen-1-yl)-2-nitropyridin-3-amine (5)
Into a 250-mL 2-neck round-bottom flask purged and maintained with an inert atmosphere of nitrogen were placed a solution of 2-nitropyridin-3-yl trifluoromethanesulfonate (1.00 g, 3.60 mmol) in toluene (100 mL), 4-cyclopropyl-naphthalen-1-amine (0.55 g, 4.00 mmol), K3PO4 (1.10 g, 5.40 mmol), Pd(dppf)Cl2 (0.13 g, 0.18 mmol), and dppf (0.020 g, 0.036 mmol). The resulting mixture was stirred for 36 h at 90 °C. The reaction mixture was cooled, dried over Na2SO4, and concentrated under vacuum. The residue was purified by eluting through a column with a 1/2 EtOAc (EA)/petroleum (PE) solvent system to provide yellow solid 5. Yield: 33.3%, mp: 95–97 °C. ESI-MS: m/z 306.4 (M + 1), C18H15N3O2 [305.12].
General procedure for the synthesis of N3-(4-cyclopropylnaphthalen-1-yl) pyridine-2,3-diamine (6)
The reaction system containing relevant compound 5 (0.20 g), Pd/C (0.040 g), and 10 mL anhydrous EtOH was vacuumed and filled with excess hydrogen gas. The reaction mixture was kept for 10 h and then filtered out Pd/C, and the solvent was evaporated under reduced pressure. The obtained crude product was washed by PE to give purified compound 6 as a purple solid. Yield: 86.7%, mp: 148–150 °C. ESI-MS: m/z 276.4 (M + 1), C18H17N3 [275.14].
General procedure for the synthesis of 1-(4-cyclopropylnaphthalen-1-yl)-1H -imidazo[4,5-b]pyridine-2-thiol (7)
Crude intermediate 6 (0.35 g, 1.20 mmol) was dissolved in anhydrous THF (15 mL) in the presence of Et3N (0.19 g, 1.90 mmol) at room temperature followed by the addition of di(1H-imidazol-1-yl)methanethione (0.34 g, 1.90 mmol). The reaction mixture was stirred at 60 °C for 5 h (monitored by TLC). The solvent was removed under reduced pressure, and water (20 mL) was added and extracted with EtOAc (3 × 10 mL), and the organic phase was washed with saturated sodium chloride (2 × 10 mL) and dried over anhydrous Na2SO4 to give the corresponding crude product, which was purified by flash column chromatography using MeOH-CH2Cl2 (1:30) system to afford the intermediate 7. Yield: 61.2%, mp: > 280 °C. ESI-MS: m/z 318.3 (M + 1), 340.4 (M + 23), C19H15N3S [317.1].
General procedure for the synthesis of target compounds (8–25)
Intermediate 7 (0.15 g, 0.63 mmol, 1 equiv), K2CO3 (0.10 g, 0.76 mmol, 1.2 equiv), and different 2-chloro-N-aryl-substituted acetamides or propanamides (1.1 equiv) were dissolved in acetone (10 mL). The reaction mixture was stirred at an ambient temperature for 4 h and then evaporated under reduced pressure. The residue was dissolved with a small amount CH2Cl2 and chromatographed on silica gel using EA–PE system. Pure fractions were collected, concentrated, and recrystallized, giving the desired compounds (8–25).
Methyl 3-bromo-4-(2-(1-(4-cyclopropylnaphthalen-1-yl)-1H-imidazo[4,5-b]pyridin-2-ylthio) acetamido)benzoate (8)
White powder, yield: 43.3%. mp: 140–142 °C. 1H NMR (400 MHz, DMSO-d6, ppm) δ: 10.17 (s, 1H, NH), 8.62 (d, 1H, J = 8.00 Hz, pyridylimidazole-H), 8.43 (d, 1H, J = 4.00 Hz, naphthaline-H), 8.14 (s, 1H, PhH), 8.09 (d, 1H, J = 8.00 Hz, pyridylimidazole-H), 7.96 (dd, 1H, J1 = 8.00 Hz, J2 = 4.00 Hz, naphthaline-H), 7.72 (d, 2H, J = 8.00 Hz naphthaline-H), 7.55 (t, 1H, J = 8.00 Hz, pyridylimidazole-H), 7.49 (d, 1H, J = 8.00 Hz, PhH), 7.31 (d, 1H, J = 8.00 Hz, naphthaline-H), 7.17 (dd, 1H, J1 = 8.00 Hz, J2 = 4.00 Hz, Naphthaline-H), 7.12 (d, 1H, J = 8.00 Hz, PhH), 4.45 (m, 2H, S-CH2), 3.85 (s, 3H, CH3), 2.62-2.55 (m, 1H, cyclopropyl-H), 1.18-1.16 (m, 2H, cyclopropyl-H), 0.85–0.81 (m, 2H, cyclopropyl-H). 13C-NMR (100 MHz, DMSO-d6, ppm) δ: 167.23 (C=O), 165.06 (C=O), 156.87, 155.66, 144.46, 142.97, 140.78, 134.31, 133.84, 131.16, 129.60, 129.53, 128.35, 128.23, 127.61, 127.39, 127.34, 125.70, 124.30, 123.41, 122.68, 118.56, 117.95, 115.56, 52.84, 36.56 (S-C), 13.43, 7.77, 7.69. ESI-MS: m/z 587.2 (M + 1), 589.3 (M + 3). C29H23BrN4O3S [586.07].
Ethyl 3-bromo-4-(2-(1-(4-cyclopropylnaphthalen-1-yl)-1H-imidazo[4,5-b]pyridin-2-ylthio) acetamido)benzoate (9)
White powder, yield: 45.2%. mp: 135–137 °C. 1H NMR (400 MHz, DMSO-d6, ppm) δ: 10.16 (s, 1H, NH), 8.62 (d, 1H, J = 8.00 Hz, pyridylimidazole-H), 8.43 (d, 1H, J = 4.00 Hz, naphthaline-H), 8.13 (s, 1H, PhH), 8.08 (d, 1H, J = 8.00 Hz, pyridylimidazole-H), 7.95 (d, 1H, J = 8.00 Hz, naphthaline-H), 7.72 (d, 2H, J = 8.00 Hz, naphthaline-H), 7.53 (t, 1H, J = 8.00 Hz, pyridylimidazole-H), 7.49 (d, 1H, J = 8.00 Hz, PhH), 7.31 (d, 2H, J = 8.00 Hz naphthaline-H), 7.17 (dd, 2H, J1 = 8.00 Hz, J2 = 4.00 Hz, naphthaline-H), 7.12 (d, 1H, J = 8.00 Hz, PhH), 4.47 (m, 2H, S-CH2), 4.33 (q, 2H, J = 4.00 Hz, CH2), 2.61–2.56 (m, 1H, cyclopropyl-H), 1.33 (t, 3H, J = 4.00 Hz, CH3), 1.19–1.16 (m, 2H, cyclopropyl-H), 0.91–0.87 (m, 2H, cyclopropyl-H). 13C-NMR (100 MHz, DMSO-d6, ppm) δ: 167.20 (C=O), 164.56 (C=O), 156.87, 155.67, 144.46, 142.98, 140.73, 134.31, 133.77, 131.16, 129.56, 129.54, 128.35, 128.24, 127.70, 127.62, 127.34, 125.70, 124.36, 123.42, 122.69, 118.56, 117.95, 115.29, 61.63 (CH2-O), 36.56 (S-C), 14.58 (CH3), 13.43 (cyclopropyl-C), 7.77 (cyclopropyl-C), 7.69 (cyclopropyl-C). ESI-MS: m/z 601.4 (M + 1), 603.3 (M + 3), 605.3 (M + 5). C30H25BrN4O3S [600.08].
N-(2-Bromo-4-cyanophenyl)-2-(1-(4-cyclopropylnaphthalen-1-yl)-1H-imidazo[4,5-b]pyridin-2-ylthio)acetamide (10)
White powder, yield: 41.3%. mp: 214-215 °C. 1H NMR (400 MHz, DMSO-d6, ppm) δ: 10.25 (s, 1H, NH), 8.62 (d, 1H, J = 8.00 Hz, pyridylimidazole-H), 8.43 (d, 1H, J = 4.00 Hz, naphthaline-H), 8.25 (d, 1H, J = 4.00 Hz, PhH), 8.11 (d, 1H, J = 8.00 Hz, pyridylimidazole-H), 7.87 (d, 1H, J = 8.00 Hz, naphthaline-H), 7.74–7.70 (m, 2H, naphthaline-H), 7.55 (t, 1H, J = 8.00 Hz, pyridylimidazole-H), 7.48 (d, 1H, J = 8.00 Hz, PhH), 7.31 (dd, 1H, J1 = 8.00 Hz, J2 = 4.00 Hz, naphthaline-H), 7.17 (dd, 1H, J1 = 8.00 Hz, J2 = 4.00 Hz, naphthaline-H), 7.11 (d, 1H, J = 8.00 Hz, PhH), 4.49–4.39 (m, 2H, S-CH2), 2.62–2.55 (m, 1H, cyclopropyl-H), 1.19–1.15 (m, 2H, cyclopropyl-H), 0.91–0.87 (m, 2H, cyclopropyl-H). 13C-NMR (100 MHz, DMSO-d6, ppm) δ: 167.41 (C=O), 156.86, 155.62, 144.44, 142.98, 140.99, 136.88, 134.31, 132.80, 131.16, 129.52, 128.33, 128.24, 127.61, 127.33, 125.70, 124.54, 123.41, 122.68, 118.58, 117.99, 117.90, 115.49, 108.63, 36.67 (S-C), 13.43 (cyclopropyl-C), 7.77 (cyclopropyl-C), 7.69 (cyclopropyl-C). ESI-MS: m/z 554.4 (M + 1), 556.3 (M + 3), 558.4 (M + 5). C28H20BrN5OS [553.06].
N-(4-Acetyl-2-bromophenyl)-2-(1-(4-cyclopropylnaphthalen-1-yl)-1H-imidazo[4,5-b]pyridin-2-ylthio)acetamide (11)
White powder, yield: 47.7%. mp: 195–196 °C. 1H NMR (400 MHz, DMSO-d6, ppm) δ: 10.16 (s, 1H, NH), 8.62 (d, 1H, J = 8.00 Hz, pyridylimidazole-H), 8.44 (d, 1H, J = 4.00 Hz, naphthaline-H), 8.17 (d, 1H, J = 4.00 Hz, PhH), 8.07 (d, 1H, J = 8.00 Hz, pyridylimidazole-H), 7.97 (d, 1H, J = 8.00 Hz, naphthaline-H), 7.74-7.70 (m, 2H, naphthaline-H), 7.55 (t, 1H, J = 8.00 Hz, pyridylimidazole-H), 7.49 (d, 1H, J = 8.00 Hz, PhH), 7.30 (d, 1H, J = 8.00 Hz naphthaline-H), 7.17 (dd, 1H, J1 = 8.00 Hz, J2 = 4.00 Hz, naphthaline-H), 7.12 (d, 1H, J = 8.00 Hz, PhH), 4.49–4.40 (m, 2H, S-CH2), 2.60–2.55 (m, 4H, cyclopropyl-H, CH3), 1.19–1.15 (m, 2H, cyclopropyl-H), 0.91–0.87 (m, 2H, cyclopropyl-H). 13C-NMR (100 MHz, DMSO-d6, ppm) δ: 167.18 (C=O), 156.87, 155.68, 144.45, 142.97, 140.54, 134.74, 134.31, 133.03, 131.15, 129.53, 128.76 (2 × C), 128.36, 128.23, 127.61, 127.33, 125.70, 124.24, 123.41, 122.69, 118.55, 117.93, 115.59, 36.69 (S-C), 27.08 (CH3), 13.43 (cyclopropyl-C), 7.77 (cyclopropyl-C), 7.69 (cyclopropyl-C). ESI-MS: m/z 571.3 (M + 1), 573.3 (M + 3), 575.4 (M + 5). C29H23BrN4O2S [570.07].
N-(2-Bromo-4-sulfonamido-phenyl)-2-(1-(4-cyclopropylnaphthalen-1-yl)-1H-imidazo[4,5-b]pyridin-2-ylthio)acetamide (12)
White powder, yield: 46.1%. mp: 185–186 °C. 1H NMR (400 MHz, DMSO-d6, ppm) δ: 10.21 (s, 1H, NH), 8.62 (d, 1H, J = 8.00 Hz, pyridylimidazole-H), 8.43 (d, 1H, J = 4.00 Hz, naphthaline-H), 8.04 (d, 1H, J = 4.00 Hz, pyridylimidazole-H), 8.01 (s, 1H, PhH), 7.81 (d, 1H, J = 8.00 Hz, naphthaline-H), 7.72–7.70 (m, 2H, naphthaline-H), 7.55 (t, 1H, J = 8.00 Hz, pyridylimidazole-H), 7.49 (m, 3H, PhH, SONH2), 7.30 (d, 1H, J = 8.00 Hz, naphthaline-H), 7.17 (dd, 1H, J1 = 8.00 Hz, J2 = 4.00 Hz, naphthaline-H), 7.12 (d, 1H, J = 8.00 Hz, PhH), 4.48–4.39 (m, 2H, S-CH2), 2.62–2.55 (m, 1H, cyclopropyl-H), 1.18–1.16 (m, 2H, cyclopropyl-H), 0.90-0.88 (m, 2H, cyclopropyl-H). 13C-NMR (100 MHz, DMSO-d6, ppm) δ: 168.25 (C=O), 156.87, 155.74, 144.45, 142.97, 141.85, 139.46, 134.31, 131.14, 130.46, 129.55, 128.37, 128.23, 127.61, 127.33, 126.05, 125.70, 125.32, 123.42, 122.70, 118.55, 117.93, 115.95, 36.61 (S-C), 13.43 (cyclopropyl-C), 7.78 (cyclopropyl-C), 7.70 (cyclopropyl-C). ESI-MS: m/z 608.2 (M + 1), 610.2 (M + 3), 612.3 (M + 5). C27H22BrN5O3S2 [607.03].
3-Bromo-4-(2-(1-(4-cyclopropylnaphthalen-1-yl)-1H-imidazo[4,5-b]pyridin-2-ylthio)acetamido)benzamide (13)
White powder, yield: 36.8%. mp: 149–151 °C. 1H NMR (400 MHz, DMSO-d6, ppm) δ: 10.09 (s, 1H, NH), 8.62 (d, 1H, J = 8.00 Hz, pyridylimidazole-H), 8.44 (d, 1H, J = 4.00 Hz, naphthaline-H), 8.15 (s, 1H, naphthaline-H), 8.05 (s, 1H, PhH), 7.94–7.74 (m, 2H, pyridylimidazole-H, naphthaline-H), 7.72 (d, 2H, J = 8.00 Hz, naphthaline-H), 7.53–7.30 (m, 3H, pyridylimidazole-H, CONH2), 7.28 (s, 1H, PhH), 7.17–7.10 (m, 2H, naphthaline-H), 7.11 (dd, 1H, J1 = 12.00 Hz, J2 = 8.00 Hz, PhH), 4.47–4.38 (m, 2H, S-CH2), 2.64–2.53 (m, 1H, cyclopropyl-H), 1.19–1.18 (m, 2H, cyclopropyl-H), 0.89–0.88 (m, 2H, cyclopropyl-H). 13C-NMR (100 MHz, DMSO-d6, ppm) δ: 166.98 (C=O), 166.37 (C=O), 156.90, 155.71, 144.43, 142.96, 138.93, 134.31, 132.45, 132.31, 131.14, 129.55, 128.37, 128.22, 127.87, 127.60, 127.33, 125.69, 124.57, 123.42, 122.70, 118.53, 117.91, 115.75, 36.62 (CH-S), 13.43 (cyclopropyl-C), 7.77 (cyclopropyl-C), 7.69 (cyclopropyl-C). ESI-MS: m/z 572.3 (M + 1), 574.4 (M + 3), 594.4 (M + 23). C28H22BrN5O2S [571.07].
3-Chloro-4-(2-(1-(4-cyclopropylnaphthalen-1-yl)-1H-imidazo[4,5-b]pyridin-2-ylthio)acetamido)benzoic acid (14)
White powder, yield: 28.6%. mp: 224–225 °C. 1H NMR (400 MHz, DMSO-d6, ppm) δ: 13.13 (s, 1H, COOH), 11.34 (s, 1H, NH), 8.62 (d, 1H, J = 8.00 Hz, pyridylimidazole-H), 8.44 (d, 1H, J = 8.00 Hz, naphthaline-H), 8.17 (d, 1H, J = 8.00 Hz, pyridylimidazole-H), 7.96 (s, 1H, PhH), 7.90 (d, 1H, J = 12.00 Hz, naphthaline-H), 7.72–7.71 (m, 2H, naphthaline-H), 7.55 (t, 1H, J = 8.00 Hz, pyridylimidazole-H), 7.48 (d, 1H, J = 8.00 Hz, PhH), 7.30 (d, 1H, J = 8.00 Hz, naphthaline-H), 7.18–7.14 (m, 1H, PhH), 7.11 (d, 1H, J = 8.00 Hz, naphthaline-H), 4.48–4.38 (m, 2H, S-CH2), 2.62–2.55 (m, 1H, cyclopropyl-H), 1.19–1.16 (m, 2H, cyclopropyl-H), 0.90-0.88 (m, 2H, cyclopropyl-H). 13C-NMR (100 MHz, DMSO-d6, ppm) δ: 167.34 (C=O), 166.24 (C=O), 156.89, 155.63, 144.49, 142.98, 139.17, 134.31, 131.13, 130.71, 129.53, 129.21, 128.34, 128.24, 128.12, 127.61, 127.34, 125.69, 124.47, 123.41 (2 × C), 122.66, 118.58, 117.94, 36.56 (S-C), 13.43 (cyclopropyl-C), 7.77 (cyclopropyl-C), 7.69 (cyclopropyl-C). ESI-MS: m/z 529.3 (M + 1), 531.2 (M + 3), 551.3 (M + 23). C28H21ClN4O3S [528.1].
Ethyl3-chloro-4-(2-(1-(4-cyclopropylnaphthalen-1-yl)-1H-imidazo[4,5-b]pyridin-2-ylthio)acetamido)benzoate (15)
White powder, yield: 42.5%. mp: 141–142 °C. 1H NMR (400 MHz, DMSO-d6, ppm) δ: 10.36 (s, 1H, NH), 8.62 (d, 1H, J = 8.00 Hz, pyridylimidazole-H), 8.44 (d, 1H, J = 4.00 Hz, naphthaline-H), 8.21 (d, 1H, J = 8.00 Hz, pyridylimidazole-H), 7.98 (s, 1H, PhH), 7.92 (d, 1H, J = 8.00 Hz, naphthaline-H), 7.72–7.70 (m, 2H, naphthaline-H), 7.53(t, 1H, J = 8.00 Hz, pyridylimidazole-H), 7.48 (d, 1H, J = 8.00 Hz, PhH), 7.31 (d, 1H, J = 8.00 Hz, naphthaline-H), 7.17 (dd, 1H, J1 = 8.00 Hz, J2 = 4.00 Hz, naphthaline-H), 7.11 (d, 1H, J = 8.00 Hz, PhH), 4.49–4.39 (m, 2H, S-CH2), 4.33 (q, 2H, J = 4.00 Hz, CH2), 2.62–2.55 (m, 1H, cyclopropyl-H), 1.33 (t, 3H, J = 4.00 Hz, CH3), 1.20–1.15 (m, 2H, cyclopropyl-H), 0.91–0.87 (m, 2H, cyclopropyl-H). 13C-NMR (100 MHz, DMSO-d6, ppm) δ: 167.20 (C=O), 164.69 (C=O), 156.98, 155.63, 144.49, 142.98, 139.53, 134.31, 131.13, 130.53, 129.53, 129.07, 128.34, 128.24, 127.61, 127.33, 127.02, 125.69, 124.49, 123.40 (2 × C), 122.66, 118.58, 117.95, 61.63 (CH2-O), 36.37 (S-C), 14.58 (CH3), 13.43 (cyclopropyl-C), 7.77 (cyclopropyl-C), 7.69 (cyclopropyl-C). ESI-MS: m/z 557.2 (M + 1), 559.3 (M + 3), 579.4 (M + 23). C30H25ClN4O3S [556.13].
N-(2-Chloropyridin-3-yl)-2-(1-(4-cyclopropylnaphthalen-1-yl)-1H-imidazo[4,5-b]pyridin-2-ylthio)acetamide (16)
White powder, yield: 40.1%. mp: 204–205 °C. 1H NMR (400 MHz, DMSO-d6, ppm) δ: 10.29 (s, 1H, NH), 8.62 (d, 1H, J = 8.00 Hz, pyridylimidazole-H), 8.44 (d, 1H, J = 8.00 Hz, naphthaline-H), 8.30 (d, 1H, J = 8.00 Hz, pyridine-H), 8.20 (d, 1H, J = 4.00 Hz, pyridylimidazole-H), 7.72–7.70 (m, 2H, naphthaline-H), 7.53 (t, 1H, J = 8.00 Hz, pyridylimidazole-H), 7.48 (d, 1H, J = 4.00 Hz, pyridine-H), 7.45 (dd, 1H, J1 = 8.00 Hz, J2 = 4.00 Hz, naphthaline-H), 7.30 (d, 1H, J = 8.00 Hz, naphthaline-H), 7.17 (dd, 1H, J1 = 8.00 Hz, J2 = 4.00 Hz, pyridine-H), 7.12 (d, 1H, J = 8.00 Hz, naphthaline-H), 4.47–4.38 (m, 2H, S-CH2), 2.62–2.55 (m, 1H, cyclopropyl-H), 1.18–1.15 (m, 2H, cyclopropyl-H), 0.91–0.87 (m, 2H, cyclopropyl-H). 13C-NMR (100 MHz, DMSO-d6, ppm) δ: 167.40 (C=O), 156.95, 155.69, 145.63, 144.45, 142.96, 134.31, 133.09, 132.32, 131.10, 129.44, 128.38, 128.22, 127.60, 127.32, 125.69, 123.93 (2 × C), 123.41, 122.69, 118.54, 117.90, 36.56 (S-C), 13.43 (cyclopropyl-C), 7.77 (cyclopropyl-C), 7.69 (cyclopropyl-C). ESI-MS: m/z 486.4 (M + 1), 489.4 (M + 3), 508.3 (M + 23). C26H20ClN5OS [485.11].
3-Chloro-4-(2-(1-(4-cyclopropylnaphthalen-1-yl)-1H-imidazo[4,5-b]pyridin-2-ylthio)acetamido)-N-methoxybenzamide (17)
White powder, yield: 41.1%. mp: 160–161 °C. 1H NMR (400 MHz, DMSO-d6, ppm) δ: 11.83 (s, 1H, CONH), 10.29 (s, 1H, NH), 8.62 (d, 1H, J = 8.00 Hz, pyridylimidazole-H), 8.44 (d, 1H, J = 8.00 Hz, naphthaline-H), 8.09 (d, 1H, J = 8.00 Hz, pyridylimidazole-H), 7.85 (s, 1H, PhH), 7.72–7.55 (m, 3H, naphthaline-H), 7.53 (t, 1H, J = 8.00 Hz, pyridylimidazole-H), 7.48 (d, 1H, J = 8.00 Hz, PhH), 7.30 (d, 1H, J = 8.00 Hz, naphthaline-H), 7.17 (m, 1H, PhH), 7.11 (d, 1H, J = 8.00 Hz, naphthaline-H), 4.48–4.38 (m, 2H, S-CH2), 3.70 (s, 3H, N-CH3), 2.62–2.55 (m, 1H, cyclopropyl-H), 1.19–1.16 (m, 2H, cyclopropyl-H), 0.90–0.88 (m, 2H, cyclopropyl-H). 13C-NMR (100 MHz, DMSO-d6, ppm) δ: 170.27 (C=O), 166.69 (C=O), 156.46, 155.15, 143.96, 142.46, 137.57, 133.80, 130.61, 129.11, 129.02, 128.10, 127.85, 127.71, 127.09, 126.81, 126.41, 125.18, 124.33, 123.29, 122.88, 122.15, 118.04, 117.40, 63.29 (O-CH), 36.11 (S-CH), 12.91 (cyclopropyl-C), 7.25 (cyclopropyl-C), 7.18 (cyclopropyl-C). ESI-MS: m/z 588.3 (M + 1), 560.3 (M + 3), 580.3 (M + 23). C29H24ClN5O3S [557.13].
Ethyl2-(3-chloro-4-(2-(1-(4-cyclopropylnaphthalen-1-yl)-1H-imidazo[4,5-b]pyridin-2-ylthio)acetamido)benzamido)acetate (18)
White powder, yield: 44.2%. mp: 221–222 °C. 1H NMR (400 MHz, DMSO-d6, ppm) δ: 10.27 (s, 1H, NH), 9.03 (t, 1H, J = 8.00 Hz, NH), 8.62 (d, 1H, J = 8.00 Hz, pyridylimidazole-H), 8.44 (d, 1H, J = 4.00 Hz, naphthaline-H), 8.11 (d, 1H, J = 12.00 Hz, pyridylimidazole-H), 7.99 (s, 1H, PhH), 7.85 (d, 1H, J = 12.00 Hz, naphthaline-H), 7.72–7.70 (m, 2H, naphthaline-H), 7.55 (t, 1H, J = 8.00 Hz, pyridylimidazole-H), 7.49 (d, 1H, J = 12.00 Hz, PhH), 7.31 (d, 1H, J = 16.00 Hz, naphthaline-H), 7.17 (dd, 1H, J1 = 12.00 Hz, J2 = 8.0 Hz, naphthaline-H), 7.12 (d, 1H, J = 8.00 Hz, PhH), 4.49–4.37 (m, 2H, S-CH2), 4.16 (q, 2H, J = 8.00 Hz, CH2), 4.04–3.99 (m, 2H, CH2), 2.63–2.55 (m, 1H, cyclopropyl-H), 1.23–1.16 (m, 5H, cyclopropyl-H, CH3), 0.91–0.86 (m, 2H, cyclopropyl-H). 13C-NMR (100 MHz, DMSO-d6, ppm) δ: 169.68 (C=O), 166.69 (C=O), 164.77 (C=O), 156.47, 155.15, 143.95, 142.46, 137.49, 133.80, 130.61, 130.54, 129.03, 128.37, 127.85, 127.71, 127.08, 126.81, 126.63, 125.17, 124.31, 123.22, 122.89, 122.15, 118.04, 117.41, 60.44 (O-CH), 41.31(N-CH), 36.13 (S-CH), 14.504 (CH3), 12.91 (cyclopropyl-C), 7.25 (cyclopropyl-C), 7.17 (cyclopropyl-C). ESI-MS: m/z 614.3 (M + 1). C32H28ClN5O4S [613.16].
Ethyl2-(3-chloro-4-(2-(1-(4-cyclopropylnaphthalen-1-yl)-1H-imidazo[4,5-b]pyridin-2-ylthio)acetamido)benzamido)propanoate (19)
White powder, yield: 48.0%. mp: 223–224 °C. 1H NMR (400 MHz, DMSO-d6, ppm) δ: 10.26 (s, 1H, NH), 8.64–8.60 (m, 2H, pyridylimidazole-H, PhH), 8.43 (d, 1H, J = 4.00 Hz, naphthaline-H), 8.06 (d, 1H, J = 8.00 Hz, pyridylimidazole-H), 7.94 (s, 1H, NH), 7.80 (d, 1H, J = 12.00 Hz, naphthaline-H), 7.72–7.70 (m, 2H, naphthaline-H), 7.55 (t, 1H, J = 8.00 Hz, pyridylimidazole-H), 7.48 (d, 1H, J = 8.00 Hz, PhH), 7.30 (d, 1H, J = 4.00 Hz, naphthaline-H), 7.17 (dd, 1H, J1 = 8.00 Hz, J2 = 4.00 Hz, naphthaline-H), 7.11 (d, 1H, J = 8.00 Hz, PhH), 4.47–4.38 (m, 2H, S-CH2), 4.09 (q, 2H, J = 8.00 Hz, O-CH2), 3.50 (q, 1H, J = 8.00 Hz, N-CH-C), 2.59–2.55(m, 3H, cyclopropyl-H), 1.19–1.15 (m, 6H, 2 × CH3), 0.91–0.87 (m, 2H, cyclopropyl-H). 13C-NMR (100 MHz, DMSO-d6, ppm) δ: 171.71 (C=O), 167.14 (C=O), 164.90 (C=O), 156.99, 155.67, 144.46, 142.97, 137.68, 134.31, 131.78, 131.12, 129.54, 128.74, 128.37, 128.23, 127.60, 127.33, 127.03, 125.70, 124.79, 123.73, 123.41, 122.67, 118.55, 117.92, 60.41 (O-CH), 36.63(N-CH), 36.06 (S-CH), 34.14 (CH3), 14.54 (CH3), 13.43 (cyclopropyl-C), 7.77 (cyclopropyl-C), 7.69 (cyclopropyl-C). ESI-MS: m/z 628.5 (M + 1), 630.4 (M + 3), 650.5 (M + 23). C33H30ClN5O4S [627.17].
3-Chloro-4-(2-(1-(4-cyclopropylnaphthalen-1-yl)-1H-imidazo[4,5-b]pyridin-2-ylthio)acetamido)benzamide (20)
White powder, yield: 47.4%. mp: 158–160 °C. 1H NMR (400 MHz, DMSO-d6, ppm) δ: 10.26 (s, 1H, NH), 8.62 (d, 1H, J = 8.00 Hz, pyridylimidazole-H), 8.44 (d, 1H, J = 4.00 Hz, naphthaline-H), 8.06 (d, 2H, J = 8.00 Hz, naphthaline-H), 7.99 (s, 1H, PhH), 7.84 (d, 1H, J = 8.00 Hz, pyridylimidazole-H,), 7.72 (d, 2H, J = 8.00 Hz, naphthaline-H), 7.53–7.31 (m, 3H, pyridylimidazole-H, CONH2), 7.30 (d, 1H, J = 8.00 Hz, PhH), 7.17–7.14 (m, 1H, naphthaline-H), 7.11 (d, 1H, J = 8.00 Hz, PhH), 4.84–4.38 (m, 2H, S-CH2), 2.62–2.55 (m, 1H, cyclopropyl-H), 1.19–1.18 (m, 2H, cyclopropyl-H), 0.91–0.87 (m, 2H, cyclopropyl-H). 13C-NMR (100 MHz, DMSO-d6, ppm) δ: 167.15 (C=O), 166.47 (C=O), 156.98, 155.67, 144.46, 142.97, 137.71, 134.31, 131.79, 131.12, 129.54, 129.09, 128.37, 128.23 (2 × C), 127.61, 127.33, 125.70, 124.75, 123.63, 123.41, 122.67, 118.55, 117.91, 36.62 (CH-S), 13.43 (cyclopropyl-C), 7.77 (cyclopropyl-C), 7.66 (cyclopropyl-C). ESI-MS: m/z 528.4 (M + 1), 531.4 (M + 3), 550.5 (M + 23). C28H22ClN5O2S [527.12].
Methyl4-(2-(1-(4-cyclopropylnaphthalen-1-yl)-1H-imidazo[4,5-b]pyridin-2-ylthio)acetamido)-3-nitrobenzoate (21)
Yellowish-white powder, yield: 47.1%. mp: 130–131 °C. 1H NMR (400 MHz, DMSO-d6, ppm) δ: 11.11 (s, 1H, NH), 8.62 (d, 1H, J = 8.00 Hz, pyridylimidazole-H), 8.45 (s, 1H, PhH), 8.41 (d, 1H, J = 8.00 Hz, naphthaline-H), 8.12 (d, 1H, J = 8.00 Hz, pyridylimidazole-H), 7.74–7.69 (m, 2H, naphthaline-H), 7.55 (t, 1H, J = 8.00 Hz, pyridylimidazole-H), 7.49 (d, 1H, J = 8.00 Hz, PhH), 7.29 (d, 2H, J = 8.00 Hz, naphthaline-H), 7.16–7.12 (m, 2H, naphthaline-H, PhH), 4.44 (s, 2H, S-CH2), 3.89 (s, 3H, CH3), 2.62–2.55 (m, 1H, cyclopropyl-H), 1.20–1.15 (m, 2H, cyclopropyl-H), 0.91–0.89 (m, 2H, cyclopropyl-H). 13C-NMR (100 MHz, DMSO-d6, ppm) δ: 167.05 (C=O), 164.69 (C=O), 156.40, 155.71, 144.34, 142.95, 140.53, 135.92, 135.09, 134.31, 131.16, 129.57, 128.37, 128.20, 127.60, 127.26, 126.63, 126.06, 125.65, 124.64, 123.39, 122.78, 118.51, 117.90, 53.11 (O-C), 36.61 (S-C), 13.43 (cyclopropyl-C), 7.78 (cyclopropyl-C), 7.67 (cyclopropyl-C). ESI-MS: m/z 554.3 (M + 1), 576.3 (M + 23). C29H23N5O5S [553.14].
N-(2-Chloro-4-sulphonylaminophenyl)-2-(1-(4-cyclopropylnaphthalen-1-yl)-1H-imidazo[4,5-b]pyridin-2-ylthio)propanamide (22)
White powder, yield: 49.9%. mp: 242–243 °C. 1H NMR (400 MHz, DMSO-d6, ppm) δ: 10.59 (d, 1H, J = 60.00 Hz, NH), 8.62 (d, 1H, J = 8.00 Hz, pyridylimidazole-H), 8.17 (dd, 1H, J1 = 8.00 Hz, J2 = 4.00 Hz, naphthaline-H), 7.89 (s, 1H, PhH), 7.76–7.67 (m, 3H, pyridylimidazole-H, naphthaline-H), 7.47–7.44 (m, 4H, pyridylimidazole-H, PhH, SO2NH2), 7.33–7.30 (m, 1H, naphthaline-H), 7.19 (dd, 2H, J1 = 8.00 Hz, J2 = 4.00 Hz, naphthaline-H), 7.11 (dd, 1H, J1 = 24.00 Hz, J2 = 8.00 Hz, PhH), 5.16–5.12 (m, 1H, S-CH), 2.57–2.55 (m, 1H, cyclopropyl-H), 1.65 (dd, 3H, J1 = 24.00 Hz, J2 = 8.00 Hz, CH3), 1.19–1.15 (m, 2H, cyclopropyl-H), 0.89–0.88 (m, 2H, cyclopropyl-H). 13C-NMR (100 MHz, DMSO-d6, ppm) δ: 170.57 (C=O), 156.37, 155.66, 144.62, 143.02, 141.44, 138.05, 134.27, 131.61, 130.92, 129.53, 128.27, 127.59, 127.34, 127.27, 125.71, 125.54, 124.64, 123.41, 122.64, 122.45, 118.70, 118.06, 45.54 (CH), 18.51(CH3), 13.42 (cyclopropyl-C), 7.80 (cyclopropyl-C), 7.66 (cyclopropyl-C). ESI-MS: m/z 578.4 (M + 1). C28H24ClN5O3S2 [577.1].
3-Chloro-4-(2-(1-(4-cyclopropylnaphthalen-1-yl)-1H-imidazo[4,5-b]pyridin-2-ylthio)propanamido)benzamide (23)
White powder, yield: 45.9%. mp: 158–160 °C. 1H NMR (400 MHz, DMSO-d6, ppm) δ: 10.47 (d, 1H, J = 68.00 Hz, NH), 8.62 (t, 1H, J = 8.00 Hz, pyridylimidazole-H), 8.46 (d, 1H, J = 4.00 Hz, naphthaline-H), 8.06–7.98 (m, 3H, pyridylimidazole-H, PhH, naphthaline-H), 7.83 (s, 1H, PhH), 7.72–7.69 (m, 2H, naphthaline-H), 7.47–7.41 (m, 3H, pyridylimidazole-H, CONH2), 7.32 (d, 1H, J = 8.00 Hz naphthaline-H), 7.19 (dd, 1H, J1 = 8.00 Hz, J2 = 4.00 Hz, naphthaline-H), 7.11 (dd, 1H, J1 = 12.00 Hz, J2 = 8.00 Hz, PhH), 5.17 (q, 1H, J = 8.00 Hz, S-CH), 2.60–2.56 (m, 1H, cyclopropyl-H), 1.65 (dd, 3H, J1 = 24.00 Hz, J2 = 8.00 Hz, CH3), 1.19–1.18 (m, 2H, cyclopropyl-H), 0.89–0.88 (m, 2H, cyclopropyl-H). 13C-NMR (100 MHz, DMSO-d6, ppm) δ: 170.33 (C=O), 166.46 (C=O), 156.45, 155.67, 144.55, 143.01, 137.57, 134.30, 131.97, 130.94, 129.53, 129.07, 128.21, 127.62, 127.32 (2 × C), 125.71, 125.25, 123.91, 123.41, 122.64, 122.45, 118.62, 118.06, 95.02, 45.53 (CH), 18.60 (CH3), 13.42 (cyclopropyl-C), 7.79 (cyclopropyl-C), 7.65 (cyclopropyl-C). ESI-MS: m/z 542.4 (M + 1), 545.4 (M + 3), 564.4 (M + 23). C29H24ClN5O2S [541.13].
N-(2-Bromo-4-sulphonylaminophenyl)-2-(1-(4-cyclopropylnaphthalen-1-yl)-1H-imidazo[4,5-b]pyridin-2-ylthio)propanamide (24)
White powder, yield: 38.7%. mp: 240–242 °C. 1H NMR (400 MHz, DMSO-d6, ppm) δ: 10.41 (d, 1H, J = 44.00 Hz, NH), 8.61 (d, 1H, J = 8.00 Hz, pyridylimidazole-H), 8.46 (d, 1H, J = 4.00 Hz, naphthaline-H), 8.05 (d, 1H, J = 4.00 Hz, PhH), 8.01 (d, 1H, J = 8.00 Hz, pyridylimidazole-H), 7.80 (d, 1H, J = 8.00 Hz, naphthaline-H), 7.72–7.67 (m, 2H, naphthaline-H), 7.55–7.45 (m, 4H, pyridylimidazole-H, PhH, SO2NH2), 7.31 (d, 1H, J = 8.00 Hz naphthaline-H), 7.19 (dd, 1H, J1 = 12.00 Hz, J2 = 8.00 Hz, naphthaline-H), 7.11 (dd, 1H, J1 = 12.00 Hz, J2 = 8.00 Hz, PhH), 5.16–5.11 (m, 1H, S-CH), 2.61–2.56 (m, 1H, cyclopropyl-H), 1.68 (dd, 3H, J1 = 24.00 Hz, J2 = 8.00 Hz, CH3), 1.19–1.15 (m, 2H, cyclopropyl-H), 0.89–0.88 (m, 2H, cyclopropyl-H). 13C-NMR (100 MHz, DMSO-d6, ppm) δ: 170.42 (C=O), 156.31, 155.75, 144.55, 142.97, 142.12, 139.34, 134.28, 130.98, 130.43, 129.55, 128.31, 127.59, 127.35, 126.03, 125.82, 125.71, 123.41, 122.64, 122.53, 118.64, 118.03, 116.53, 45.76 (CH), 18.84(CH3), 13.42 (cyclopropyl-C), 7.80 (cyclopropyl-C), 7.65 (cyclopropyl-C). ESI-MS: m/z 622.4 (M + 1), 624.4 (M + 3), 626.3 (M + 5). C28H24BrN5O3S2 [621.05].
3-Bromo-4-(2-(1-(4-cyclopropylnaphthalen-1-yl)-1H-imidazo[4,5-b]pyridin-2-ylthio)propanamido)benzamide (25)
White powder, yield: 46.1%. mp: 155–157 °C. 1H NMR (400 MHz, DMSO-d6, ppm) δ: 10.30 (d, 1H, J = 52.00 Hz, NH), 8.62 (d, 1H, J = 8.00 Hz, pyridylimidazole-H), 8.46 (d, 1H, J = 4.00 Hz, naphthaline-H), 8.14 (d, 1H, J = 4.00 Hz, pyridylimidazole-H), 8.05 (d, 1H, J = 4.00 Hz, PhH), 7.88–7.85 (m, 2H, PhH, naphthaline-H), 7.72–7.67 (m, 2H, naphthaline-H), 7.55–7.43 (m, 3H, pyridylimidazole-H, CONH2), 7.32 (d, 1H, J = 8.00 Hz naphthaline-H), 7.19 (dd, 1H, J1 = 12.00 Hz, J2 = 8.00 Hz, naphthaline-H), 7.11 (dd, 1H, J1 = 12.00 Hz, J2 = 8.00 Hz, PhH), 5.15 (m, 1H, S-CH), 2.57–2.55 (m, 1H, cyclopropyl-H), 1.68 (dd, 3H, J1 = 24.00 Hz, J2 = 8.00 Hz, CH3), 1.19 (m, 2H, cyclopropyl-H), 0.89-0.88 (m, 2H, cyclopropyl-H). 13C-NMR (100 MHz, DMSO-d6, ppm) δ: 170.33 (C=O), 166.44 (C=O), 156.39, 155.75, 144.51, 142.96, 138.76, 134.28, 132.71, 132.31, 130.95, 129.54, 128.31, 128.16, 127.84, 127.57, 127.35, 125.70, 125.10, 123.41, 122.54, 118.62, 118.01, 116.53, 45.86 (CH), 18.88(CH3), 13.42 (cyclopropyl-C), 7.79 (cyclopropyl-C), 7.64 (cyclopropyl-C). ESI-MS: m/z 586.4 (M + 1), 590.4 (M + 5). C29H24BrN5O2S [585.08].
In vitro anti-HIV assay
Evaluation of the antiviral activity and cytotoxicity of the compounds in MT-4 cells was performed using the MTT assay as previously described 36, 37. Stock solutions (10 × final concentration) of test compounds were added in 25 μL volumes to two series of triplicate wells so as to allow simultaneous evaluation of their effects on mock- and HIV-infected cells at the beginning of each experiment. Serial fivefold dilutions of test compounds were made directly in flat-bottomed 96-well microtiter trays using a Biomek 3000 robot (Beckman instruments, Fuller-ton, CA, USA). Untreated control HIV- and mock-infected cell samples were included for each sample.
HIV-1 (IIIB), K103N/Y181C double RT-mutant strain (RES056), or HIV-2 (ROD) 38 stock (50 μL) at 100–300 CCID50 (cell culture infectious dose) or culture medium was added to either the infected or mock-infected wells of the microtiter tray. Mock-infected cells were used to evaluate the effect of test compound on uninfected cells in order to assess the cytotoxicity of the test compound. Exponentially growing MT-4 cells were centrifuged for 5 min at 95 g, and the supernatant was discarded. The MT-4 cells were resuspended at 6 × 105 cells/mL, and 50-μL volumes were transferred to the microtiter tray wells. Five days after infection, the viability of mock- and HIV-infected cells was examined spectrophotometrically by the MTT assay. The 50% cytotoxic concentration (CC50) was defined as the concentration of the test compound that reduced the absorbance (OD540) of the mock-infected control sample by 50%. The concentration achieving 50% protection from the cytopathic effect of the virus in infected cells was defined as the 50% effective concentration (EC50).
HIV-1 RT inhibition assay
A reverse transcriptase (RT) assay kit produced by Roche was selected for the RT inhibition assay. All the reagents for performing the RT reaction came with the kit, and the ELISA procedures for RT inhibition assay were carried out following the description in the kit protocol. Briefly, the reaction mixture containing template/primer complex, viral nucleotides (dNTPs), and RT in the incubation buffer with or without inhibitors was incubated for 1 h at 37 °C. After that, the reaction mixture was transferred to a streptavidin-coated microtiter plate and incubated for another 1 h at 37 °C to make sure that retranscriptional cDNA chain consisted of biotin-labeled dNTPs bound to streptavidin. Then, unbound dNTPs were removed using washing buffer and anti-DIG-POD working solution was added. After incubation for 1 h at 37 °C, the DIG-labeled dNTPs incorporated into cDNA were bound to the anti-DIG-POD antibody. The unbound anti-DIG-PODs were removed and the peroxide substrate (ABST) solution was added to the MTPs. A colored reaction proceeded during the cleavage of the substrate catalyzed by POD. The absorbance of the sample was determined at OD 405 nm using a microtiter plate ELISA reader. The percentage inhibitory activity of RT inhibitors was calculated by formula as given below:
%Inhibition = [OD value with RT but without inhibitors – OD value with RT and inhibitors]/[OD value with RT and inhibitors – OD value without RT and inhibitors].
The IC50 values corresponded to the concentrations of the inhibitors required to inhibit biotin-dUTP incorporation by 50%.
Molecular simulation experiment
The molecule 12 for docking was optimized for 2000 generations until the maximum derivative of energy became 0.005 kcal/(mol*A), using the Tripos force field. Charges were computed and added according to Gasteiger–Huckel parameters. The published three-dimensional crystal structures of RT complexes with GW564511 (PDB code: 3DLG) were retrieved from the Protein Data Bank and were used for the docking experiment by means of Surflex-docking module of sybyl-x 1.1. The protein was prepared by using the biopolymer application accompanying sybyl: The bound ligand was extracted from the complexes, water molecules were removed, hydrogen atoms were added, side chain amides and side chains bumps were fixed, and charges and atom types were assigned according to amber 99. After the protomol was generated, the optimized molecule 12 was Surflex-docked into the binding pocket of NNRTIs, with the relevant parameters set as defaults. Top-scoring pose was shown by the software of pymol version 1.5 (www.pymol.org.). The secondary structure of RT is shown in cartoons, and only the key residues for interactions with the inhibitor were shown in sticks and labeled. The potential hydrogen bonds were presented by dashed lines.
Results and Discussions
Chemistry
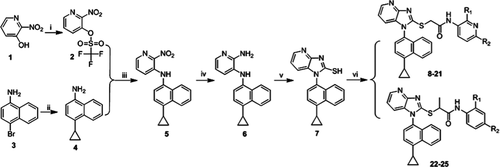
The general synthetic steps adopted to obtain the desired compounds 8–25 were straightforwardly outlined in Scheme 1. The commercially available 2-nitropyridin-3-ol (1) was converted to intermediate 2-nitropyridin-3-yl trifluoromethanesulfonate (2) by the acylation reaction with trifluoromethanesulfonic anhydride in the presence of triethylamine (Et3N)1. Then, 4-cyclopropylnaphthalen-1-amine (4) was prepared from commercially available 4-bromonaphthalen-1-amine (3) and cyclopropylboronic acid by the classical Suzuki-coupling reaction 392. The metal-catalyzed cross-coupling reaction of 4 with already prepared intermediate 2 afforded N-(4-cyclopropylnaphthalen-1-yl)-2-nitropyridin-3-amine (5)3. Then, the nitro group of intermediate 5 was treated with excess hydrogen gas in the presence of hydrogenation-catalyzed Pd/C (20%) to afford N3-(4-cyclopropylnaphthalen-1-yl)pyridine-2,3-diamine (6)4. Treatment of 6 with 1,1′-thiocarbonyldiimidazole in tetrahydrofuran (THF) under reflux afforded 1-(4-cyclopropylnaphthalen-1-yl)-1H-imidazo[4,5-b]pyridine-2-thiol (7) 40. The final imidazo[4,5-b]pyridin-2-ylthioacetanilides 8–25 were obtained by reaction of 7 with a set of 2-chloro-N-aryl-substituted acetamides or propanamides in a rapid and convenient way 41, 42. Both analytical and spectral data of all the newly synthesized compounds are in full agreement with the proposed structures.
Anti-HIV activity evaluation
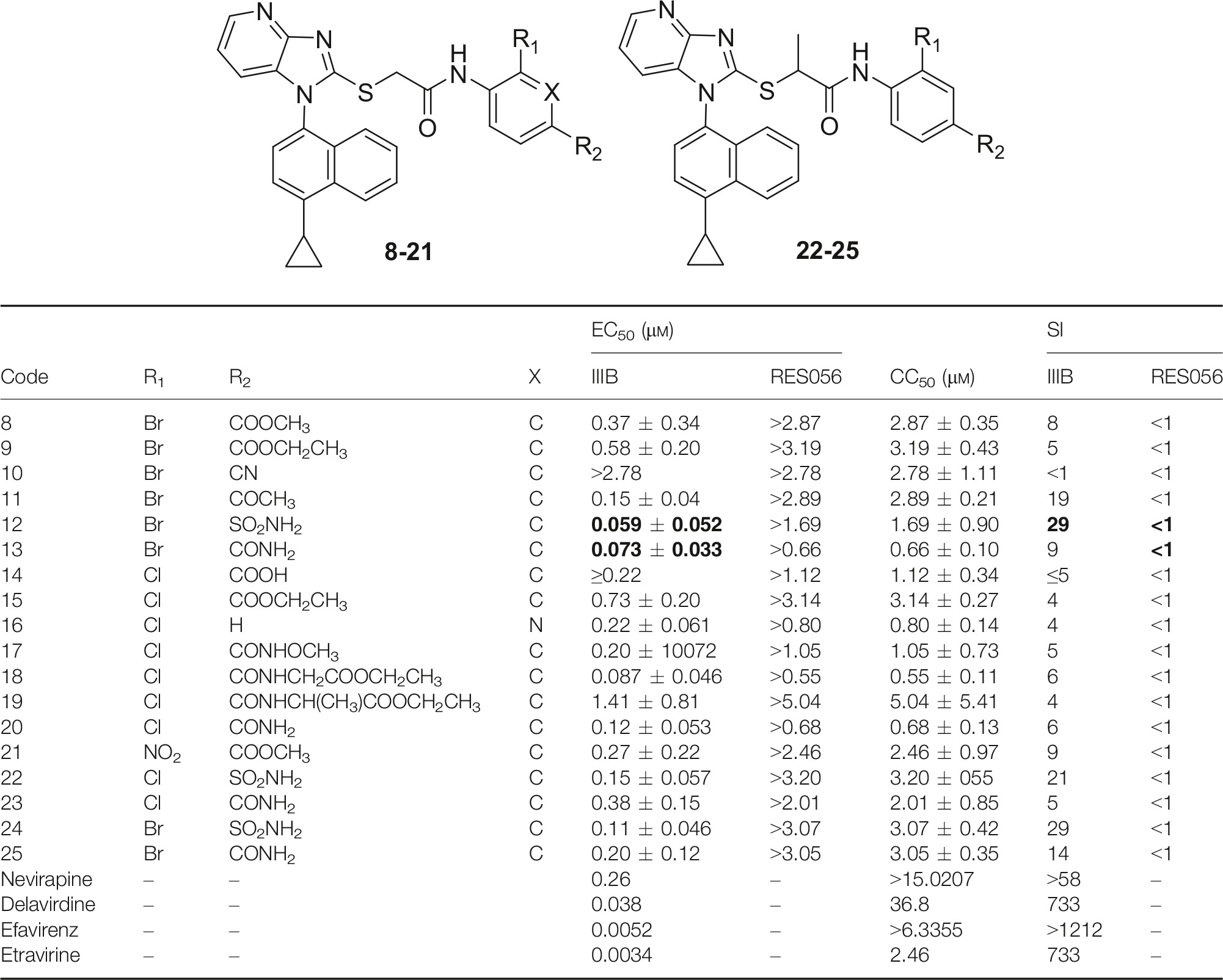
The newly synthesized imidazo[4,5-b]pyridin-2-ylthioacetanilides were evaluated for in vitro anti-HIV activity and cytotoxicity in MT-4 cell cultures infected by HIV-1 IIIB strain (wt), HIV-1 mutant strain RES056 (K103N/Y181C double RT mutant), and HIV-2 strain ROD, respectively. The FDA-approved drugs nevirapine (NVP), delavirdine (DLV), efavirenz (EFV), and etravirine (ETR) were chosen as reference drugs. Comparisons of the antiviral inhibitory concentration (EC50), cytotoxic concentration (CC50), and SI (selectivity, given by the CC50/EC50 ratio) values for different compounds are depicted in Table 1.
| Compound | 12 | NVP | ETR |
|---|---|---|---|
| IC50 (μm)a | 1.50 | 0.69 | 0.37 |
- a IC50: Inhibitory concentration of tested compounds required to inhibit biotin deoxyuridine triphosphate (biotin-dUTP) incorporation into the HIV-1 RT by 50%.
As shown in Table 1, except for compounds 10 and 14, the rest derivatives demonstrated a moderate to good anti-HIV-1 (IIIB) activity with EC50 values ranging from 1.41 to 0.059 μm, while none of them was active against HIV-2 (data were not shown). Encouragingly, the most potent two compounds 12 and 13 possessing EC50 values of 0.059 and 0.073 μm, respectively, were much better than NVP (0.26 μm) and similar to DLV (0.038 μm), but were still inferior to EFV (0.0052 μm) and ETR (0.0036 μm). Besides, inhibitory activities against wt HIV-1 of some other compounds (8, 9, 11, 15, 16, 17, 20–25) could reach the same order of magnitude as NVP. Unfortunately, none of these compounds was active against the double-mutant HIV-1 strain (RES056). In general, compared with our previously disclosed imidazo[4,5-b]pyridine and imidazo[4,5-c]pyridine derivatives, these novel imidazo[4,5-b]pyridine counterparts demonstrated a significant improvement in anti-HIV-1 (IIIB) activity (by comparing the most active compounds in two series 5b16 and 12, EC50 = 0.21, 0.059 μm, respectively), which will pave the way for the next-round rational design of AATAs.
Based on the above results, the preliminary SAR analysis was summarized in the following section. Most notably, the anti-HIV activities of compounds 8–25 are strongly dependent on the nature of the para position of the anilide moiety (R2). In the case of the 2-bromophenyl series, the order of R2 substitution for potency ranked as follows: (a) acetamides: -SO2NH2 > -CONH2 > -COCH3 > -COOCH3 > -COOCH2CH3 > -CN; (b) propanamides: -SO2NH2 > -CONH2. Similarly, obvious regularity of antiviral activity also can be found in 2-chlorophenyl compounds with sequence: (a) acetamides: -CONHCH2COOCH2CH3 > -CONH2 > -CONHOCH3 > -COOCH2CH3 > -CONHCH(CH3)COOCH2CH3 > -COOH; (b) propanamides: SO2NH2 > CONH2. Based on the activity order of para substituents, some important pieces of information can be obtained: (i) Generally, amide and sulfamide were the preferred substituents at the para position, which was in agreement with the previously reported results 27; especially, compounds bearing the sulfamide group possessed a favorable inhibitory activity, suggesting that the sulfamide group with appropriate polarity and hydrophilicity can better accommodate the chemical environment in this region of RT and form potential interactions with surrounding residues. (ii) It is interesting to note that the bioactivity of 18 was dramatically decreased when the methyl group was introduced into the R2 position (18, EC50 = 0.087 μm; 19, EC50 = 1.41 μm). The increased hydrophobicity could probably lead to unfavorable interactions with the RT–solvent interface. (iii) Among all the substituent groups in the para position of the anilide moiety, the cyan looks like unfavorable groups, resulting in absolute deactivation of the compound 10. (4) Regarding the derivatives bearing hydrophilic carboxyl group, in theory, it is regarded as a favorable group directing to the solvent-exposed region. However, mediocre activity was observed in compound 14. Probably, the cause was not the suitability with the binding site, but the poor membrane permeability in cell lines. These results indicated that the binding site of the HIV RT is very sensitive to inhibitors; meanwhile, they also confirmed proof of concept that the overall physicochemical properties of molecules was also one of the most prominent effects on the expected bioactivities. (5) Pyridine derivative 16 with a hydrogen atom in R2 position (EC50 = 0.22 μm) showed a comparable level of anti-HIV-1 activities as other substituted phenyl counterparts.
Then, we focused on the ortho substitution at the phenyl ring of the anilide moiety. Considering our prior experience that the presence of electron-withdrawing groups is essential to improve the potency against HIV-1, only Cl, Br and NO2 groups were selected as preferred substituents in this work. The results demonstrated that apart from big difference found between compounds 13 and 20 (13: R1 = Br, EC50 = 0.073 μm; 20: R1 = Cl, EC50 = 0.12 μm), these three substituents only have a marginal impact on anti-HIV-1 activity with a general order: NO2 > Br > Cl, through the pairwise comparison of the EC50 values, namely 21 (R1 = NO2, EC50 = 0.27 μm) and 8 (R1 = Br, EC50 = 0.37 μm), 9 (R1 = Br, EC50 = 0.58 μm) and 15 (R1 = Cl, EC50 = 0.73 μm), 24 (R1 = Br, EC50 = 0.11 μm) and 22 (R1 = Cl, EC50 = 0.15 μm), 25 (R1 = Br, EC50 = 0.20 μm) and 23 (R1 = Cl, EC50 = 0.38 μm).
Looking at the modification of the acetamide linker, the introduction of a methyl group (i.e. propanamide) resulted in perceptible deactivation of activities by comparing the potency of acetamide to its propanamide counterpart (12: EC50 = 0.059 μm, 24: EC50 = 0.11 μm; 13: EC50 = 0.073 μm, 25: EC50 = 0.30 μm; 20: EC50 = 0.12 μm, 23: EC50 = 0.38 μm), which might reflect a spatial restriction in the target site of the HIV RT and indicate the crucial function of the acetamide linkage for maintaining the anti-HIV activity.
All the target compounds were also screened for their inhibition against HIV-2 (strain ROD) and K103N/Y181C double RT-mutant strain (RES056) in MT-4 cells, but none was found effective at a subtoxic concentration, indicating that the newly synthesized compounds were specific for HIV-1. Besides, the reason of absolute deactivation to RES056 may be explained by following molecular modeling study.
Inhibition of HIV-1 RT
With the aim to further confirm that the title compounds target HIV-1 RT, the most active compound 12 was selected to be evaluated in recombinant HIV-1 RT inhibitory assays, which use hybrid poly (A)·oligo (dT)15 (9A260 nm/mL), lyophilizate as template primer (RT kit; Roche Basel, Switzerland). The result indicated that compound 12 exhibited a moderate inhibition of enzymatic activity with an IC50 value of 1.50 μm, which is slightly lower than those of the reference drug NVP (IC50 = 0.69 μm) and ETR (IC50 = 0.37 μm). Thus, the newly synthesized imidazo[4,5-b]pyridinylthioacetanilides do indeed act as typical HIV-1 NNRTIs.
Molecular modeling
With the aim to obtain deeper insight into the molecular basis of the inhibitory potency and to rationalize the SARs, a molecular modeling of the most potent compound 12 and our previously reported compound 5b16 23-29 was carried out by means of Surflex-Dock module sybyl-x 1.1 (Certara Inc, Princeton, NJ, USA) software and the docking results were shown by pymol 1.5 (DeLano Scientific LLC, San Carlos, CA, USA).
As shown in Figure 3A, notable features of 12 in the NNIBP were summarized as follows: (i) The cyclopropyl-naphthalene moiety fitted into a hydrophobic subpocket formed by the aromatic side chains of Tyr181, Tyr188, and Trp229, giving rise to a π–π stacking interaction. (ii) One crucial hydrogen bond was formed between amide carbonyl with the backbone N-H of Lys103, greatly improving the affinity between RT and inhibitor as well as the stability of RT–inhibitor complex. (iii) The 2-bromo-4-sulfonamidephenyl of 12 is close to Pro236, and the sulfonamide moiety points toward the solvent-exposed region. More surprisingly, the hydrogen of sulfonamide forms a potential hydrogen bond with Lys104, which is a new feature for the binding mode of AATAs. (iv) The imidazopyridine ring of 12 is located in the middle of NNIBP, anchoring the two substitutions on the ring in a favorable conformation for binding. However, there is no expected interaction formed between imidazopyridine and surrounding residues according to docking results.
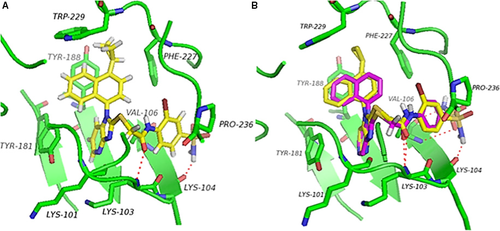
In addition, as shown in Figure 3B, compounds 12 and 5b16 are likely to share a likely binding pose. Meanwhile, these two compounds undergo the same crucial hydrogen bond with the backbone N-H of Lys103 through amide carbonyl. The difference is that the additional cyclopropyl of 12 was closer to conserved amino acid Trp229. Besides, the sulfonamide moiety of these two compounds formed different hydrogen bonds with different residues. Compound 5b16 formed two hydrogen bonds with Val106 through oxygen and hydrogen of sulfonamide moiety, while only one hydrogen bond was built between sulfonamide of 12 and Lys104.
Overall, the new compounds showed a good activity against wt HIV-1 as they can bind into the NNIBP of wt RT and exhibit interactions with NNIBP. However, these new compounds displayed an absolute deactivation to K103N/Y181C-resistant mutant strain of HIV-1 because of the absence of crucial hydrogen bond with Lys103. All these analyses were in accordance with the biological data. Further structural optimization will consider these aspects analyzed above.
Conclusion
In summary, through a structure-guided core-refining approach, a series of novel imidazo[4,5-b]pyridin-2-ylthioacetanilides were designed, synthesized, and evaluated for their biological activities as potent NNRTIs, which is an extension of our unremitting efforts toward the discovery of novel potent HIV-1 NNRTIs. Most of target compounds exhibited a moderate to good anti-HIV-1 potency. Thereinto, 12 and 13 were identified as the most promising leads with favorable inhibitory activities against HIV-1 (IIIB) (12: EC50 = 0.059 μm; 13: EC50 = 0.073 μm). Moreover, preliminary SARs based on anti-HIV-1 activities were discussed in detail and docking studies were carried out to investigate the interactions between these inhibitors and HIV-1 RT. These valuable pieces of information and the initial results provide us with valuable insights for further optimization. It was also recognized that the relative simplicity and tractability of the synthetic approach make these compounds attractive for further development and optimization. Consequently, the follow-up preparation of more synthetically feasible molecules and bioactivity investigation is underway in our laboratory and will be reported in due course.
Acknowledgments
We thank K. Erven, K. Uyttersprot, and C. Heens for technical assistance with the HIV assays. This work was financially supported by the National Natural Science Foundation of China (NSFC No. 81273354, No. 81573347), Key Project of NSFC for International Cooperation (No. 81420108027), Major Project of Science and Technology of Shandong Province (2015ZDJS04001), Research Fund for the Doctoral Program of Higher Education of China (Nos. 20110131130005), The Natural Science Foundation of Shandong Province (No. ZR2009CM016), and KU Leuven (GOA 10/014).




