Unveiling the nexus: Decoding interactions between regulated cell death and systemic lupus erythematosus pathogenesis for innovative therapeutic avenues
Edited by Zhiyu Wang and Lishao Guo.
Abstract
Systemic lupus erythematosus (SLE) is characterized by disruptions in cell death pathways and impaired clearance of apoptotic cells, resulting in immune dysregulation and tissue damage. This review explores the complex interplay of regulated cell death (RCD) mechanisms, including apoptosis, necroptosis, pyroptosis, NETosis, autophagy, and ferroptosis, in the pathogenesis of SLE. These pathways release autoantigens and danger signals, triggering autoimmune reactions and inflammation. Six various RCDs have mutual associates to support immune dysregulation and are associated with SLE. Apoptosis intrinsically induces immune tolerance by packaging dying cells into immunologically inert fragments. Deficiencies in apoptotic clearance will result in impaired tolerance. Necroptosis, pyroptosis, NETosis, and ferroptosis lead to cell membrane destruction, production of intracellular immunostimulatory components, and triggering a strong inflammatory immune reaction. Abnormal autophagic activity affects the development, differentiation, function, and metabolism of many immune cell subpopulations. Investigating the interconnections between cell death pathways and SLE sheds light on the disease's underlying mechanisms and provides opportunities for novel therapeutic interventions. The convergence of precision medicine and innovative strategies targeting these intricate pathways holds promise for expanding the landscape of SLE treatment.
Key points
-
Several regulated cell death products initiate activation of autoreactive B cells, leading to the formation of autoantibodies.
-
Apoptotic disturbances and abnormal clearance of apoptotic cells enhance immune recognition of modified autoantigens.
-
PANoptosis combines pyroptosis, apoptosis, and necroptosis, linking regulated cell death to lupus pathogenesis and inflammation.
1 INTRODUCTION
Systemic lupus erythematosus (SLE) is a heterogeneous autoimmune disorder characterized by disrupted cell death pathways and impaired apoptotic cell clearance.1 Regulated cell death (RCD), a genetically guided process, eliminates infected, damaged, or diseased cells, releasing intracellular components that trigger pro-inflammatory gene expression in immune cells. Excessive debris externalization activates the immune system, fostering antigen–antibody immune complex (IC) formation across various tissues.2 Addressing flawed cell death and clearance mechanisms may prevent SLE-related immunogenic nuclear autoantibodies and immune abnormalities. The review delves into the six aberrant cell death pathways intrinsic to SLE's pathogenesis, aiming to unveil their interplay and identify therapeutic targets.
2 CELL DEATH PATHWAY INTERPLAY IN SLE: NOVEL PERSPECTIVES
2.1 Apoptosis and its dysregulation
Apoptosis, a fundamental programmed cell death process, has a pivotal role in maintaining both the physiological equilibrium and pathological responses, imparting it with a central significance in SLE. The intricate signaling mechanisms orchestrating apoptosis ensure cellular integrity and curtail aberrant immune reactions that fuel inflammation (Figure 1A).
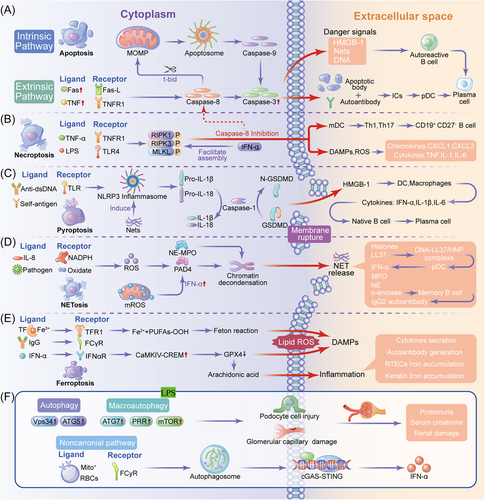
Within the intricate landscape of SLE, aberrations in the apoptotic pathway intricately entwine with the genesis of autoimmunity, marking a critical juncture where self-tolerance falters.3 At the heart of this paradigm lies extrinsic and intrinsic apoptotic pathways finely tuned to distinct triggers, orchestrating regulatory cues that profoundly shape the immunological milieu.
The presence of Fas ligand and Fas receptors in epidermal keratinocytes from skin biopsies of individuals diagnosed with chronic cutaneous lupus erythematosus indicates dysregulation of the extrinsic apoptotic pathway.4 An imbalance in this pathway contributes to inadequate elimination of apoptotic cells, thereby promoting the survival and proliferation of autoreactive B and T cells, and forming ICs in a lupus-prone milieu.5, 6 Mice harboring mutations in the Fas gene (MRL/lpr) or FasL gene (C3H/HeJ-gld/gld) manifest lymphoproliferation, generate autoantibodies, and display glomerulonephritis mediated by ICs.7 In individuals diagnosed with autoimmune lymphoproliferative syndrome, mutations in the FAS gene are frequently observed. Although autoimmune lymphoproliferative syndrome is primarily distinguished by lymphoproliferation and autoimmune cytopenias, certain patients may also exhibit lupus-like manifestations,8 shedding light on mechanistic parallels between aberrant Fas signaling and the induction of lupus-like manifestations. The heightened levels of apoptosis observed in lupus nephritis (LN) highlight the engagement of both Fas and caspase-3.9 However, their implications transcend mere cellular demise. Beyond its apoptotic role, caspase-3 orchestrates cyclic GMP-AMP synthase (cGAS) cleavage, thereby exerting influence over the type I interferon (IFN-I) axis. This uncovers the intricate nexus connecting apoptosis to innate immune effectors, revealing a novel aspect of its contribution to SLE.10
2.2 Necrosis and its contribution to inflammation
Among cell death pathways, necroptosis is an alternative when traditional apoptotic routes falter. Initiated by receptor-interacting serine/threonine-protein kinase (RIPK) 3 and mixed lineage kinase domain-like protein (MLKL) interplay, necroptosis diverges from the usual path by causing rapid organelle and cellular swelling instead of shrinking (Figure 1B).11
As the main initiators, RIPK3 and RIPK1 respond to tumor necrosis factor-α (TNF-α) to trigger this unique cell death.12 RIPK3 is overexpressed in podocytes in renal biopsies from patients with class IV LN and in diseased kidneys from lupus-prone mice, demonstrating that necroptosis contributes to LN pathogenesis. Additionally, RIPK3 activation is specifically blocked by GSK872 in murine models, implying that inhibition of RIPK3 is a novel therapy for LN.13 RIPK1's catalytic role in cytokine synthesis shapes SLE inflammation and immune responses.14, 15 IFN-α promotes RIPK1/RIPK3 assembly coupled with increased MLKL expression, fostering necroptosis and tissue damage.16, 17 Inhibitors such as necrostatin-1 (Nec-1) that suppress RIPK1 exhibit promising prospects beyond the mere control of inflammation, encompassing the regulation of neutrophil apoptosis in SLE.18
In the immune microenvironment, macrophages phagocytize mitochondria from necroptotic cells, resulting in cytokine secretion and facilitating dendritic cell (DC) maturation.19 In SLE, mature DCs differentiate and stimulate B-cells by polarizing naive T cells into Th1 and Th17 cells, contributing to lymphopenia and autoantibody production.20 Necroptosis leads to the release of reactive oxygen species (ROS) and damage-associated molecular patterns (DAMPs), such as high-mobility group box protein 1 (HMGB1), and the release of pro-inflammatory chemokines (e.g., CXC motif chemokine ligand 1 [CXCL1] and CXCL2) and cytokines (e.g., TNF, interleukin [IL]-1, and IL-6), causing tissue inflammation and neutrophil infiltration.21 Furthermore, sustained activation of the IFN signaling pathway contributes to upregulation of MLKL expression in SLE, leading to necroptosis and subsequent tissue damage. Elevated levels of necroptosis have been observed in cutaneous keratinocytes of lupus patients with interface dermatitis upon stimulation with IFN-γ and TNF-α.22
2.3 Pyroptosis and pro-inflammatory outcomes
Pyroptosis, a programmed cell death mechanism, arises from pathological triggers, leading to pore formation, cellular swelling, and eventual rupture. This process significantly contributes to SLE-associated inflammation (Figure 1C).
Pyroptosis initiation involves pro-caspase-1 orchestrating the inflammasome complex via apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC) adaptor binding, which is intricately linked to SLE through autoantibodies targeting ASC specks.23 The nucleotide-binding domain leucine-rich repeat-containing protein 3 (NLRP3) inflammasome, consisting of NLRP3, pro-caspase-1, and ASC, generates mature caspase-1 and cleaved gasdermin D (GSDMD), driving IL-1β and IL-18 maturation.24, 25 Elevated NLRP3/ASC/caspase-1 activity drives LN, impacting podocyte function and autoinflammatory disorders.26, 27 NLRP3 and ASC deficiencies mitigate proliferative LN.28, 29
Physiologically, Toll-like receptor (TLR) 2 triggers IL-1, IL-6, and TNF-α release via the HMGB1 nuclear complex, causing lysosomal rupture, cathepsin-B activation, and caspase-1-mediated pyroptosis.25, 30 HMGB1, an SLE alarmin, induces macrophage pyroptosis and is also released by pyroptotic cells, acting as a DAMP. HMGB1 binds to NLRP3 and facilitates its oligomerization, leading to caspase-1 activation and subsequent pyroptotic cell death.31 Elevated caspase-1 cleavage characterizes SLE macrophages.32 Neutrophil extracellular traps (NETs) containing LL-37 amplify NLRP3 inflammasome activation, driving IL-1β and IL-18 release, and exacerbating systemic inflammation.33 HMGB1 regulates T-cell responses, leading to heightened proliferation and cytotoxic activity. This ultimately promotes T-cell activation and a shift towards a more pro-inflammatory phenotype, thereby exacerbating the manifestations of SLE.34 Additionally, through its interaction with receptors for advanced glycation end products (RAGE) on B cells, HMGB1 enhances B-cell activation and autoantibody production, further fueling the autoimmune response.35 Furthermore, HMGB1 induces endothelial cell activation and contributes to a procoagulant state, thereby contributing to the vascular abnormalities commonly observed in SLE patients.36
2.4 NETosis and its role in IC formation
Neutrophils function through phagocytosis, antimicrobial secretion, and NETs–protein–DNA networks immobilizing pathogens (Figure 1D).37, 38
Compensatory mitochondrial NETosis during nicotinamide adenine dinucleotide phosphate oxidase impairment worsens lupus in animal models and humans, highlighting the role of NETs in SLE.39 Elevated peptidyl arginine deiminase (PAD) 4 in MRL/lpr mice and its inhibition suppressing NETs underscore its relevance in SLE.40, 41 Targeting PAD4 with inhibitors shows the potential to attenuate autoimmunity, dampening NET-induced tissue damage and managing cardiovascular SLE complications.42, 43
Mitochondrial NETosis releases unprotected mitochondrial DNA (mtDNA), promoting mtDNA-carrying cells. Anti-mtDNA antibodies in SLE patients signal immunogenicity of mtDNA-containing NETs, triggering IFN-α secretion.40 Targeting mtDNA and anti-mtDNA antibodies is a potential therapeutic strategy for SLE. NET release induces liberates DAMPs including HMGB1. Subsequently, extracellular HMGB1 interacts with its corresponding receptors (e.g., RAGE, TLR2, and TLR4) located on the neutrophil surface, thereby establishing a self-sustaining loop of inflammation and tissue injury.44 The HMGB1-NET DNA complex, which is a robust inducer of immune responses, is specifically identified by antibodies in individuals with SLE, leading to the formation of ICs, activating complement, and additional internalization by DCs to intensify the immune response.45
NETs might shape SLE autoantibodies by DNA exposure during cell death and altered protein interactions. Mitochondrial ROS activate cGAS–stimulator of interferon genes and IFN-I via NET-incorporated oxidized DNA.46 Some drugs (e.g., procainamide) induce lupus-like symptoms by promoting NET formation.47 Kinase-mediated disassembly and envelope rupture of the nuclear lamina amplify entry of decondensation enzymes, facilitating nuclear chromatin decondensation and extracellular NET release.48 Protein kinase C (PKC)α and cyclin-dependent kinases (CDK)4/6 affect the nuclear alterations and mechanisms that precipitate nuclear envelope rupture, a pivotal event in NETosis. PKCα directly facilitates phosphorylation and dismantling of lamin B, resulting in rupture of the nuclear envelope.49 CDK4/6 appears to primarily contribute to broader regulation of the cell cycle and the associated nuclear processes that contribute to NET formation.50 Essentially, dysregulation of these nuclear lamin enzymes may contribute to lupus pathogenesis by augmenting the formation of problematic NETs and this intricate NET–SLE interplay offers novel insights.
2.5 Ferroptosis and linkage to iron overload and oxidative stress
Ferroptosis, an iron-triggered nonapoptotic cell death pathway activated by iron overload and lipid peroxidation, involves distinct mitochondrial alterations (Figure 1E).51
At its core, glutathione peroxidase 4 (GPX4) mitigates lipid hydroperoxides via glutathione during oxidative stress. Myeloid-specific GPX4-haploinsufficient mice exhibit lupus-like manifestations.52 GPX4 inhibits lipoxygenases and cyclooxygenases, thereby suppressing pro-inflammatory cascades from lipid oxidative mediators.53 Selenium-induced elevation of glutathione/glutathione peroxidase activity in lupus patients has been noted, whereas cytokine activation, encompassing IFN-α, IFN-γ, IL-6, serum IgG, and autoantibodies, exploits the CaMKIV/CREM axis to induce ferroptosis.52
Ferroportin, the iron transporter, mediates iron uptake through transferrin receptor, and ferritin heavy/light chain can increase iron levels, both of which can promote ferroptosis. The systemic iron equilibrium is governed by hepcidin and ferroportin.54 Hepcidin's potential to mitigate kidney inflammation in LN has gained prominence,55 alongside pro-inflammatory IL-6's disruption of the iron balance through ferroportin inhibition.56 Glomerular hepcidin elevation correlates to IC accumulation and the inverse serum transferrin–SLE activity correlation underscores iron's significance.57 Hepcidin modulation has emerged as a prospective strategy for LN management.58
Kidney dysfunction and skin inflammation associated with SLE are linked to ferroptosis. Cutaneous lupus may be associated with dysregulation of iron metabolism and ultraviolet-induced ferroptosis, owing to the light sensitivity of lupus and the accumulation of ROS.59 As the disease advances, renal tubular epithelial cells experience ferroptosis due to the excessive absorption of iron by the kidneys and the subsequent pathological accumulation of iron.60
Notably, neutrophil-driven ferroptosis, characterized by GPX4 downregulation and lipid ROS elevation, induces autoreactive B cells, plasmacytoid DCs, and IFN-I production, influencing disease pathogenesis.52 Ferroptosis-mediated release of DAMPs and lipid oxidation products regulates immune responses to cellular debris, amplifying inflammation and organ damage inherent to SLE.
2.6 Autophagy and its dual roles in SLE
Autophagy is essential for innate and adaptive immunities, as well as the SLE lymphocyte balance. It also preserves the cellular equilibrium via self-degradation.61 Macroautophagy, microautophagy, and chaperone-mediated autophagy constitute its cargo capture forms (Figure 1F).
Elevated autophagy-related gene expression in SLE macrophages underscores pathological relevance.62 ATG5 gene variants near genome-wide association study-identified loci are associated with SLE susceptibility and clinical traits, including specific single-nucleotide polymorphisms, such as rs6568431 and rs2245214, correlating to anemia, renal involvement, and increased anti-DNA autoantibodies.63
Metabolically, dysregulated macrophage autophagy may aggravate lupus pathogenesis through TNF-α and IL-6 amplification.62 Mitochondrial dysfunction also significantly contributes to SLE. Deficiencies in mitophagy trigger IFN-α signal transduction and autoimmunity in murine models, underscoring its relevance to lupus.64 The involvement of mitochondrial interferon in erythrocyte development facilitated by hypoxia-inducible factor-mediated adjustments underscores the importance of red blood cell mitochondria.64 Mitochondria play a critical role in maintaining the immune equilibrium and shaping disease progression.
3 INTERPLAY OF CELL DEATH MECHANISMS AND PANOPTOSIS IN SLE PATHOGENESIS
3.1 Complex interconnections: Apoptosis, necroptosis, and pyroptosis interplay in lupus
Comparing the attributes of six RCDs associated with lupus establishes a foundation to investigate their interconnectedness. PANoptosis uniting pyroptosis, cell death, and necroptosis under P, A, and N components has emerged as an important convergence point. PANoptosome assembly initiated by sensor molecules provides a framework for interactions governing pyroptosis (inflammasome sensors, ASC, and caspase-1), apoptosis (caspase-8), necroptosis (RIPK3 and RIPK1), and related pathways.
PANoptosomes coordinate downstream apoptosis effectors pyroptosis (caspase-1 and GSDMD), apoptosis (caspase-3 and -7), and necrosis (RIPK3 and MLKL). Pyroptotic NLRP3 inflammasome activation triggers caspase-1, processing IL-1β and IL-18.65, 66 The NLRP3/IL-1 axis enhances naive T-cell differentiation, intensifying T- and B-cell responses, causing inflammation, and organ damage.67 Necroptotic RIPK3 activation catalyzes NLRP3 inflammasome activation. Inhibiting RIPK3 mitigates autoimmunity, ameliorating LN.13 Pyroptosis cleaves caspases and PARP1, exerting downstream control over caspase-1. Apoptotic caspase-8 is crucial for NLRP3-dependent inflammasome initiation,68 highlighting intricate pyroptosis, apoptosis, and necrosis interplay. Moderate PANoptosis aids immune cell infiltration against viral infections, whereas excessive PANoptosis may trigger harmful inflammation and lung injury.69
3.2 Converging pathways: Important interplay in cell death regulation and implications in lupus
In SLE, central cell death regulatory crosstalk emerges. Apoptotic cells generate histone-modified microparticles, which enhances pDC and myeloid DC activation, driving NET formation.70 HMGB1 regulates triggers of pyroptosis and NETosis, shaping inflammatory responses. IL-1β and IL-18 from pyroptotic cells initiate inflammation cascades, activating NETosis-affected neutrophils and reinforcing SLE-specific inflammation.71 HMGB1 prompts adhesion molecule synthesis, fostering neutrophil recruitment and transmigration, which is amplified by HMGB1-induced NETosis.72 Excessive NET formation underlies tissue injury, contributing to SLE and rheumatoid arthritis.73
Autophagy inhibition restrains NETosis, promoting apoptosis-like outcomes.74 NETs enriched with tissue factor and IL-17A drive thrombo-inflammation via the REDD1/autophagy pathway in SLE.75 Zhang et al.76 proposed neutrophil ferroptosis and NETosis synergy in SLE pathogenesis. Excessive ROS production exacerbates both ferroptosis and NETosis.
The interplay between ferroptosis and necroptosis is pivotal for SLE pathogenesis. Recent research has revealed their connections, expanding our understanding. MLKL mediates susceptibility to both necroptosis and ferroptosis.77, 78 Glutathionylation-triggered caspase-8 inactivation induces TNF-α-independent necroptosis in GPX4-deficient cells.79 Elevated heat shock protein 90 in SLE patients is linked to neuropsychiatric symptoms, as indicated by mouse model findings.80 Autophagy amplifies ferroptosis through heightened activity influenced by ferritin's role as an iron reservoir.81 Reciprocal inhibition between ferroptosis and autophagy is seen in multiple myeloma cells.82 These insights deepen our understanding of SLE mechanisms, offering therapeutic possibilities.
The evolving comprehension of the interplay between ferroptosis and necroptosis in SLE highlights their complexity. Major components, such as MLKL, GPX4, heat shock protein 90, and ferritin, along with intricate autophagy interactions enhance our understanding of SLE's basis and potential treatment prospects. Continued research is crucial to unravel these intricate dynamics and their relevance to managing SLE and related conditions.
3.3 Intersecting trajectories: The multifaceted effect of extracellular vesicles (EVs) on the pathogenesis and prognosis of autoimmune diseases
EVs are membranous particles produced by nearly all types of cells and have roles in facilitating intercellular communication. Both exosomes and membrane microvesicles (MVs) can be released by cells undergoing apoptosis. Apoptotic bodies are the corpse of apoptotic cells formed in the end stage of apoptosis.83 Serving as carriers that facilitate intercellular communication, EVs encapsulate various contents, such as proteins, lipids, and nucleic acids, which they subsequently transport and deliver to target cells.84 The accumulation of nuclear autoantigens (e.g., nucleosomal DNA, Ro, and La) in the late stages of apoptosis is a major feature of SLE.85 Cells experiencing pyroptosis or necroptosis may discharge EVs carrying nuclear and mitochondrial components, potentially acting as autoantigens. MVs containing acetylated chromatin drive ROS-independent NET release in SLE patients with active LN.86 NETotic neutrophils release EVs devoid of nuclear DNA, but they might carry proteins and citrullinated histone autoantigens.87 Neutrophil-derived PR3 or myeloperoxidase (MPO)-positive EVs induce vascular endothelial cell damage and serve as autoantigens a related to anti-neutrophil cytoplasmic antibody-associated vasculitis.88 Additionally, endothelial damage mediated by MPO and the formation of ICs (EV-ICs) further exacerbate podocyte injury, foot process effacement, and mesangial cell proliferation.89 Pyroptotic monocytes are capable of releasing heterogeneous populations of EVs, among which certain MVs contain cleaved GSDMD and active caspase-1, thereby also inducing injury to vascular endothelial cells.90 Extensive research conducted on EVs91 has demonstrated that the release of subcellular EVs by various cell types undergoing cell death significantly contributes to autoimmune responses and the pathogenesis of lupus through diverse mechanisms.
4 NOVEL THERAPEUTIC STRATEGIES
4.1 Potential of targeting RCD pathways for SLE treatment
The complexity of RCD pathways presents novel opportunities for SLE treatment. Notably, inhibitors such as Nec-1 have emerged as promising candidates to suppress necroptosis, a mechanism intricately linked to SLE pathogenesis. Nec-1's ability to block RIPK1 activation highlights its potential to halt necroptotic cell death and subsequent inflammatory cascades. By mitigating necroptosis-driven tissue damage and cytokine release, Nec-1 may curb SLE-associated inflammation and organ damage.
Another strategy for targeted therapy in SLE is modulation of NETs through PAD inhibitors. PAD4 inhibition may control excessive NETosis, a contributor to IC formation and tissue damage. Regulating PAD4 activity may temper the release of NETs and attenuate SLE-associated inflammation, offering a unique approach to disease management.
In SLE, leveraging RCD pathways as therapeutic targets has both potential and challenges. Defective clearance of apoptotic cells in SLE suggests enhancing efferocytosis by targeting the Mer tyrosine kinase receptor.92 It also underscores the critical need to understand the balance between sufficient clearance and avoiding potential off-target effects that might amplify autoimmunity. The use of inhibitors involved in pyroptosis, such as GSDMD and caspase-1, is tempered by concerns about suppressing necessary inflammatory responses that fight infections. Similarly, curtailing necroptosis via molecules, such as RIPK1 and RIPK3, appears promising, but ensuring specificity will be paramount to avoid disrupting other essential cellular processes. Newly recognized PANoptosis with its amalgamation of several RCDs offers a novel therapeutic strategy, but its exact role and regulation in SLE is incompletely understood. The role of DAMPs, especially HMGB1, in SLE-related inflammation emphasizes the need for precision in modulating immune responses without inducing immunosuppression. The challenge is identifying therapeutic targets in these pathways and fine-tuning therapies to ensure efficacy, minimize side effects, and provide long-lasting relief for SLE patients. Future directions should focus on rigorous clinical trials, personalized medicine approaches, and continuous monitoring of the evolving understanding of RCD in SLE.
4.2 Cutting-edge therapeutic advances: RCD pathways in SLE treatment
A comprehensive examination of the six RCDs in relation to SLE has potential for innovative therapies. Such exploration summarizes the intricate mechanisms and underscores the potential for targeted treatments to address SLE's complexities. In the context of SLE, constitutive IFN signaling is linked to MLKL expression and necroptosis-triggered tissue damage.41 Notably, the application of Nec-1, an inhibitor of RIPK1, in a chronic kidney disease model in mice reduces major regulators IL-17, IL-1β, IL-6, and TNF-α, mitigating tissue damage.93 However, challenges persist because of the lack of clear necrosis markers.
The association between pyroptosis and SLE has been established, and various medications, including melatonin, oleuroside, baicalein, piperine, and MCC950, have demonstrated their ability to attenuate the activation of the NLRP3 inflammasome.54-58 Consequently, these interventions have been observed to reduce the development of murine LN and proteinuria. Recent studies underscore the multifaceted roles of absent in melanoma 2 inflammasome, P2X7 receptor, and GSDMD in SLE, presenting both pyroptosis and protective effects.94-97
In SLE, NETs with factor- and IL-17A-bearing components contribute to thrombin production and fibrotic potential.75 Thrombin's ability to cleave pro-IL-1α activates the immune system.98 Drugs such as tofacitinib and polydatin suppress NET formation and ROS, ameliorating lupus-like manifestations.99, 100 However, these do not match the efficacy of liproxstatin-1 (LPX-1) for ferroptosis, highlighting the distinct role of neutrophil ferroptosis.
Neutrophil ferroptosis-induced neutropenia in SLE is addressable through targeted therapy. Inhibitors such as LPX-1 and deferoxamine have shown potential in reducing serum-induced neutrophil death in SLE patients.52
Early B-cell autophagy activation in SLE influences plasmablast development, affecting autoantibody production.101 Autophagy-related genes (e.g., ATG7, IRGM, and LRRK2) have been implicated in SLE susceptibility.102-104 Therapies involving chloroquine and vitamin D partially modulate autophagy,105, 106 demanding in-depth exploration for effective SLE treatment.
This enigma of autoimmune diseases lies in immune self-reactivation. Dysregulated cell death pathways and inefficient clearance of cell debris amplify inflammatory responses. This leads to accelerated cell death and exposure to nuclear/cytoplasmic autoantigens, releasing DAMPs fueling SLE progression.
Recent therapeutic advancements have focused on mitigating NLRP3 inflammasome activation, a critical driver of SLE-related inflammation. Compounds such as melatonin, oleuropein, and MCC950 have the potential to dampen NLRP3 activation, thereby reducing IL-1β and IL-18 production. These compounds target upstream events triggering inflammasome assembly, presenting a novel means of curbing pro-inflammatory cytokine release and mitigating SLE-associated tissue damage.
Advancements in SLE therapy have also explored mtDNA and anti-mtDNA antibodies as potential targets. Elevated levels of mtDNA contribute to immune activation in SLE. Inhibiting mtDNA release from damaged mitochondria may restrain the subsequent IFN-α response and autoimmunity. Additionally, targeting anti-mtDNA antibodies offers a unique approach to curb immune responses driven by mtDNA-containing ICs, addressing a crucial aspect of SLE pathogenesis.
4.3 Precision medicine and challenges in innovative SLE therapies: A dual perspective
Precision medicine is pivotal, spotlighting individual SLE complexities and tailoring treatments to unique disease profiles for optimal efficacy and minimal side effects. By targeting relevant pathways, this approach recognizes SLE's diversity and the importance of discerning distinct molecular drivers. Personalized therapies based on genetics, molecules, and clinical data have the potential to revolutionize SLE treatment by precisely addressing mechanisms.
As SLE therapeutic horizons expand into RCD pathways, inhibitors curbing necroptosis and modulating NETosis have potential. Recent advancements targeting NLRP3 activation, mtDNA responses, and anti-mtDNA antibodies underscore innovative strategies. Precision medicine further sharpens therapeutic approaches, emphasizing tailored treatments for diverse SLE manifestations.
5 CONCLUSION
This comprehensive review delves into the complex interplay between SLE and RCD, revealing novel insights that have significant therapeutic implications. The convergence of various forms of RCD, such as apoptosis, necroptosis, pyroptosis, NETosis, autophagy, and ferroptosis, underscores their collective influence on immune dysregulation and their associations with SLE. These intricate interactions contribute to the pathogenesis of SLE and the dynamics of inflammation.
By unraveling these multifaceted regulatory pathways, this review sheds light on the intricate nature of SLE and discusses innovative therapeutic strategies. With a deep comprehension of these diverse mechanisms, a range of novel approaches emerges. These approaches have the potential to revolutionize SLE treatment by improving patient outcomes and raising the standards of disease management. The therapeutic avenues explored in this review have the potential to untangle the complexities of SLE, ushering in a novel phase of precise and effective disease management.
AUTHOR CONTRIBUTIONS
Conceptualization: Shuilian Yu and Wenhui Huang. Investigation: Qing Tan. Writing—original draft: Qing Tan. Writing—review and editing: Shuilian Yu and Wenhui Huang. The manuscript's published form was approved by all authors once they had read it.
ACKNOWLEDGMENTS
The authors express their gratitude to the clinical staff of the Department of Rheumatology at the Second Affiliated Hospital of Guangzhou Medical University. Project supported by the Guangdong Basic and Applied Basic Research Foundation of Guangdong Province, China (Grant/Award Numbers: 2019A1515011094, 2022A1515010471); the Guangzhou Science and Technology Planning Project of Guangdong Province, China (Grant/Award Number: 202102010139).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
Not applicable.
Open Research
DATA AVAILABILITY STATEMENT
None.