Journal list menu
Export Citations
Download PDFs
Systemic lupus erythematosus
Unveiling the nexus: Decoding interactions between regulated cell death and systemic lupus erythematosus pathogenesis for innovative therapeutic avenues
- First Published: 25 February 2024
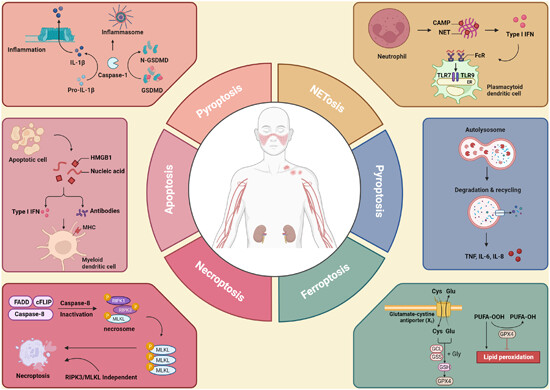
Multidimensional RCD interactions and therapeutic interventions in SLE: The graphical abstract illustrates the convergence of various regulated cell death (RCD) pathways—apoptosis, necroptosis, pyroptosis, NETosis, autophagy, and ferroptosis—in the pathogenesis of systemic lupus erythematosus (SLE). It highlights key molecular targets within these pathways and their linkage to inflammatory and autoimmune responses.
Mitochondrial aberrations in systemic lupus erythematosus pathogenesis: Insights and therapeutic implications
- First Published: 16 April 2024
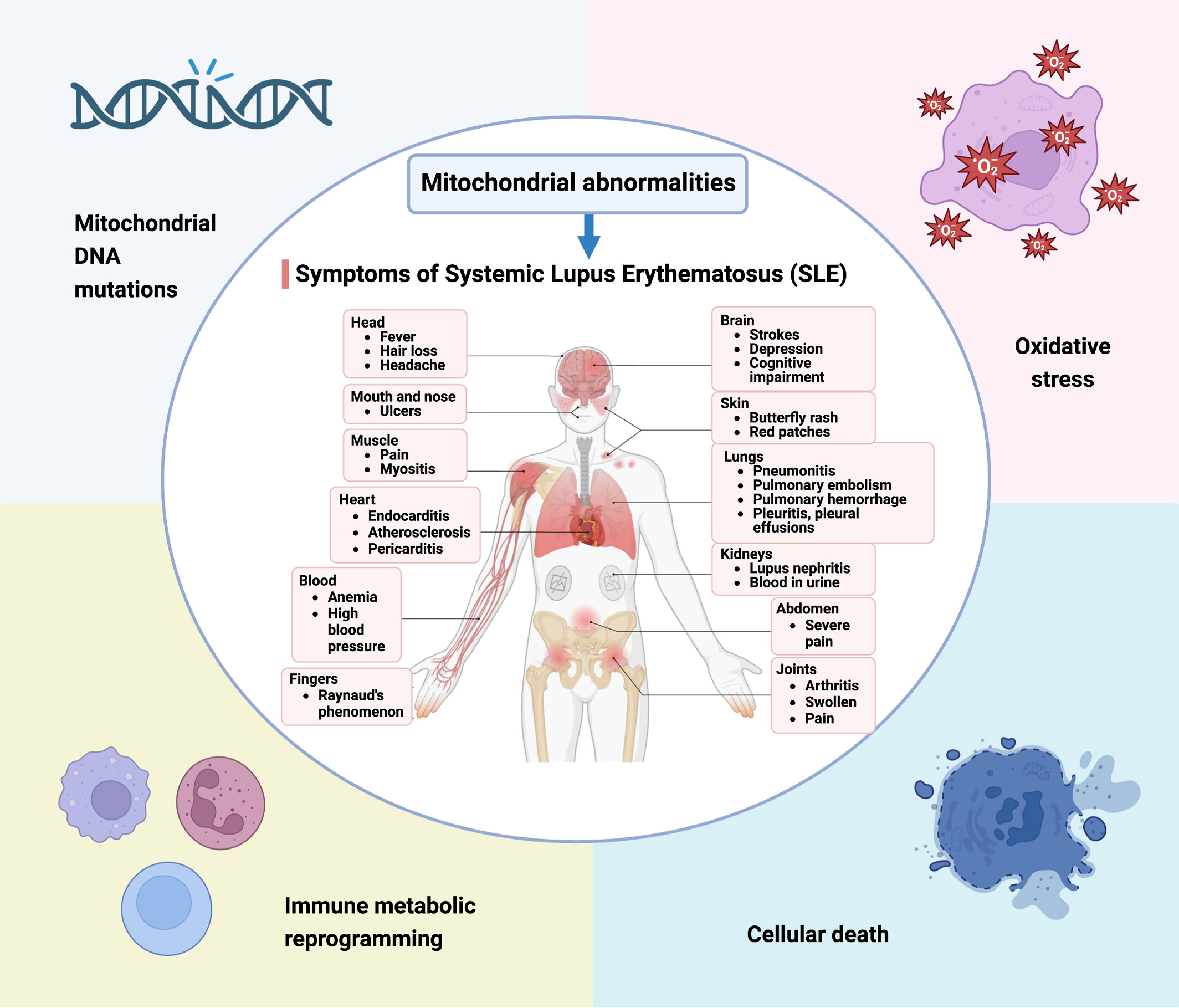
Mitochondrial abnormalities are crucial in the progression of systemic lupus erythematosus (SLE), with key elements like mitochondrial DNA mutations, oxidative stress, and immune metabolic reprogramming leading to cellular death. These dysfunctions result in an array of SLE symptoms, pointing toward mitochondrial pathways as potential therapeutic targets for improved patient outcomes.
CircEPSTI1 in peripheral blood as a novel potential biomarker for childhood-onset systemic lupus erythematosus
- First Published: 09 November 2023
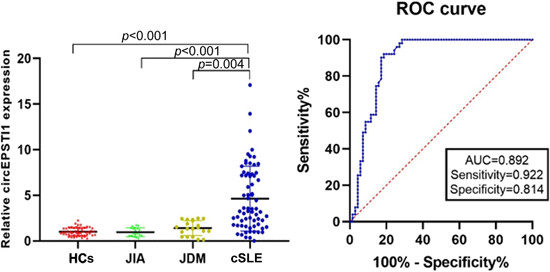
The expression levels of circEPSTI1 were significantly higher in childhood-onset systemic lupus erythematosus (SLE) patients compared with the healthy controls (HCs), juvenile dermatomyositis (JDM), and juvenile idiopathic arthritis (JIA) patients. CircEPSTI1 may represent a prospective biomarker of childhood-onset SLE.
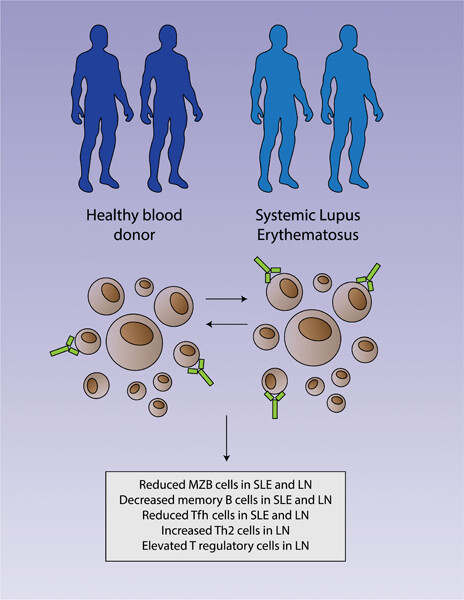
Immunophenotyping identifies distinct cellular signatures for systemic lupus erythematosus and lupus nephritis
- First Published: 23 November 2022
Rheumatoid arthritis
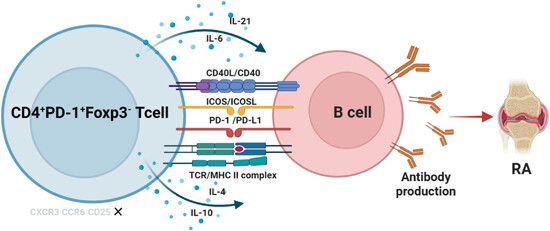
Pathologically expanded peripheral CD4+PD-1+Foxp3− T-cell subset promotes B-cell hyperactivity in patients with rheumatoid arthritis
- First Published: 11 March 2024
SLAMF8 as a potential biomarker for rheumatoid arthritis identified by comparing peripheral blood mononuclear cells, fibroblast-like synoviocytes, and synovial tissue using bioinformatics analysis
- First Published: 08 May 2024
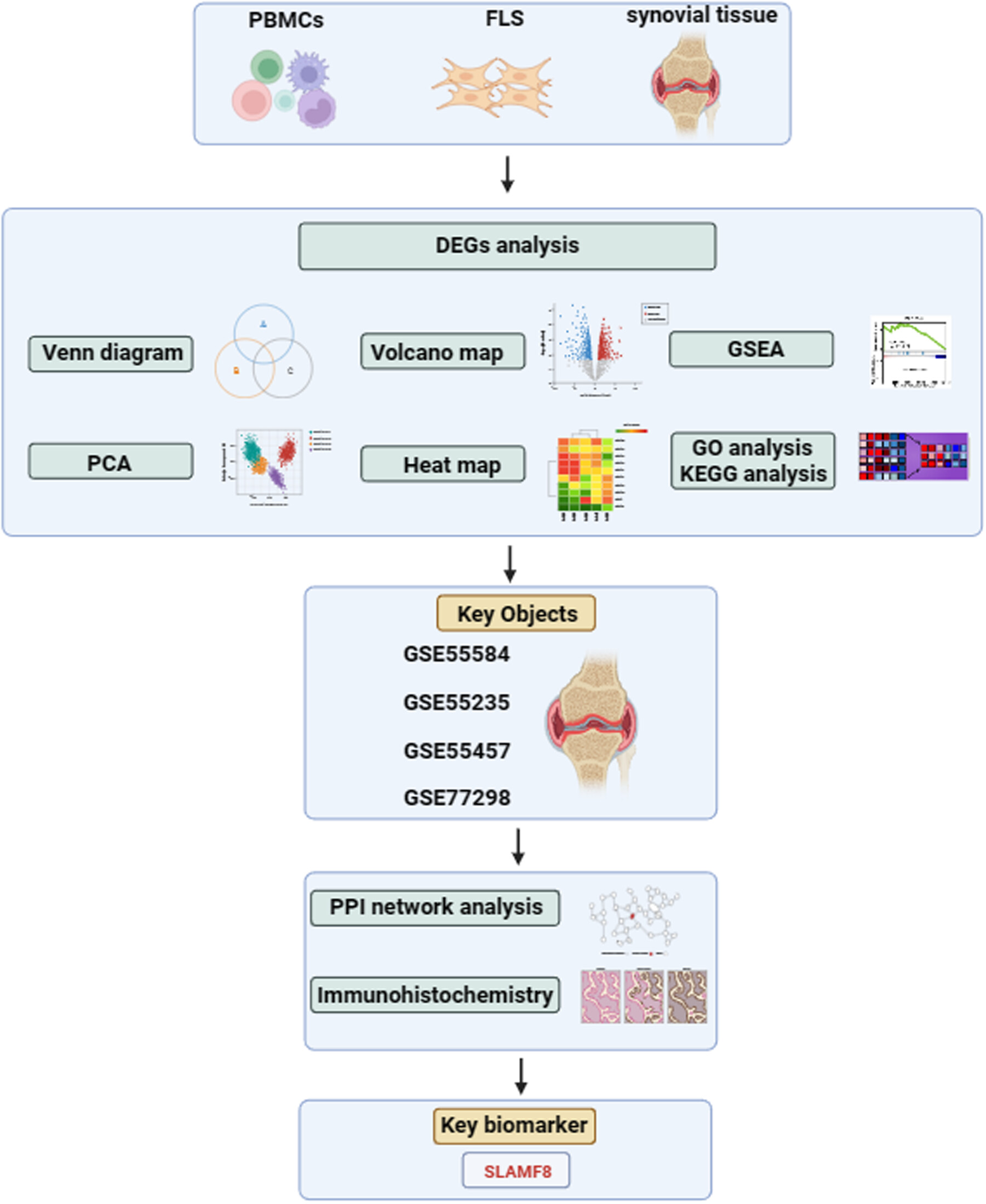
Microarray datasets of rheumatoid arthritis (RA), osteoarthritis, and healthy control were downloaded from the Gene Expression Omnibus database. Venn diagram, principal component analysis, heat map, volcano map, gene set enrichment analysis, gene ontology, and Kyoto encyclopedia of genes and genomes were used to analyze the data. Synovial tissue was identified as the key research objects. Further by protein–protein interaction network analysis of the four synovial tissue datasets and validation with immunohistochemistry of patients with RA and collagen-induced arthritis mice models, we identified that SLAMF8 may be a key biomarker for RA.
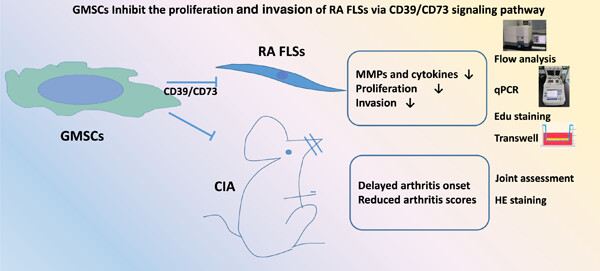
Human gingival tissue-derived mesenchymal stem cells inhibit proliferation and invasion of rheumatoid fibroblast-like synoviocytes via the CD39/CD73 signaling pathway
- First Published: 14 May 2023
Autoimmunity
Inflammasomes and their roles in autoimmune diseases
- First Published: 15 December 2024
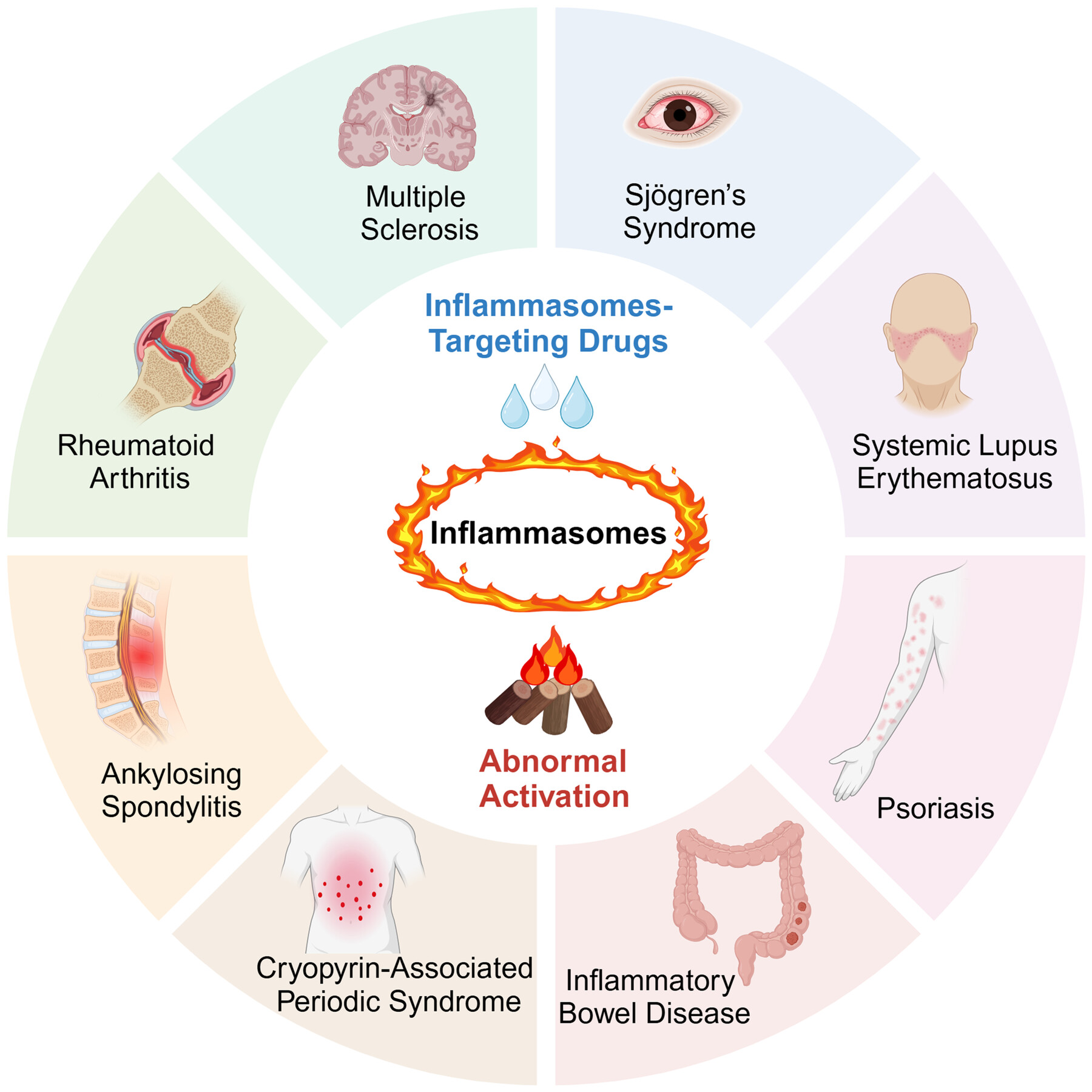
This review offers an introduction to the immunological functions of inflammasomes and elucidates their pivotal roles in the pathogenesis of autoimmune diseases, including rheumatoid arthritis, systemic lupus erythematosus, multiple sclerosis, inflammatory bowel disease, cryopyrin-associated periodic syndrome, psoriasis, Sjögren's syndrome, and ankylosing spondylitis. Additionally, this review highlights the importance of developing drugs targeting inflammasomes as promising therapeutic avenues.
Expanding the scope of mast cell disease: Does mast cell-derived TNF play a role in immune-mediated chronic illness and autoimmunity?
- First Published: 31 December 2024
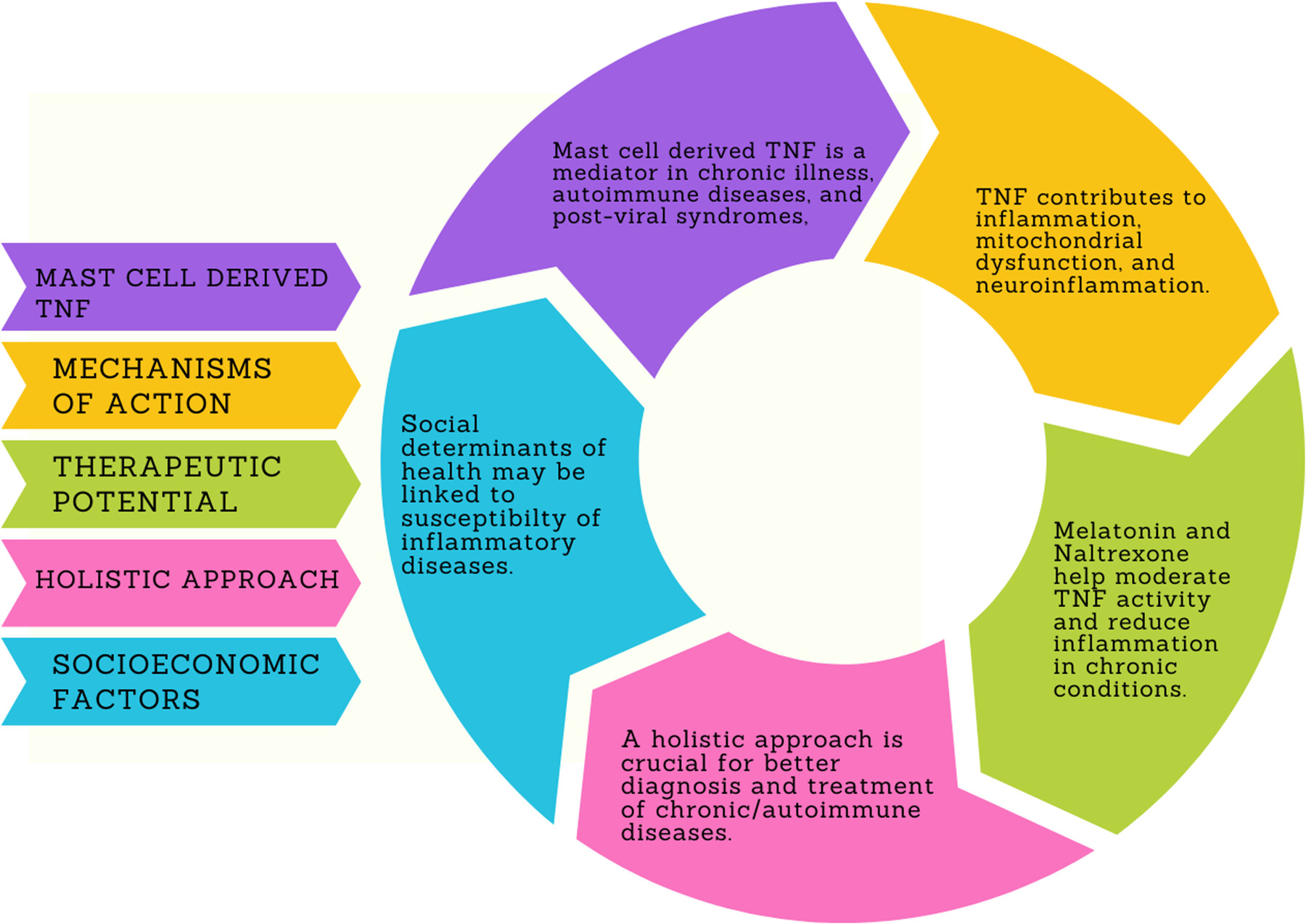
Mast cells (MCs) and MC-derived tumor necrosis factor (TNF) are important in chronic inflammatory and autoimmune diseases and post-viral syndromes, having important mechanisms of action, and therapeutic potential. MC and MC-derived TNF activation are involved in conditions like COVID-19, and neuroinflammation, linking TNF to various pathological processes such as apoptosis, thrombosis, and mitochondrial dysfunction. Potential therapeutic benefits of treatments, like high-dose melatonin and low-dose naltrexone (LDN) for modulating MC activation and reducing TNF-induced inflammation, seem to be promising therapies. They could help prevent or treat immune-mediated illnesses. Future research is encouraged to explore holistic approaches for better treatment of chronic/auto-immune diseases and also consider social determinants of health.
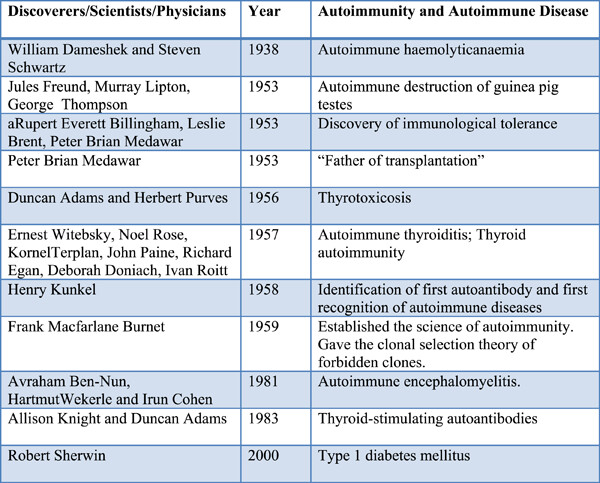
Origins and history of autoimmunity—A brief review
- First Published: 21 September 2022
Antiphospholipid syndrome
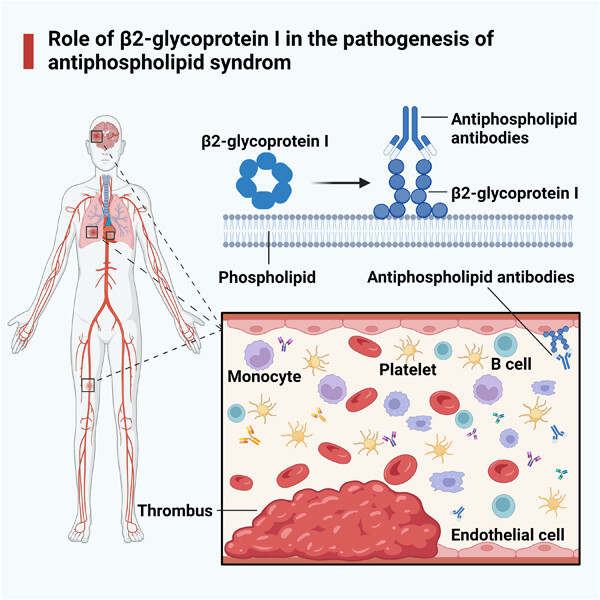
Role of β2-glycoprotein I in the pathogenesis of the antiphospholipid syndrome
- First Published: 17 May 2023