Expanding the scope of mast cell disease: Does mast cell-derived TNF play a role in immune-mediated chronic illness and autoimmunity?
Edited by Zhiyu Wang and Lishao Guo.
Abstract
Increasing evidence underscores the vital significance of diverse mast cell mediators in inflammatory illness, yet diagnostic criteria for mast cell activation syndrome still focus predominantly on a solitary mediator, tryptase. Therapeutic interventions, conversely, tend to prioritize histamine, prostaglandins, and leukotrienes. This article, via a review of both experimental and clinical findings, spotlights the mechanisms of action of an important mast cell mediator – tumor necrosis factor (TNF), and elucidates the intricate linkages between mast cell dysfunction, chronic illness, and autoimmunity. After providing an overview of the role of mast cell-derived TNF in inflammatory illnesses and viral infections, we hypothesize the interplay between mast cell-derived TNF and mitochondrial dysfunction, microglial activation, and the hypothalamic-pituitary-adrenal axis and the pathways of action on mast cells of treatments such as high-dose melatonin and low-dose naltrexone.
Key points
-
Mast cell activation syndrome exhibits diverse clinical presentations, often overlapping with symptoms of other illnesses while being underrecognized for its role in hyperinflammation, including viral infections.
-
Mast cells store and release potent pro-inflammatory cytokines implicated in various physiological and pathological processes that are associated with chronic illnesses and autoimmune conditions, while its inhibitors have shown efficacy in treating autoimmune and inflammatory disorders.
1 INTRODUCTION
According to the broader consensus on mast cell (MC) activation syndrome (MCAS), MC involvement in disease varies widely, with extreme heterogeneity in clinical presentations.1 This hypothesis is born out in the fact that hyper-inflammation in the novel coronavirus disease 2019 (COVID-19) is highly individualized and that MCs are at play in long-term COVID, which has a wide range of overlapping symptoms, as recent evidence demonstrates that most COVID-19 hyperinflammation is consistent with MC-driven inflammation.2, 3 Poor social determinants of health can be linked to MC activation,4 heightened inflammatory response,5 and immune dysfunction.6 Despite evidence that MC mediators play a significant role in chronic illness and disease, patients experiencing MC activation outside of the narrow consensus on MCAS vis a vis tryptase,7 or histamine,8 currently fall into the category of “functional” or “medically unexplained illness.” For this reason, it is vital that we connect MC dysfunction to chronic illness and autoimmunity by tracing mechanisms of action of various MC mediators beyond tryptase, histamine, chymase, and carboxypeptidase, among others;9 the former two are the focus of some MCAS diagnoses.
As MCs have been implicated in a wide range of autoimmune and chronic illnesses, we may be able to discern the specific and sometimes contradictory behavior of MCs (e.g., their involvement in both thrombosis and anti-coagulation) by looking at hypothesized mechanisms of action of treatments such as high-dose melatonin and low-dose naltrexone (LDN).
This article focuses on one MC mediator compound—tumor necrosis factor (TNF)—and traces its role in the inflammatory cascade in chronic illness and autoimmune disease. It also presents a theoretical synthesis of clinical trials and lab studies that elucidate mechanisms of action for melatonin and LDN in autoimmune disease. The relationship between MCs and TNF is complex, as MCs release pre-formed TNF during an inflammatory response, synthesize TNF, and are activated by TNF through both immunoglobulin and nonallergic receptors.10 Our aim is to describe the roles of TNF in various pathways, which might be a plausible explanation for MCs' involvement in chronic disease and autoimmunity. We begin with an overview of MCs and then proceed to discuss the multiple ways in which TNF is implicated.
MCs are relatively long-lived cells that are a part of the immune system which originate from hematopoietic stem cells in the bone marrow and mature in the interstitial tissue of various organs.4 They are prevalent in the skin, gastrointestinal tract, and respiratory tract and play a role in allergies,11 anaphylaxis, hypersensitivity reactions, chronic inflammatory12 and autoimmune disorders, auto-immune thyroid disease, multiple sclerosis (MS), rheumatoid arthritis, chemical sensitivity, insulin-dependent type II diabetes mellitus, as well as acid-mediated eosinophilic esophagitis, endometriosis,13 infertility,14 various tumors,15 and cancer.16, 17 MCAS, or MC hyperactivity, is a chronic illness that causes inflammation and other issues leading to fibrosis18 of the lungs, liver, kidneys, heart, and/or large intestines.4 As we describe below, there are several MC chronic-disease pathways that implicate particular endogenous markers.
2 MCs AND TNF
MCs store and release pre-formed mediators including histamine, heparin, cyclooxygenase-1, cyclooxygenase-2, and cytokines, including TNF.
Upon IgE stimulation, MCs store and rapidly secrete pre-formed TNF,19 particularly TNF-α.20 In a mouse model, MC-derived TNF directly played a role in activating white blood cells, such as neutrophils,21 and mouse peritoneal MCs have been shown to contain large amounts of TNF.19 Tracing the role of MCs in neuroinflammation, Caraffa et al.22 report that MC-derived TNF is a very powerful pro-inflammatory cytokine that mediates sensitization of the meningeal nociceptors.
TNF is a “a multifunctional cytokine that influences physiological and pathological mechanisms.”19 There are many types of TNF, which includes TNF-α, TNF-β, CD40 ligand (CD40L), Fas ligand (FasL), and TNF-related apoptosis-inducing ligand (TRAIL), among others,23 each with unique differences. For example, TNF-α is mainly produced by macrophages, whereas TNF-β is mainly produced by T lymphocytes.23 Other cells can also express TNF-α and TNF-β at low levels.23
TNF has a variety of pathological and physiological actions. For instance, TNF induces tumor cells to undergo necrosis, which is a process that involves the swelling of cells, destruction of organelles, and cell lysis.23 It is also involved in apoptosis, which is a process that causes cells to shrink, form condensed bodies, and fragment DNA.23
TNF also plays a role in blood clotting, pulmonary damage, insulin resistance, cardiovascular failure, and other conditions.24 High levels of TNF are linked to a variety of chronic illnesses and autoimmune conditions including psoriasis, type 2 diabetes, Crohn's disease, and rheumatoid arthritis. TNF is also dominant in ulcerative colitis, ankylosing spondylitis, and MS.25 Furthermore, while TNF plays an important role in fighting viruses, recent evidence suggests TNF-α is implicated in certain cancers,26 while high serum TNF is linked to low blood pressure and syncope,27 muscle aches,28 fever,29 muscle wasting,30 loss of appetite,31 and thrombosis.32 Elevated levels of TNF have also been linked to increased risk of COVID-19 fatality.32
TNF inhibitors have efficacy in a broad range of autoimmune and other disorders32 in countering TNF-induced inflammatory effects in many of the aforementioned conditions. Anti-TNF treatment reduces vascular endothelial growth factor and interleukins (IL) such as IL-6 and IL-1.32 When clinical trials of therapeutic immunomodulation targeting IL-6 and dexamethasone were disappointing, Robinson et al.32 proposed that TNF blockade may be effective in COVID-19. More recently, other researchers have proposed that blocking TNF signaling may save lives in COVID-19 infection.33, 34 Furthermore, a review of 84 studies found that “TNF-α inhibitors are associated with a lower probability of hospitalization and severe COVID-19 when compared to any other treatment for an underlying inflammatory disease.”35
3 VIRAL INFECTION, ELEVATED TNF, AND DOWNSTREAM INFLAMMATORY ILLNESS
TNF plays a vital role in modulating viral infections;36 therefore, it is not surprising that people undergoing anti-TNF treatment have a heightened risk of infections.32, 37 People with hepatitis B infection are at particular risk of viral reinfection, as are carriers of the herpes zoster virus.38 Therefore, chronic use of anti-TNF-α treatments may lead to viral replication that would then require antiviral therapy.39
Conversely, post-viral syndromes are associated with high TNF. A study of serologically confirmed West Nile virus patients with post-infectious symptoms all had abnormally elevated TNF,40 and post-acute sequelae of COVID-19 is associated with high IL-1β, IL-6, and TNF levels.41
Chronic viral infections are associated with elevated concentrations of inflammatory cytokines, including TNF. These elevated concentrations of cytokines are linked to further downstream pathological responses and variations in the encompassing inflammatory response.42 Beyer et al.42 find that chronic viral infections are also associated with a divergence from functional memory and effector T cell differentiation, which leads to the emergence of dysfunctional T cells, also called “T cell exhaustion.” This link between chronic inflammation and T cell dysfunction is mediated by TNF.
So and Ishii43 and others describe a process through which TNF superfamily receptors and TNF superfamily molecules function as costimulatory receptors and ligands in T cell function, augmenting T cell responses to bacteria,44 viruses, and parasite infections.45 They argue that this interaction “drives the development of autoimmunity and inflammation in many different animal models of asthma, colitis, graft-versus-host disease, diabetes, MS, rheumatoid arthritis, atherosclerosis, and transplantation.”43
Based on estimates that MC activation exhibits broadly in the population,1, 46 it is worth considering whether MC-derived TNF inaugurates a vicious circle of chronic illness. Health stressors brought on by poor social determinants of health lead to greater susceptibility to viral infections,47 including colds and flu.48 Therefore, people working multiple jobs and living with housing and food insecurity and higher stress may succumb to viruses more often, leading to higher TNF and downstream inflammatory conditions.
4 MCs, TNF, AND MITOCHONDRIAL DYSFUNCTION
MC-derived TNF may play a role in mitochondrial dysregulation, which in turn triggers MC activation and accelerates a pro-inflammatory state. A review of the role of mitochondrial dynamics in MC high-affinity IgE receptor-dependent activation showed “strong correlation between the loss of mitochondrial adenosine triphosphate and cytokine secretion during MC exocytosis.”49 A study found that cardiac mitochondria of TNF-treated mice “became irregular in shape and smaller, and the cristae were decreased and appeared disorganized, with breaks,” indicating that the pathological effects of TNF on the heart may be mediated by changes in mitochondria.50
In turn, dysregulated mitochondria are pro-inflammatory and can up-regulate MCs. Theoharides et al.51 found that MC activation is “accompanied by mitochondrial fission and translocation to the cell surface from where they secrete at least adenosine triphosphate and DNA outside the cell” and hypothesized that these extracellular mitochondrial components further stimulate MCs and other immune cells.
5 MCs, MICROGLIA, TNF, AND TOLL-LIKE RECEPTOR 4 (TLR4)
Current research points to the coordinated role of microglia, the brain's resident macrophages, and MCs in neuroinflammation. In their review of MC-microglia communication in neurodegenerative diseases, Sandhu and Kulka.52 found that active communication between MCs and microglia is involved in neuroinflammation and neurodegeneration. Microglia respond to pro-inflammatory signals from non-neuronal immune cells, including MCs.53 MCs alert glial cells to initiate neuroinflammatory processes, and activated brain MCs “can cause phenotypic changes and activation of microglial cells” through the release of histamine, proteases (such as tryptase), and pro-inflammatory cytokines (such as chemokine ligand 2, TNF-α and IL-1β).52 Microglia are found adjacent to amyloid deposits, and drugs that suppress the inflammatory response in microglia also attenuate symptoms in a mouse model of Alzheimer's disease.54
Both MCs and microglia can be triggered by the same signal and thus jointly induce brain inflammation. For example, IL-6 released by microglia may upregulate MCs by affecting TLR2 and TLR4 and upregulating chemokines, which in turn would induce further inflammation in microglia.54 MCs and microglia both contain inflammasomes, innate immune system receptors within the cytoplasm which are triggered by infection or cell damage.52 Dong et al.55 show that stabilizing MCs can reduce neuroinflammation by inhibiting microglia inflammation. In this context, TNF and the NOD-like receptor P3 inflammasome are also both important targets for anti-inflammatory intervention.52
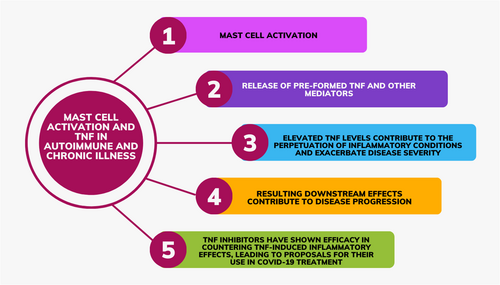
TLR2 and TRL4 on both MCs and microglia respond to damage-associated molecular patterns. When activated via TLR2 or TRL4, MCs produce significant levels of TNF and IL-6.56 MCs rapidly secrete TNF when endotoxins are recognized by TLR4 receptors located in MC membranes.57 The activation of MC TLR4 “leads to the rapid secretion of pre-synthesized TNF from intracellular pools” and to the activation of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), all of which is necessary for de novo synthesis of TNF and other cytokines.58 Singh et al.'s rodent model shows that MC activation via TLR4 leads to colonic barrier dysfunction.59 Based on the foregoing, we summarize the importance of mast-cell-derived TNF in the pathophysiology of various autoimmune and chronic illnesses in Figure 1.
6 TNF AND THE HYPOTHALAMIC-PITUITARY-ADRENAL (HPA) AXIS
In a review of the impact of circadian rhythms and glucocorticoids in the HPA axis, Spies et al.60 trace the role that TNF plays in initiating the induction of chronic inflammation. There is substantial evidence that chronic inflammation associated with HPA dysfunction may not be an adaptation to chronic stress, as originally thought, but rather to the negative feedback of active cortisol on the HPA axis.60
The recognition of the involvement of the liver and kidneys in glucocorticoid metabolism has led to the terming of the HPA axis as the hepato-hypothalamic-pituitary-adrenal-renal axis. Inactive cortisone is converted to cortisol in the liver via 11β-hydroxysteroid dehydrogenase (11β-HSD) type 1, and active cortisol is converted back to cortisone in the kidneys via 11β-HSD type 2.60 While cortisone is converted to cortisol in many other tissues in the body, the liver is a key organ in systemic inflammation, as TNF and other inflammatory cytokines upregulate the expression of 11β-HSD type 1, thus greatly increasing the conversion of cortisone to cortisol in the liver. It is hypothesized that this TNF- and cytokine-induced increase in 11β-HSD type 1 and the resulting increase in cortisol in chronic illness leads to increased negative feedback of cortisol on the HPA axis, inducing changes and eventually dysfunction in the HPA axis and chronic inflammation.60
7 MELATONIN, TNF, AND THE IMMUNE PINEAL AXIS
TNF alters the immune-pineal axis and reduces the nocturnal melatonin surge by stimulating pinealocytes TNF receptor 1.61 TNF-induced reduction in melatonin is implicated in an array of inflammatory conditions. High TNF levels are associated with dysfunctional sleep breathing, difficulty with initiating and maintaining sleep, and sleep hyperhidrosis. Children with autism spectrum disorders (ASD) have higher TNF levels overnight than during the day, while children without ASD do not show a variance in TNF levels from night to day. Children with ASD commonly experience the aforementioned sleep disruptions.61
While TNF reduces melatonin, melatonin is a powerful anti-inflammatory hormone, which itself reduces TNF and other inflammatory factors. Melatonin decreases IL-6, TNF,62, 63 IL-8 and nitrous oxide and IL-1.64 Melatonin inhibits TNF and IL-1β in a dose-dependent manner through several signaling pathways, including phosphoinositide-3-kinase–protein kinase B, extracellular signal-regulated kinase, and NF-κB signaling pathways.65 Dos Santos et al.66 demonstrate that melatonin supplementation reverses insulin resistance and high TNF in the context of pinealectomized rats. Further, melatonin replacement normalizes the lipid profile. Chahbouni et al.67 found that two daily doses of melatonin at 20 mg at 21:00 and 10 mg at 9:00 reduced elevations in lipid peroxidation and IL-1β, IL-2, IL-6, TNF-α, and interferon (IFN)-γ in Duchenne muscular dystrophy in a time-dependent manner. In an in vitro experiment, Song et al.68 induced hepatocyte damage with TNF and then dose-dependently inhibited TNF-induced damage with melatonin. In addition, melatonin inhibits stress-induced mitochondrial dysfunction.69
8 MCs, TNF, AND LDN
Thus far, the above-noted discussion on MC-derived TNF has focused on various disease mechanisms and pathways of action. We now shift the discussion to therapeutic products that are currently used in the management of diseases that could implicate MC-derived TNF, such as naltrexone. Naltrexone is a nonspecific opioid receptor antagonist, which is used at higher doses (~50 mg) to manage opioid and alcohol addiction and at lower doses (~4.5 mg) to treat inflammatory conditions. At higher doses, opioids are known to provoke MC and microglial activation, exacerbating pro-inflammatory and pro-nociceptive processes,70 while lower doses have been shown to have the opposite effect.71, 72
LDN has been used to treat MCAS,73, 74 and HPV vaccine-triggered escalation of underlying MC disease.75 LDN has been proposed for the prevention and treatment of immunothrombosis in COVID-19 based on its anti-inflammatory effect in intestinal cells and reduction in systemic IL-6, IL-12, C-reactive protein, and TNF, which is associated with a reduction in symptoms in COVID-19.76 LDN has also performed well on small-scale trials in MS, Crohn's disease, and rheumatoid arthritis, and a 2017 10-week, single-blind, crossover trial study of eight participants with fibromyalgia showed reduced TNF after 8 weeks of treatment with LDN.71
While its exact mechanisms of action have not been proven, research reveals two distinct (although possibly related) mechanisms by which LDN reduces inflammation and immune and cancer cell proliferation. Both hypothesized mechanisms involve a bidirectional effect based on the dose and duration of receptor blockade, with higher doses (and therefore constant blockade) resulting in increased inflammation and cell proliferation and lower doses (and therefore intermittent blockade) resulting in reduced inflammation and cell proliferation.
One mechanism of action involves microglia and the antagonism of TLR4. As reviewed above, TLR4 downstream signaling leads to inflammatory factors such as IL-1, TNF, IFN-β, and nitric oxide.71 Xu et al.72 report that LDN downregulates inflammatory cytokines IL-6, IL-17, TNF, and IFN-γ and upregulates anti-inflammatory cytokines IL-4 and IL-10. They hypothesize that LDN may inhibit the release of pro-inflammatory factors by microglia.72
Considering the relationship between microglia and MCs reviewed above, downregulation of microglia could in turn downregulate MCs and decrease MC-derived TNF. In low doses from 1 to 5 mg, LDN binds to TLR4 and acts as a glial modulator.71, 77 LDN also binds to certain opioid receptors to reduce the expression of TLR4.72 LDN has also been shown to bind to the lipopolysaccharides binding pocket of myeloid differentiation protein 2 to block TLR4 signaling.78
The second mechanism by which LDN reduces inflammation and immune cell proliferation involves the upregulation of opioid growth factor (OGF) and OGF receptors. By intermittently blocking opioid receptors, LDN creates a “blockade of opioids from opioid receptors for a short term (4–6 h) each day provides an 18–20 h window where the elevated opioids and opioid receptors can interact to elicit an exaggerated response (e.g. depression in cell proliferation).”79 Conversely, a higher dose of naltrexone creates a continuous blockade of opioid receptors from endogenous opioids, leading to oncogenesis and accelerated tumor growth.72, 80
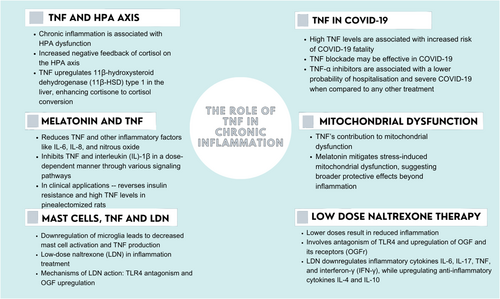
This mechanism relies on the upregulation of OGF to reduce inflammation. When OGF was administered to mice with experimental autoimmune encephalomyelitis, an experimental model of MS, their spinal cords showed a decrease in the numbers of T lymphocytes, microglia, and activated astrocytes.80 Thus, both LDN mechanisms of action involve a reduction in TNF and the downregulation and/or numerical reduction of microglia, which are upregulated in situations of MC activation. Other therapeutics, such as glucocorticosteroids, are not as promising because they have minimal effect on inhibiting MC degranulation.81 Based on the foregoing, we summarize the role of TNF in chronic inflammation in Figure 2.
9 CONCLUSIONS
This review suggests that MC-derived TNF may play an important role in the initiation and progression of immune-mediated illness. Future research on, and diagnosis of, MC disease should consider MC mediators beyond tryptase. From a clinical perspective, it may be worth testing for serum TNF and other relevant MC mediators in inflammatory illness, even in the absence of confirmed autoimmune disease typically involving high TNF. However, one of the limitations of this strategy is that TNF expression is not specific to MCs and could also be indicative of a variety of clinical manifestations of inflammation that may or may not be tied back to the initial activation of MCs. Thus, while elevated TNF is important in identifying certain variants of MC disease, there would also be a need for parallel molecular, clinical, toxicological, or other evidence in conjunction with the former.
In addition, further research into the role of MCs in immune-mediated chronic illness and autoimmune disease should consider whether it may be possible to prevent autoimmune disease through earlier intervention to stabilize MCs or through other MC-modulating treatments such as high-dose melatonin or LDN. There is emerging evidence that genetic ablation and pharmacological inhibition of MCs ameliorate lymphatic dysfunction and reduce inflammatory-erosive arthritis in TNF-transgenic mice.82 Accordingly, MC silencing may be a novel therapeutic approach for chronic urticaria and other MC-mediated diseases using investigational agents, specifically inhibitors of MC mediators like TNF blockers such as Adalimumab, Etanercept, and Infliximab.9 These drugs are currently in the Phase 2 stage of clinical development for asthma, chronic spontaneous urticaria, and eosinophilic esophagitis, respectively.9 MCs are “first responder” cells that react to a full array of environmental triggers. The latter includes food, pathogens, temperature, and vibrations; therefore, further study on MC mediators, such as TNF, may provide opportunities for new insights into connections between social and environmental determinants and inflammatory disease.
AUTHOR CONTRIBUTIONS
Conceptualization: Rachel da Silveira Gorman. Literature curation: Rachel da Silveira Gorman. Writing—original draft preparation: Rachel da Silveira Gorman and Iffath Unissa Syed. Writing—review and editing: Rachel da Silveira Gorman and Iffath Unissa Syed. Both authors have read and agreed to the published version of the manuscript.
ACKNOWLEDGMENTS
The authors have nothing to report.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
No new data were created or analyzed in this study.