Human gingival tissue-derived mesenchymal stem cells inhibit proliferation and invasion of rheumatoid fibroblast-like synoviocytes via the CD39/CD73 signaling pathway
Abstract
Background
Gingiva-derived mesenchymal stem cells (GMSCs) suppress immune and inflammatory responses in experimental arthritis models. Here, we determined whether GMSCs suppress the proliferation and invasion of rheumatoid arthritis fibroblast-like synoviocytes (RA FLSs).
Methods
Surface markers of GMSCs were analyzed by flow cytometry. mRNA expression of matrix metalloproteinases and pro-inflammatory cytokine in RA FLSs was measured by quantitative polymerase chain reaction (PCR). RA FLS proliferation was analyzed by a 5-ethynyl-2′-deoxyuridine assay. To explore the molecular mechanisms, we assessed the effect of GMSCs on RA FLS proliferation by adding indoleamine-2,3-dioxygenase (IDO), CD39, or CD73 inhibitors. The invasion was analyzed by a transwell assay. The anti-inflammatory effects of GMSCs were assessed in a type II collagen-induced arthritis (CIA) mouse model.
Results
Compared with human dermal fibroblast, GMSCs displayed a higher expression of CD39. Interleukin (IL)-8, IL-6, MMP-1, MMP-3, MMP-13, and monocyte chemoattractant protein-1 mRNA were all decreased in RA FLSs after incubation with GMSCs. GMSCs significantly reduced RA FLS proliferation in a dose-dependent manner in vitro, which was partly dependent on CD39/CD73 signaling. GMSCs significantly impeded the invasive capacity of RA FLSs in a dose-dependent manner in vitro. GMSCs infusion delayed arthritis onset and reduced arthritis scores in the CIA model.
Conclusion
GMSCs are effective to inhibit the aggressive behavior of RA FLSs and treat experimental arthritis, implying their therapeutic potential in RA patients.
Key points
-
Gingiva-derived mesenchymal stem cells (GMSCs) suppress the proliferation and invasion of rheumatoid arthritis fibroblast-like synoviocytes (RA FLSs) from RA patients.
-
GMSCs are effective in treating experimental arthritis.
1 INTRODUCTION
Rheumatoid arthritis (RA) is a classic chronic inflammatory autoimmune disease characterized by bone erosion and joint destruction. It is driven by environmental factors and genetic background, leading to articular synovial inflammation and bone destruction.1, 2 Type II collagen-induced arthritis (CIA) is a valuable animal model to investigate the pathological development of RA and evaluate potential therapeutic agents.3 The CIA model exhibits similar manifestations to RA patients, such as joint swelling, the histological presence of synovitis, inflammatory cell infiltration, articular bone erosion, and osteopenia.4 This model is used to evaluate potential therapies and pathophysiological responses.5-7 Joint inflammation can be observed visually. Compelling evidence supports that activated fibroblast-like synoviocytes (FLSs) are major pathological cells in RA development.7-10 RA FLSs generate inflammatory cytokines, and matrix metalloproteinases (MMPs) are involved in the degradation and remodeling of the extracellular matrix. Furthermore, RA FLSs have highly proliferative and invasive behaviors, which are similar to tumor cells, leading to the formation of pannus in the synovial membrane.11 RA FLSs migrate, invade, and penetrate the synovial matrix, resulting in synovial inflammation and bone destruction. Interestingly, RA FLSs retain their proliferative and aggressive behaviors without exogenous stimulation in vitro. Strong evidence suggests that RA FLSs invade and degrade human synovial tissue implanted in severe combined immunodeficiency (SCID) mice on the same side and erode into contralateral synovial tissue without inserting RA FLSs. Thus, RA FLSs are a major factor in the formation of erosive synovium and persistent synovial inflammation, joint swelling, and bone destruction. RA FLSs survive in vitro for a long time, suggesting resistance to apoptosis.8 Therefore, targeting RA FLSs by regulating their proliferation and invasiveness may be a promising therapeutic approach for RA.9, 12
Biological disease-modifying anti-rheumatic drugs are currently in use (e.g., adalimumab, certolizumab pegol, etanercept, golimumab, and infliximab) and targeted synthetic disease-modifying antirheumatic drugs such as JAK inhibitors are highly effective to reduce inflammation and improve joint symptoms.13 However, these drugs are insufficient and not a cure.14 Patients may undergo reactivation of hepatitis B or tuberculosis and malignancy.15 Additionally, many RA patients are difficult to treat.16 Therefore, finding a new therapeutic strategy is essential.
Mesenchymal stem cells (MSCs) have a promising therapeutic effect on several autoimmune diseases because of their immunomodulatory roles in innate and humoral immunity. MSCs have been isolated from almost all adult tissues, including bone marrow, umbilical cord blood, peripheral blood, dental tissue, adipose tissue, skin, and placenta.17-19 Gingiva-derived MSCs (GMSCs) are a novel MSC population that exhibits self-renewal, multipotent differentiation, immunomodulatory, and anti-inflammatory properties.20-22 Bone mesenchymal stem cells (BMSCs) have been considered to be a potential clinical therapy. However, BMSCs are difficult to harvest and have a tumorigenesis potential.23 Unlike BMSCs, GMSCs are easy to isolate from abandoned gingiva tissue, proliferate rapidly in vitro, and most importantly, have a lower tendency to develop into tumors.24
Our previous studies have shown that GMSCs can treat murine arthritis, osteoclastogenesis, bone erosion, streptozotocin-induced type 1 diabetes, xenogenic graft-versus-host disease (GVHD), and murine lupus nephritis disease via CD39/CD73 or indoleamine-2,3-dioxygenase (IDO) signaling.21, 22, 25-27 These data suggest that GMSCs have a promising potential to treat RA patients. CD39 promotes the hydrolysis of ATP and ADP to generate AMP, which is then hydrolyzed by CD73 to adenosine. Adenosine suppresses inflammation via its receptor. We further explored whether GMSCs inhibited the inflammatory response, proliferation, and invasion of RA FLSs depending on the same CD39/CD73 or IDO signaling.
In the present study, we demonstrate that GMSCs inhibit the inflammatory response and aggressive behavior of RA FLSs and significantly attenuate inflammatory arthritis in an experimental CIA model. The therapeutic roles of GMSCs appear to be mainly dependent on CD39/CD73 signaling. These results indicate that the manipulation of GMSCs may provide a promising therapeutic approach for the treatment of RA patients.
2 METHODS
2.1 Chemicals
Human recombinant tumor necrosis factor (TNF)-α and platelet-derived growth factor (PDGF) were purchased from R&D Systems (Minneapolis, USA). An IDO inhibitor [1-methyl-l-tryptophan (1-MT)] and CD73 inhibitor (α,β-methylene ADP, APCP) were purchased from Sigma-Aldrich (St Louis, USA). A CD39 inhibitor (sodium polyoxotungstate, POM1) was supplied by Tocris Bioscience (Bristol, UK). In some experiments, GMSCs were pretreated with 1-MT (500 μmol/L), APCP (100 μmol/L), and POM1 (100 μmol/L).
2.2 GMSC, human dermal fibroblast, and BMSC culture
Human gingiva tissue samples were obtained through routine dental procedures at the Department of Dentistry at Penn State University Hershey College of Medicine and the First Affiliated Hospital, Zhejiang University School of Medicine. Human GMSCs were prepared from these samples in accordance with the protocol in our previous study.21 All experimental protocols were approved by the Medical Ethics Committees of the Institutional Review Boards at both institutes. Informed consent was obtained from donors before the study. GMSCs were used in experiments from the third to sixth passage.
A human dermal fibroblast cell line (ATCC, PCS-201-012) and BMSCs (ATCC, PCS-500-012) were obtained from ATCC (https://www.atcc.org/). They were used as negative and positive controls of GMSCs.
2.3 Flow cytometry
The following fluorescent dye-conjugated mouse antibodies were used for flow cytometric analysis: mouse anti-human CD90, anti-CD29, anti-CD44, anti-CD73, anti-CD105, anti-CD39, anti-CD4, anti-CD34, anti-CD45, anti-CD14, and anti-CD11b (Biolegend, San Diego, USA). Cell subsets were stained with monoclonal antibodies and the isotype control and analyzed on a FACSCalibur flow cytometer using CellQuest Software (Becton Dickinson). Plots were generated using FlowJo Software (Treestar Inc, Ashland, USA).
2.4 RA FLSs
Synovial tissue specimens were collected from six female patients with active RA, who fulfilled the ACR 1987 revised criteria for the classification or the 2010 ACR/European League Against Rheumatism criteria of RA28, 29 and underwent synovectomy or joint replacement surgery. All patients provided written informed consent before the procedure. RA fibroblast-like synoviocytes were isolated from the synovial tissue of each RA patient separately. The synovial tissue was finely minced and digested with 1 mg/mL type I collagenase (Sigma-Aldrich) in serum-free Dulbecco's modified Eagle's medium (DMEM) at 37°C with 5% CO2 for 2 h. The cell suspension was filtered through a sterile cell strainer (BD Biosciences, USA). Synoviocytes were collected and rinsed by centrifugation. The pellet was resuspended in DMEM containing 10% fetal bovine serum (FBS), 100 U/ml penicillin, and 100 μg/mL streptomycin, and transferred to a 100 mm dish. At 90% confluence, RA FLSs were trypsinized and passaged to another dish. Passage 4–6 RA FLSs were used in experiments.
2.5 Semiquantitative real-time PCR
RA FLSs were stored in TRIzol reagent (Invitrogen, China) at −80°C. Total RNA was extracted from the RA FLSs. cDNA was prepared from 1 μg RNA and amplified by semiquantitative real-time PCR using SYBR Green (Takara, Japan). GAPDH was used for normalization. Data were analyzed by the 2−ΔΔCT method. Each sample was analyzed in triplicate, and the analysis was repeated three times. The primers for target genes were as follows.
IL-8, forward: 5ʹ-GACCACACTGCGCCAACAC-3ʹ
reverse: 5ʹ-CTTCTCCACAACCCTCTGCAC-3ʹ,
IL-6, forward: 5ʹ-ACTCACCTCTTCAGAACGAATTG-3ʹ
reverse: 5ʹ-CCATCTTTGGAAGGTTCAGGTTG-3ʹ,
MMP-1, forward: 5ʹ-GCTAACAAATACTGGAGGTATGATG-3ʹ
reverse: 5ʹ-ATTTTGGGATAACCTGGATCCATAG-3ʹ,
MMP-3, forward: 5ʹ-AGCAAGGACCTCGTTTTCATT-3ʹ
reverse: 5ʹ-GTCAATCCCTGGAAAGTCTTCA-3ʹ,
MMP-13, forward: 5ʹ-TCCTGATGTGGGTGAATACAATG-3ʹ
reverse: 5ʹ-GCCATCGTGAAGTCTGGTAAAAT-3ʹ
MCP-1, forward: 5ʹ-CAGCCAGATGCAATCAATGCC-3ʹ
reverse: 5ʹ-TGGAATCCTGAACCCACTTCT-3ʹ,
GAPDH, forward: 5ʹ-GGAGCGAGATCCCTCCAAAAT-3ʹ
reverse: 5ʹ-GGCTGTTGTCATACTTCTCATGG-3ʹ,
2.6 Transwell invasion assay
RA FLSs were starved for 24 h in serum-free DMEM. A transwell assay was performed in 6.5 mm transwell chambers with 8 μm pores. Briefly, the bottom surface of each membrane was precoated with matrigel. Then, RA FLSs (1 × 104) were added to the upper chamber, and complete DMEM with 10% FBS and GMSCs or human dermal fibroblast cell was added to the lower chamber. After incubation for 24 h at 37°C, the upper surface of each membrane was removed and cleaned with a cotton swab. Cells that had migrated to the bottom side were carefully stained with crystal violet and counted under a light microscope.
2.7 5-Ethynyl-2′-deoxyuridine (EdU) assay
RA FLSs (1 × 104) were starved in a serum-free medium for 24 h in the upper chamber of a transwell plate. GMSCs were seeded in the lower chamber. RA FLSs were treated with PDGF (20 ng/mL) for 72 h and then treated with 50 μmol/L EdU for 2 h. RA FLSs were fixed with paraformaldehyde and then stained with Apollo fluid and Hoechst 33342. EdU incorporation was assessed under a LEICA DMIRB fluorescence microscope in accordance with the manufacturer's instructions (RiboBio Co., Guangzhou, China). Image-Pro Plus software was used for analysis.
2.8 Mice
Seven to eight-week-old male DBA/1J mice were purchased from The Jackson Laboratory (Bar Harbor, USA). Mice were handled in accordance with National Institutes of Health guidelines for the use of experimental animals with approval from the Penn State University Hershey Medical Center for the use and care of animals. Our study followed standard biosecurity and institutional safety procedures (SUZ15-01-2).
2.9 Arthritis induction
Bovine type II collagen (CII; Condrex Inc) was emulsified in an equal volume of complete Freund's adjuvant (Sigma) containing 4 mg/mL heat-denatured mycobacterium (BD Biosciences). DBA1/J mice were intradermally injected at the base of the tail with 100 μg CII per mouse. Mice were euthanized by CO2 asphyxiation and cervical dislocation at the indicated times. To determine whether GMSCs can treat CIA, mice received an intravenous injection of GMSCs (2 × 106) in the tail vein 14 days after immunization. As negative and positive controls, human dermal fibroblast (2 × 106) and BMSCs (2 × 106) were intravenously injected into mice at the same time.
2.10 Clinical assessment and histopathological evaluation of experimental arthritis
Clinical signs of arthritis were evaluated every 2–3 days to determine arthritis incidence after immunization. Each paw was assessed and scored individually for arthritis severity using a scoring system (scale 0–4).3, 21 The total scores were calculated for all paws per mouse with a maximum score of 16.
Hind limbs were harvested from mice after sacrifice on Day 56. Tissue sections were prepared and stained with hematoxylin and eosin following fixation, decalcification, and embedding in paraffin. All slides were evaluated in a blinded fashion. Evidence of synovitis, pannus formation, and bone/cartilage erosion was assessed using a graded scale.21
2.11 Statistics
Data are expressed as means ± standard error of the mean (SEM). Data were analyzed using the unpaired t-test (Mann–Whitney) between two groups or one-way analysis of variance (ANOVA) for comparison among multiple groups followed by Tukey's test as appropriate. Differences were considered statistically significant at P < 0.05.
3 RESULTS
3.1 CD39 expression is higher in GMSCs
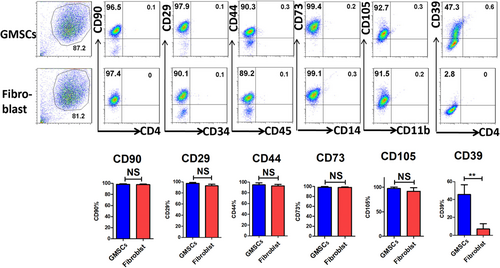
We analyzed classic surface markers of GMSCs and found that CD90, CD29, CD44, CD73, and CD105 were all highly expressed in GMSCs and human dermal fibroblasts. CD4, CD34, CD45, and CD11b were all weakly expressed in GMSCs and human dermal fibroblasts. Most importantly, CD39 was highly expressed in GMSCs, but not in human dermal fibroblasts (Figure 1).
3.2 GMSCs decrease mRNA levels of MMPs and pro-inflammatory cytokines of RA FLSs
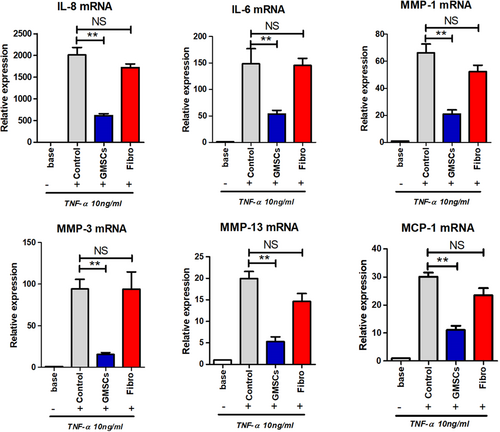
High levels of pro-inflammatory cytokines and MMPs are crucial characteristics of RA FLSs. Therefore, we examined whether GMSCs regulated the expression of MMPs and pro-inflammatory cytokines in TNF-α-stimulated RA FLSs. IL-8, IL-6, MMP-1, MMP-3, MMP-13, and MCP-1 mRNAs were all decreased in the GMSC-treated group compared with the human dermal fibroblast group (Figure 2).
3.3 GMSCs suppress the proliferation of RA FLSs via CD39/CD73 signaling
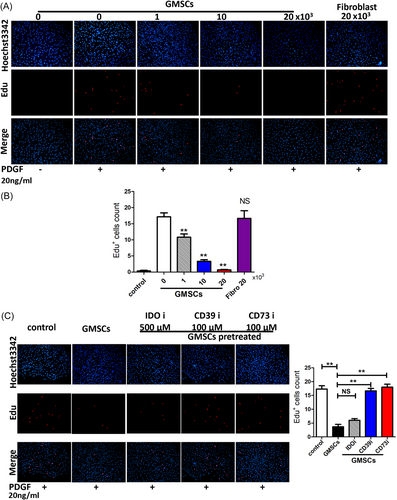
To assess the influence of GMSCs on RA FLS proliferation, RA FLSs were stimulated by PDGF and incubated with various numbers of GMSCs (0, 1, 10, and 20 × 103). Proliferation was then assessed by an EdU incorporation assay. GMSCs suppressed the proliferation of RA FLSs in a dose-dependent manner (Figure 3A,B). Furthermore, when the GMSCs were pretreated with CD39i or CD73i, such suppression was abrogated. GMSCs pretreated with IDOi still had the capacity to suppress RA FLS proliferation (Figure 3C).
3.4 GMSCs suppress RA FLS invasion
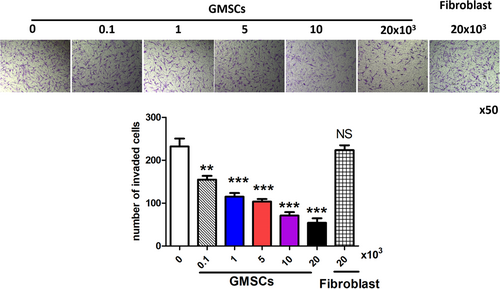
Next, we investigated the effect of GMSCs on RA FLS invasion. RA FLSs were incubated with GMSCs, and then a transwell assay was conducted to measure the effects on invasion. GMSCs markedly suppressed the FBS-induced invasion of RA FLSs through matrigel in a dose-dependent manner (Figure 4), indicating that GMSCs suppressed RA FLS invasion.
3.5 GMSC therapy delays arthritis onset and reduces arthritis scores in the CIA model
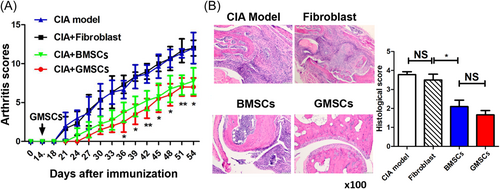
GMSCs and BMSCs exert anti-inflammatory effects in autoimmune diseases. Therefore, we investigated the effect of GMSCs in the CIA model. GMSCs and BMSCs significantly delayed arthritis onset and markedly reduced arthritis scores in the CIA model, whereas human dermal fibroblast did not have such effects. GMSCs were comparable to BMSCs in reducing arthritis scores (Figure 5A). To determine whether GMSCs prevented bone destruction in CIA mice, we stained joints with HE. A significant decrease in histological changes of synovitis, pannus formation, and destruction of cartilage and bone was observed in the ankle joints after incubation with GMSCs. However, there was no difference in histological changes between GMSC and BMSC groups (Figure 5B).
4 DISCUSSION
Our data demonstrated that GMSCs suppressed the expression of IL-8, IL-6, MMP-1, MMP-3, MMP-13, and MCP-1 in RA FLSs. Notably, GMSCs significantly reduced RA FLS proliferation in a dose-dependent manner in vitro, which was partly dependent on CD39/CD73 signaling. GMSCs significantly restrained the invasive capacity of RA FLSs in a dose-dependent manner in vitro. We also found that GMSC therapy delayed arthritis onset and reduced arthritis scores in the CIA model. These results imply that GMSCs should be evaluated as a treatment option for RA patients.
RA FLSs play a major role in RA pathogenesis by producing multiple cytokines that facilitate persistent inflammation, cartilage destruction, and bone erosion.8 IL-8 regulates the inflammatory response, mainly through the recruitment of neutrophils into an inflamed joint space.30 Serum IL-6 is positively associated with RA disease activities, and IL-6 is detected in synovial fluid of RA patients.31 Serum IL-6 induces B cell differentiation into plasma cells that produce autoantibodies in RA patients. IL-6 also facilitates the generation of Th17 cells from naïve CD4+ T cells. Local IL-6 recruits white blood cells into joints, promotes the maturation and activation of osteoclasts, and stimulates cell proliferation in synovial tissue. IL-6 also promotes the expression of MMP-1, MMP-3, and cytokines in RA FLSs. The IL-6 inhibitor tocilizumab has shown good efficacy in RA patients.32 MCP-1 was increased in the synovial fluid and serum of RA patients.33, 34 Synovial tissue macrophages constitutively produce MCP-1 that has a chemotactic activity in monocytes, activates monocytes and macrophages, and releases other cytokines, suggesting that MCP-1 is related to RA pathogenesis.35 In this study, we demonstrated that GMSC therapy suppressed the expression of IL-8, IL-6, MMP-1, MMP-3, MMP-13, and MCP-1 in RA FLSs, suggesting a strong suppressive effect of GMSCs on inflammation mediated by RA FLSs.
RA FLSs have strong proliferation ability even in the absence of exogenous stimulators. The reason is that RA FLSs are protected from apoptosis by strong survival signaling.8 RA FLSs are resistant to endoplasmic reticulum stress-induced apoptosis, probably because of increased autophagy and proteasomal activity.36 Overexpression of histone deacetylase 1 is associated with increased cell proliferation and survival of RA FLSs.37 Furthermore, RA FLSs have an aggressive and invasive capacity to invade the extracellular matrix, mediating inflammation and further exacerbating joint damage. RA FLSs that are co-implanted with human cartilage into SCID mice invade and degrade human cartilage. This aggressive behavior of RA FLSs is mainly due to increased expression of MMPs and IL-6.8, 9 We found that GMSCs significantly reduced the proliferation and invasive behavior of RA FLSs in a dose-dependent manner in vitro, which indirectly inhibited the inflammatory response and aggressiveness of RA FLSs.
We found that CD39 was highly expressed in GMSCs, but not in human dermal fibroblasts. This suggests that CD39 is a crucial biomarker for GMSCs. CD39 promotes the hydrolysis of ATP and ADP to generate AMP, which is then hydrolyzed by CD73 to adenosine which inhibits the immune response. Our previous studies have shown that GMSCs can treat the CIA model, murine osteoclastogenesis and bone erosion, a type 1 diabetes model, and xenogenic GVHD model mainly via CD39/CD73 signaling.21, 25-27 GMSC infusion also increases CD39+ Treg cells that suppress arthritis in mice.21 Another study has demonstrated that CD39 facilitates adenosine generation in Treg cells.38 Here, we confirmed that GMSCs inhibited the aggressiveness, proliferation, and inflammatory response of RA FLSs depending on CD39/CD73 signaling. These preclinical data suggest that GMSCs have a promising potential to treat RA patients.
Taken together, our study demonstrates that GMSCs suppress inflammation, proliferation, and invasiveness of RA FLSs through CD39/CD73 signaling. GMSCs also ameliorate experimental arthritis and protect against bone damage. These observations may be the basis to design clinical trials of GMSCs for RA patients.
AUTHOR CONTRIBUTIONS
Song Guo Zheng and Jin Lin: Conception and design of the study, final approval of the manuscript. Weiqian Chen, Ye Yu, and Jin Lin: Performed experiments. Weiqian Chen, Song Guo Zheng, and Jin Lin: Data analysis, interpretation. Weiqian Chen, Song Guo Zheng, and Jin Lin: Wrote the manuscript.
ACKNOWLEDGMENTS
This work was supported in part by the National Natural Science Foundation of China (No. 82171768), and the Zhejiang Provincial Natural Science Foundation of China (No. LQ17H100001).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
The protocol was approved by the medical ethics committees of Institutional Review Boards (IRB) at Penn State University Hershey College of Medicine and the First Affiliated Hospital, Zhejiang University School of Medicine.
Open Research
DATA AVAILABILITY STATEMENT
The data set used in this study is not publicly available but shall be available from the corresponding author upon reasonable request.