Journal list menu
Export Citations
Download PDFs
Cover Picture
Cover Picture: Synthesis and Photovoltaic Properties of Polythiophene Incorporating with 3,4-Difluorothiophene Units (Chin. J. Chem. 11/2013)
- Page: 1357
- First Published: 18 November 2013

The cover picture shows a new polythiophene derivative PHTDFT designed for polymer solar cells. The introduction of fluorine atoms to the polymer backbones simultaneously lowers the HOMO energy level and narrows the bandgap. Benefiting from the lower HOMO, PHTDFT:PC61BM (1:1) polymer solar cells obtain a power conversion efficiency of 1.11% and an impressed open-circuit voltage of 0.79 V under solar illumination AM1.5 (100 mW/cm2). More details are discussed in the article by Zhang et al. on page 1385–1390.
Editorial
Contents
Contents: Chin. J. Chem. 11/2013
- Pages: 1360-1364
- First Published: 18 November 2013
Full Papers
Donor-Acceptor Oligomers and Polymers Composed of Benzothiadiazole and 3-Hexylthiophene: Effect of Chain Length and Regioregularity
- Pages: 1367-1379
- First Published: 16 September 2013
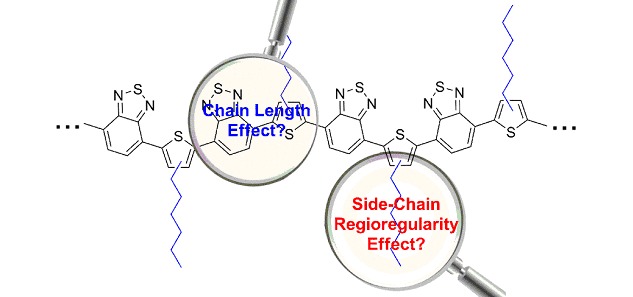
A series of donor-acceptor oligomer OBTThn (n=1–7) and polymer PBTTh1 and PBTTh2 composed of alternative 2,1,3-benzothiadiazole and 3-hexylthiophene have been designed and synthesized. The different orientation pattern of the side hexyl chains endows the polymers different regioregularity. It was found that the chain length of the oligomers and polymers has great impact on their photophysical, electrochemical and photovoltaic properties. Whereas, the side-chain regioregularity has less influence on their basic physical properties and photovoltaic performance.
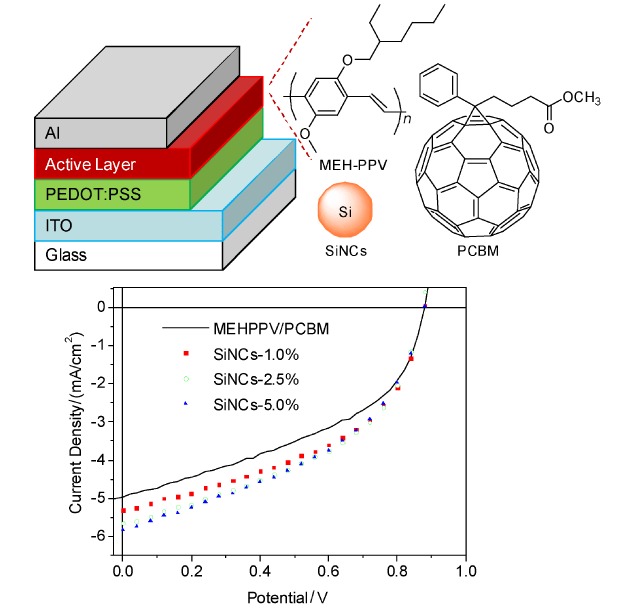
Improved Photovoltaic Performance of MEH-PPV/PCBM Solar Cells via Incorporation of Si Nanocrystals
- Pages: 1380-1384
- First Published: 17 June 2013
Synthesis and Photovoltaic Properties of Polythiophene Incorporating with 3,4-Difluorothiophene Units
- Pages: 1385-1390
- First Published: 16 September 2013
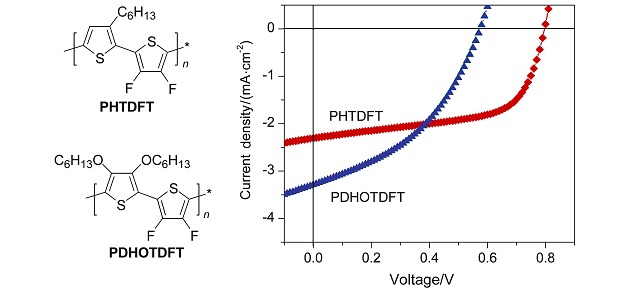
Two new polythiophene derivatives incorporating with 3,4-difluorothiphene units, PHTDFT and PDHOTDFT, show lower HOMO and narrower bandgap than that of P3HT. Benefiting from the lower HOMO, PHTDFT:PC61BM (1:1) polymer solar cells obtain a power conversion efficiency of 1.11% and an impressed open-circuit voltage of 0.79 V under solar illumination AM1.5 (100 mW/cm2).
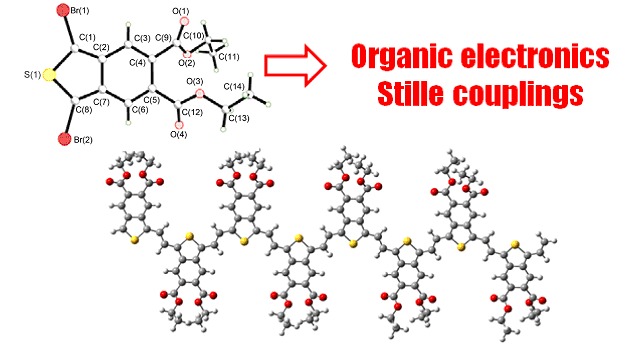
Bromination of Isothianaphthene Derivatives towards the Application in Organic Electronics
- Pages: 1391-1396
- First Published: 17 October 2013
Synthesis and Physical Properties of Benzopyridazine-Based Conjugated Molecules
- Pages: 1397-1403
- First Published: 16 September 2013
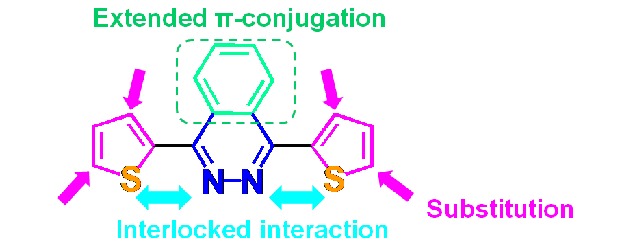
A series of organic conjugated molecules containing 2,3-benzopyridiazine as electron-withdrawing core and thiophene derivatives as electron-donating arms have been synthesized successfully. The optical properties and electrochemical behaviors of these molecules can be fine-tuned through changing the position of alkyl chains in the thiophene rings.
Dithieno[a,e]pentalene Based Conjugated Polymers: Synthesis and Characterization
- Pages: 1404-1408
- First Published: 02 October 2013
![Dithieno[a,e]pentalene Based Conjugated Polymers: Synthesis and Characterization](/cms/asset/8e34ef20-e27c-4342-b347-7fb8a5330a09/mcontent.jpg)
The concept of introducing π-conjugated but anti-aromatic repeating units into conjugated polymers has been explored. The thermal stability, optical and electrochemical properties of dithieno[a,e]pentalenes (DTP) based polymers have been characterized. The effect of introducing electron withdrawing repeating units into DTP based polymer on the physical properties of polymer has also been investigated. The new polymers showed broad absorption in visible and near-infrared region.
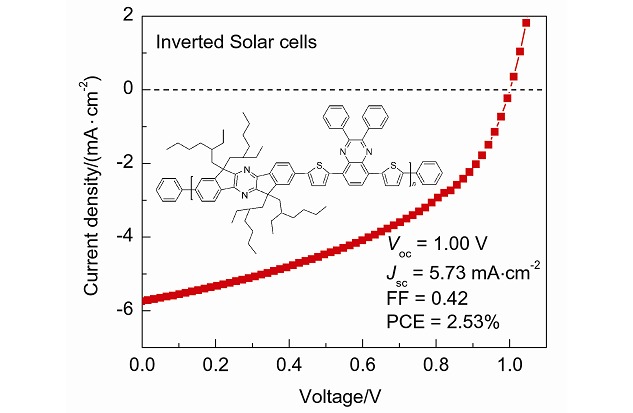
Ladder-type Diindenopyrazine Based Conjugated Copolymers for Organic Solar Cells with High Open-circuit Voltages
- Pages: 1409-1417
- First Published: 02 October 2013
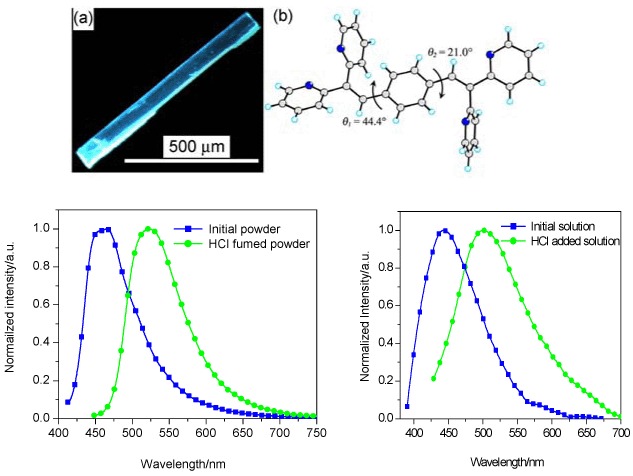
Organic Fluorescent Molecule with High Solid State Luminescent Efficiency and Protonation Stimuli-response
- Pages: 1418-1422
- First Published: 04 November 2013
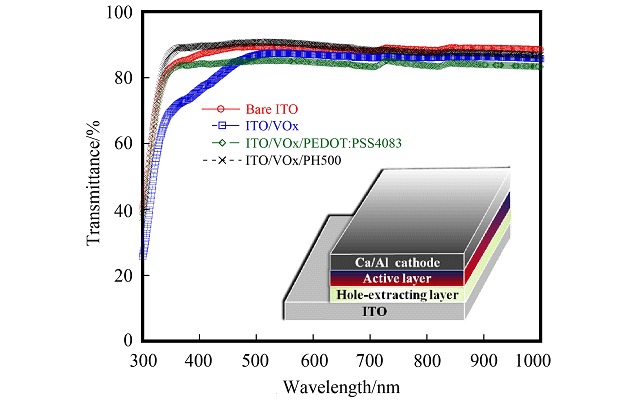
Efficient Polymer Solar Cells Based on Solution-processed Vanadium Oxide as Hole-extracting Layer
- Pages: 1423-1427
- First Published: 14 November 2013
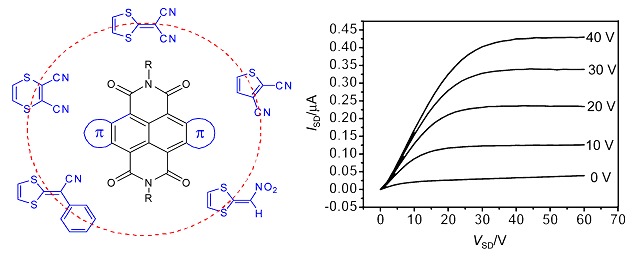
New Core-Expanded Naphthalene Diimides for n-Channel Organic Thin Film Transistors
- Pages: 1428-1438
- First Published: 02 October 2013
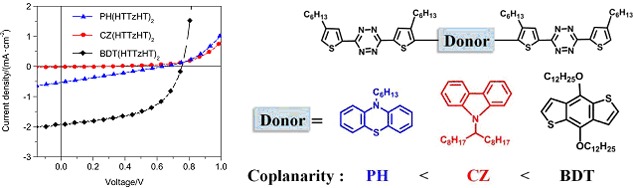
Controlling Morphology of Active Layer by Tuning Coplanarity of the Centrality in Acceptor-Donor-Acceptor Small Molecules for Photovoltaic Application
- Pages: 1439-1448
- First Published: 02 October 2013
Thickness Uniformity Adjustment of Inkjet Printed Light-emitting Polymer Films by Solvent Mixture
- Pages: 1449-1454
- First Published: 17 October 2013
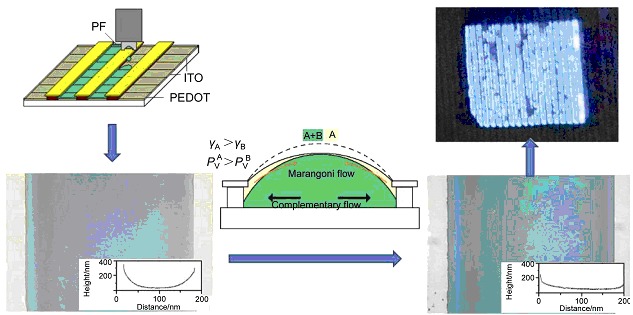
Solvent mixtures (a solvent with higher volatility, higher surface energy and lower viscosity, with another solvent with lower volatility, lower surface energy and higher viscosity) were used to improve thickness uniformity of inkjet printed polymer films. More flat films were obtained instead of films with concave-lens like cross-section. Combination of intense Marangoni flow at early drying process and weak complementary flow at the later drying process was used to explain this improvement. Array of pixels with higher effective light-emitting area proportion was obtained.
Novel Donor-Acceptor Copolymers Based on Dithienosilole and Ketone Modified Thieno[3,4-b]thiophene for Photovoltaic Application
- Pages: 1455-1462
- First Published: 04 November 2013
![Novel Donor-Acceptor Copolymers Based on Dithienosilole and Ketone Modified Thieno[3,4-b]thiophene for Photovoltaic Application](/cms/asset/2312cc34-fc36-4fc9-b794-25c0e0cd6ac1/mcontent.jpg)
Two novel donor-acceptor copolymers PDTSTT and PDTSDTTT containing dithienosilole and ketone modified thieno[3,4-b]thiophene are synthesized for the polymer solar cells. The two copolymers have broad absorption bands and low-lying HOMO energy levels. The insertion of thiophene bridges in PDTSDTTT can well relieve the steric hindrance between two units to induce a more compact packing and more favorable morphology. Without any treatment, the PDTSDTTT exhibits efficiency of 1% and relatively high open-circuit voltage of 0.75 V with blending PC61BM in a typical bulk heterojunction.