Journal list menu
Export Citations
Download PDFs
Original Article:
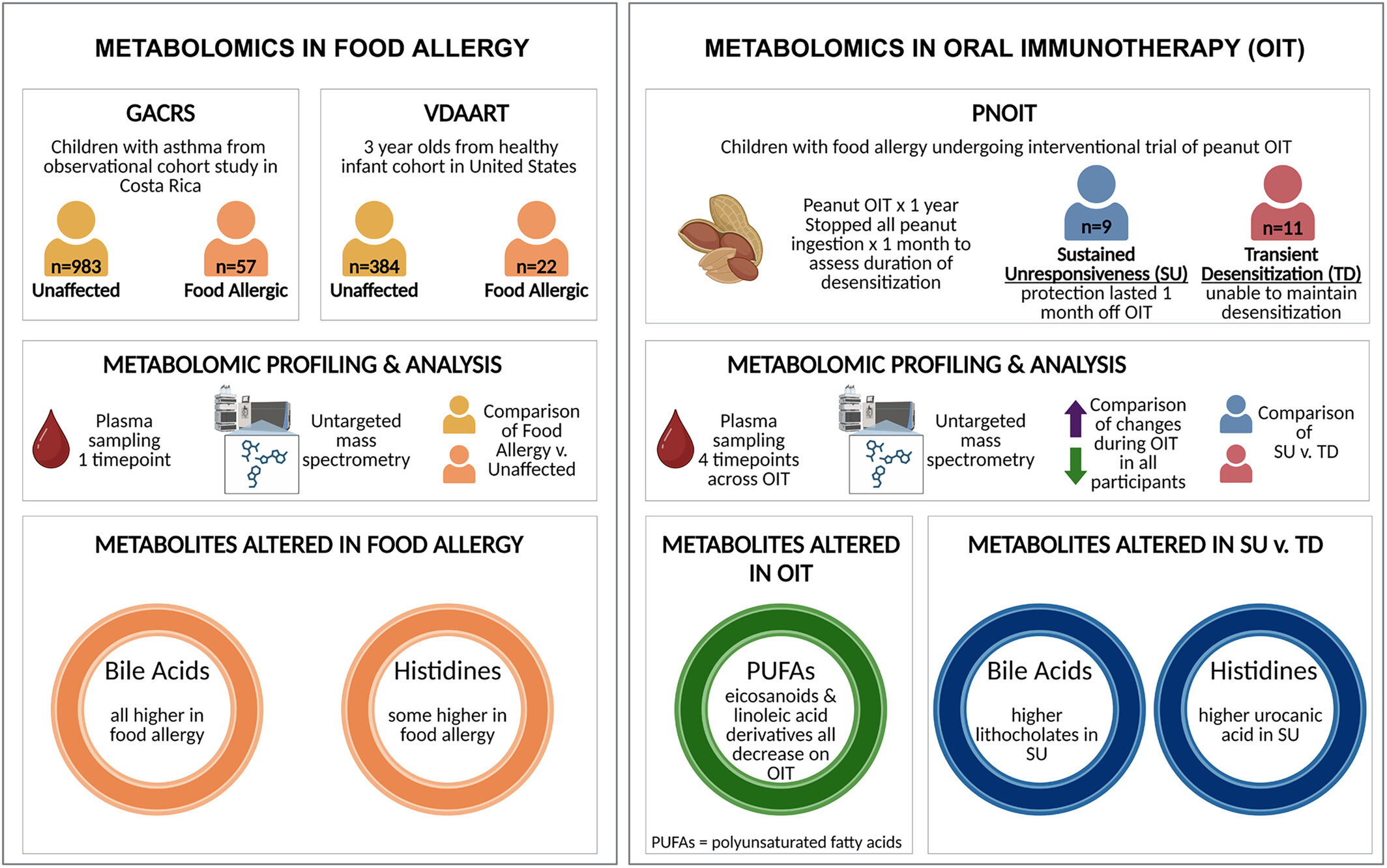
Immunomodulatory metabolites in IgE-mediated food allergy and oral immunotherapy outcomes based on metabolomic profiling
- First Published: 12 November 2024
Association of prenatal vitamin E levels with child asthma and wheeze
- First Published: 01 August 2024
Risk and protective factors of asthma and mental health condition multimorbidity in a national sample of Canadian children
- First Published: 02 August 2024

Viral and non-viral episodes of wheezing in early life and the development of asthma and respiratory phenotypes among urban children
- First Published: 17 July 2024
Parent's perception of respiratory syncytial virus and subsequent wheezing burden: A multi-country cross-sectional survey
- First Published: 04 June 2024
Innate immune responses are increased in children with acute asthma exacerbation
- First Published: 14 June 2024
Looking for ALPS: The value of a combined assessment of biochemical markers
- First Published: 03 May 2024
Benralizumab in children with severe eosinophilic asthma: Pharmacokinetics and long-term safety (TATE study)
- First Published: 16 March 2024
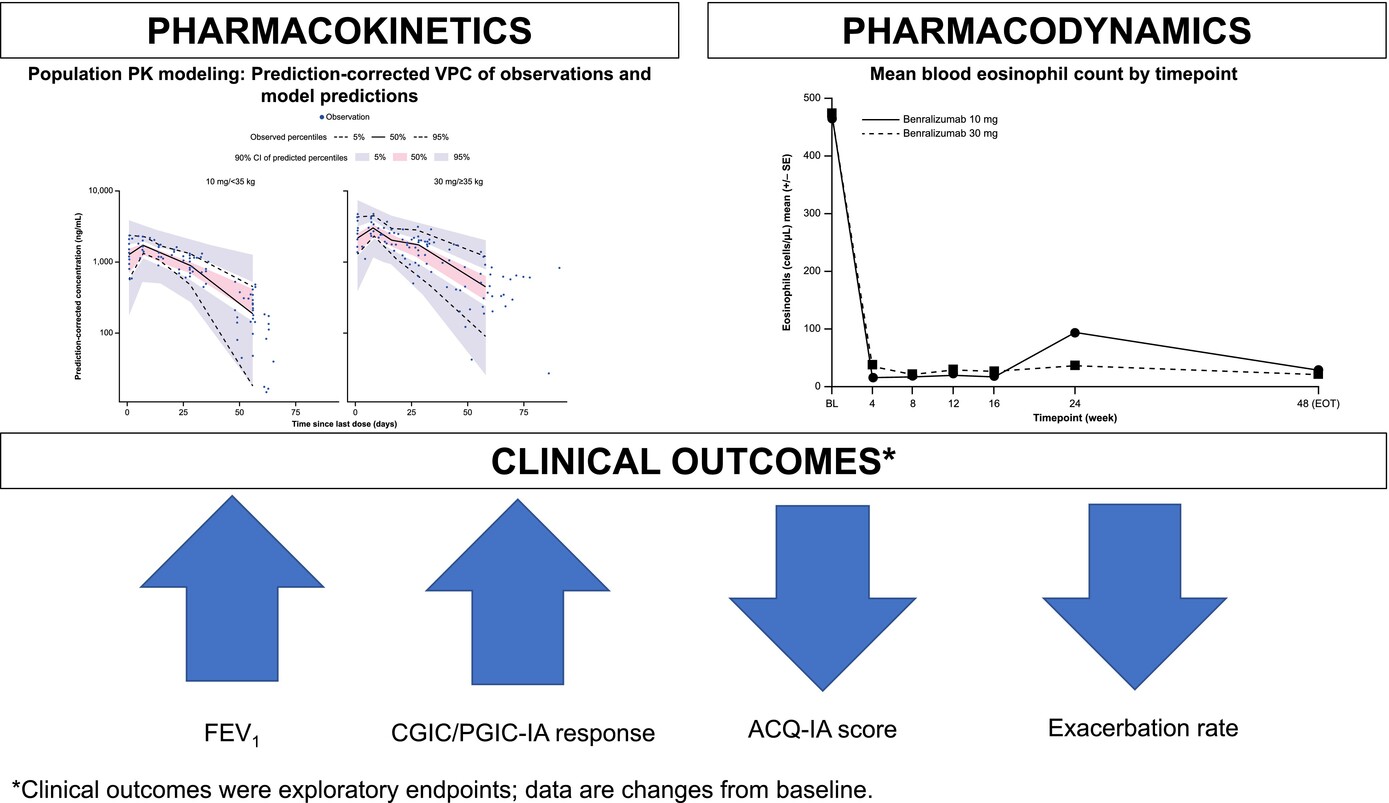
Benralizumab treatment in children demonstrated predictable PK parameters and a reassuring safety profile, consistent with previous studies in adolescents and adults. Treatment with benralizumab resulted in a near-complete reduction in blood eosinophil counts from baseline. Exploratory clinical outcomes indicated numerical improvements in FEV1, CGIC, and PGIC-IA responses, as well as ACQ-IA score and exacerbation rates. Abbreviations: ACQ-IA, Asthma Control Questionnaire-Interviewer administered; BL, baseline; CGIC, Clinician Global Impression of Change; CI, confidence interval; EOT, end of treatment; FEV1, forced expiratory volume in 1 second; PGIC-IA, Patient Global Impression of Change-Interviewer administered; PK, pharmacokinetics; SE, standard error; VPC, visual predictive check.
Design of the Intervention to Reduce Early Peanut Allergy in Children (iREACH): A practice-based clinical trial
- First Published: 02 April 2024
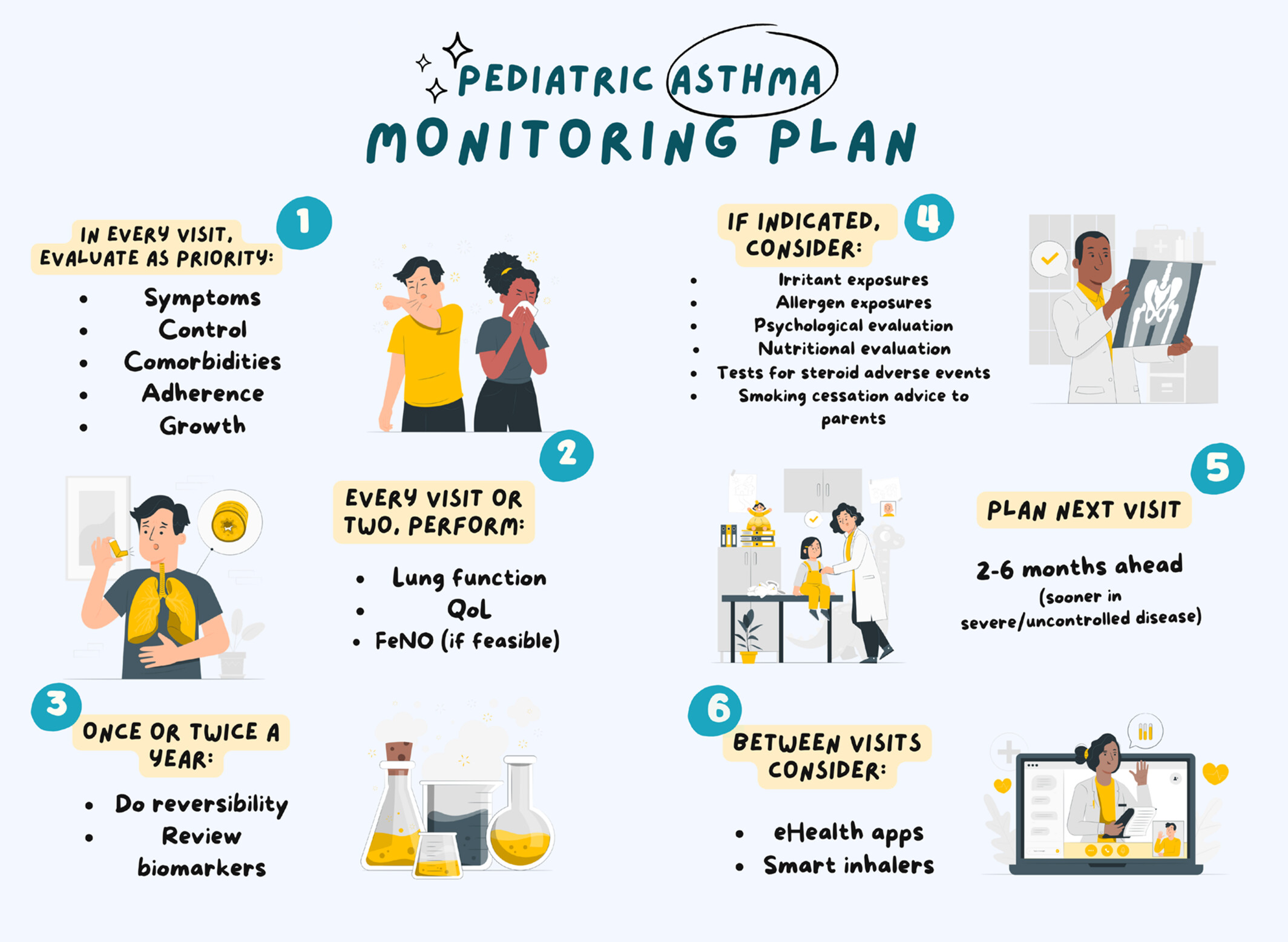
Recommendations for asthma monitoring in children: A PeARL document endorsed by APAPARI, EAACI, INTERASMA, REG, and WAO
- First Published: 25 April 2024
Pediatric penicillin allergy labels: Influence of race, insurance, and Area Deprivation Index
- First Published: 12 April 2024
Asthma and rhinitis control in adolescents and young adults: A real-world MASK-air study
- First Published: 02 February 2024
Letter:
Social and dietary impacts of food allergies in adolescents: Insights from a US teen survey
- First Published: 23 June 2025
The 5–10–15 plan: An approach to managing atopic dermatitis flares in pediatric patients
- First Published: 09 April 2025
Unmet social needs and greater symptom burden among children with eosinophilic asthma
- First Published: 24 February 2025
Early age peanut oral immunotherapy is safe and effective at achieving desensitization in 27 pediatric patients with peanut allergy
- First Published: 02 November 2024
Clinical Letter:
Omalizumab pretreatment in a case of pediatric carboplatin desensitization
- First Published: 23 June 2025