Immunophenotyping identifies distinct cellular signatures for systemic lupus erythematosus and lupus nephritis
Yi Tong V. Aw and Phillip J Whiley are co-first author.
Abstract
Background
Systemic lupus erythematosus (SLE) is a complex systemic autoimmune disease characterized by development of autoantibodies and multiorgan involvement. Kidney involvement, termed lupus nephritis, has major impact on life expectancy. It is increasingly recognized that SLE is likely a common clinical manifestation of pathophysiologically diverse processes, and lupus nephritis has similarly been associated with several distinct immunological processes. We compared the immune cell phenotypes of individuals with SLE in the presence or absence of nephritis.
Methods
Cryopreserved peripheral blood mononuclear cells from SLE patients with and without kidney involvement underwent flow cytometric analysis to identify major populations in T cells, B cells and myeloid lineages.
Results
We compared the frequencies of lymphocyte populations in 69 SLE patients without nephritis, 20 SLE patients with nephritis, and 92 healthy blood donors. Patients with SLE and lupus nephritis (LN) had reduced marginal zone B cells (P < 0.0001 in SLE; P = 0.001 in LN), memory B cells (P = 0.002 in SLE; P = 0.001 in LN) and circulating T follicular helper (Tfh) memory cells (P < 0.0001 in SLE and LN) compared to healthy donors. Patients with lupus nephritis had increase Th2 (P < 0.0001) and T regulatory cells (P < 0.0001) compared to both SLE patients without nephritis and healthy donors.
Conclusion
SLE patients with and without lupus nephritis have distinct immunologic differences that may reflect the unique pathophysiological processes contributing to disease manifestations.
Key points
-
Patients with SLE had reduced marginal zone B cells, memory B cells and circulating T follicular helper cells compared to healthy blood donors.
-
Patients with lupus nephritis had increased Th2 and T regulatory cells compared to SLE patients without lupus nephritis and healthy blood donors.
1 INTRODUCTION
Systemic lupus erythematosus (SLE) is the prototypic systemic autoimmune disease manifesting with end-organ inflammation arising from immune complex deposition. SLE is a consequence of diverse mechanisms culminating in a type 1 interferon mediated breakdown in peripheral tolerance with extensive dysregulation of the innate and adaptive arms of the immune system.1
Immune complex-mediated inflammation of the kidney, called lupus nephritis (LN), is one of the most significant manifestations of SLE. LN occurs in half of all patients and has the greatest impact on mortality of any SLE manifestation.2, 3 The glomerulonephritis of LN is classified into six classes of histopathological lesions based on the glomerular location, extent, and pattern of injury.4 The dysregulated immune response in SLE underpins LN pathogenesis,5 although emerging evidence over the past decade points toward a less direct role of autoantibodies and a more prominent role of infiltrating innate cells in driving local renal inflammation.5
The development of LN appears to have associations with particular ethnicities,6, 7 human leukocyte antigen genotypes,8-10 and the presence of specific autoantibodies11, 12 and autoantigens.13, 14 However, the precise immunological mechanisms which predispose some individuals with SLE to develop LN, and not others, remains unclear. To date, studies examining the peripheral lymphocyte population from patients with SLE, and especially LN, have focused on single lineages such as B cell or T cell subsets and not simultaneously evaluated all major lineages. Individual analysis of these compartments have resulted in several cellular phenotypes being observed in patients with SLE and LN. In the T cell compartment, SLE is associated with increased CD28− CD4+ effector T cells and with CD4+ lymphopaenia.15 Both clinically active disease and duration of activity are associated with initial recruitment of naive CD45RA+ T cells that gradually exhibit features of activation.16 Natural Killer (NK) cells may be decreased in active disease but the expression of interferon (IFN) by NK is strongly elevated. T regulatory cells have been reported are generally lower when disease activity is higher and glucocorticoid therapy is effective at raising Treg levels.17 Th17 lymphocytes have been shown to play an important role in numerous chronic autoimmune diseases including rheumatoid arthritis, multiple sclerosis and SLE.18 Th17 cells have previously been shown to be expanded in SLE patients and interleukin (IL)-17 levels reported to correlate with lupus disease activity.18 B cell phenotypes are more heterogeneous in SLE, yet most studies have noted an increase in plasmablasts, CD21− CD27− subsets19 and IgD CD95 expressing memory B cells.20
We investigated the peripheral immuno-phenotypes of individuals with SLE who developed LN and those without LN, in an observational study of a SLE/LN cohort using flow cytometry. We hypothesized that intrinsic differences in the underlying peripheral immuno-phenotype of some individuals with SLE predisposes to a proinflammatory response in renal tissue leading to the development of LN.
2 METHODS
The protocol was approved by the Sydney Local Health District Human Research Ethics Committee (HREC) at Concord Repatriation General Hospital, ACT Health HREC and Australian National University HREC. Written informed consent was obtained as part of the Australian Point Mutation in Systemic Lupus Erythematosus study, the Centre for Personalized Immunology program, the Healthy Blood Donors register and Genetic Mutation in Immune Mediated Kidney Disease study (The Canberra Hospital, Canberra, Australia). This study was carried out in accordance with the recommendations of the National Statement on Ethical Conduct in Human Research (2007), National Health and Medical Research Council with written informed consent from all subjects. All subjects gave written informed consent in accordance with the Declaration of Helsinki.
We processed cells collected from the subjects detailed above for flow cytometry. Blood was collected into acid citrate dextrose 9 ml collection tubes and processed within 24 h of collection. Peripheral blood mononuclear cells (PBMCs) were purified by layering blood over Ficoll-Paque, resuspended in Roswell Park Memorial Institute media, before freezing in 10% dimethylsulfoxide in foetal calf serum, then stored in liquid nitrogen before analysis.
Immunophenotyping of PBMCs was performed as previously described21 using 1 × 106 cells for each staining panel. Cells were thawed at 37°C and washed with FACS buffer (PBS/2% bovine serum/0.05% sodium azide). Fc receptors were blocked using Human TruStain FcX (Cat #422302; Biolegend, USA) and dead cells discrimination performed with LD Fixable Dead Cell stain (Cat #L34962; Invitrogen, USA) according to the manufacturer's instructions. PBMCs were stained with four antibody cocktails as described previously21 enabling identification of up to 54 cell parameters (Figure 1). Samples were fixed with eBioscience FoxP3 Fixation/Permeabilization reagent (Cat #00-5523-00; Invitrogen) according to the manufacturer's protocol. Flow cytometric acquisition was performed using a BD Fortessa FACS analyser at the Microscopy and Cytometry Resource Facility, John Curtin School of Medical Research and data analysis performed in Flowjo (v10; TreeStar, USA). Repeated analysis of stored PBMCs from a single control was included with every flow cytometry (FCM) run, which allowed experimental variation in each parameter to be measured.
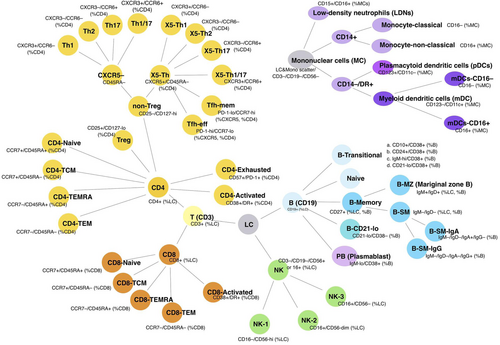
The FCM analysis was conducted as described previously21; initial gating was on viable singlet cells. Lymphocytes were gated by scatter; for nonlymphocyte populations, a broad leukocyte light scatter gate was used after excluding CD3, CD19, and CD56 expressing cells. Subpopulations were quantitated as a proportion of various parent populations as previously described21 (Figure 1 and Figures S1 and S2).
All comparisons were performed in GraphPad Prism 8.0 for Mac, using Mann–Whitney nonparameteric comparison of nonpaired values; P values below 0.05 were considered significant but were adjusted for multiple comparisons using Bonferroni correction.
3 RESULTS
We performed flow cytometric analysis of PBMCs from a cohort of 69 clinically inactive SLE individuals (herein referred to as SLE), 20 SLE individuals with LN (herein referred to as LN) and 92 healthy blood donors (HBD) as controls (HBD) (Table 1). Patients were required to meet Systemic Lupus International Collaborating Clinics (SLICC) criteria for enrollment and required biopsy-confirmed nephritis to be classified as having LN. Patient treatment was according to their treating physicians. Patients were not undergoing clinical flares of SLE at the time of blood sampling to minimize the contribution of inflammatory responses to alterations in PBMC phenotypes. Where multiple samples were collected for individual patients, the sample closest to the date of diagnosis was used in the study to better reflect the untreated state. This strategy also assumes that the known immunomodulatory role of vitamin D322 supplementation had not been initiated but has not controlled for potential seasonal variations in vitamin D3. Baseline demographics are described in Table 1 and there were/were not significant differences between cohorts (Table 2).
| Patient groups | n | Mean age (years, SD) | Sex (n) |
|---|---|---|---|
| HBD | 92 | 39.9 (13.4) | Female 60, Male 32 |
| SLE | 69 | 43.5 (18.2) | Female 56, Male 13 |
| LN | 20 | 44.6 (16.8) | Female 14, Male 6 |
| Class 2–3 | 6 | ||
| Class 4 | 11 | ||
| Class 5 | 3 | ||
| Ethnicity of SLE | |||
| European | 54 | ||
| Asian | 9 | ||
| Arabic | 3 | ||
| Latino | 3 |
- Abbreviations: HBD, healthy blood donor; LN, lupus nephritis; SD, standard deviation; SLE, systemic lupus erythematosus.
| NZM2410 | SLE patients |
|---|---|
| Reduced T regs23 | Reduced17 or increased24 T regs |
| –Rise with flares17 | |
| Increased activated CD4+23 | Increased activation over course of disease16 |
| Decreased naïve CD4+23 | Increased initially, but diminishing over time16 |
| Increased Tfh25 | Increased Tfh26 |
| mDC expansion23 | Reduced,27 normal,28 or increased29 mDC |
| pDC expansion23 | Reduced30 or increased31 pDC |
| Increased B cell activation23 | Increased plasmablast19 and memory B cell20 |
| Increased GC formation32 | Increased GC formation33 |
- Abbreviations: GC, germinal centers; mDC, myeloid dendritic cells; pDC, plasmacytoid dendritic cells; SLE, systemic lupus erythematosus; Tfh, T follicular helper cells.
PBMC immunophenotypes were classified into five broad groups (B cells, T helper [Th] cells, T cells, antigen presenting cells, and NK cells). Each group was further sub-classified into specific cellular types based on a previously reported gating strategy21 (see Section 2 and Figure 1). We compared between SLE, LN, and HBD groups both quantitative differences in cell populations as well as qualitative differences in membrane expressed proteins on circulating lymphocytes to ascertain potential contributions of these immune populations to SLE and LN.
3.1 B cells
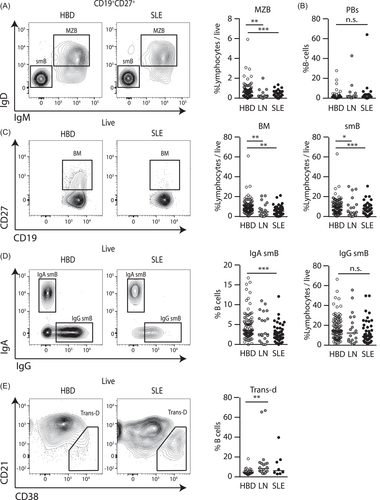
Both LN and SLE patients were noted to have reduced marginal zone B-cells (MZB, CD19+CD27+IgM+IgD+) (Figure 2A). MZBs are the first point of B cell interaction with antigen in secondary lymphoid tissues, are enriched toward autoreactivity, and are associated with SLE in murine models of disease.34, 35 We hypothesized that due to the low activation threshold and inherent autoreactivity of MZB, reductions in MZB may reflect sustained autoantigen presentation and with MZB development into effector B cell populations. Although we did not observe an increase in CD19+IgMloCD38+ plasmablast (PB) cells (Figure 2B), activated MZB form short-lived PBs which typically do not leave secondary lymphoid organs36 and therefore may not be detected in peripheral blood. In contrast, we observed significantly decreased proportions of CD19+CD27+ memory B cells (Bm) and total CD19+CD27+IgM−IgD− class-switched memory B cells (smB) (Figure 2C). Interestingly, we noted reductions in IgA smB (CD19+CD27+IgM−IgD−IgA+IgG−) in SLE (Figure 2D) with a similar, albeit nonsignificant, trend in LN. We did not observe a significant difference in CD19+CD21loCD38lo anergic B cells and other smB subtypes (Figure S1) although we did observe a modest increase in CD19+CD21loCD38+ transitional-d B cells (Trans-d) in patients with SLE (Figure 2E). Cumulatively these findings suggest a possible defect in MZ B cell responses with associated impairment in memory B cell responses in SLE and LN patients.
3.2 T cells
Consistent with our prior observation of possible aberrant MZB cells which contribute to extra-follicular responses, we observed alterations to populations of T follicular helper cells (Tfh). Tfh memory cells (CD4+CD25−CD127hiCD45RA−CXCR5+PD-1loCCR7hi) were modestly reduced in patients with SLE and LN (Figure 3A,i). When comparing these populations as a percentage of CD4+ cells, the effects were especially pronounced (Figure 3A,ii). However, we did not observe a difference in Tfh effector (CD4+CD25−CD127hiCD45RA−CXCR5+PD-1hiCCR7lo) populations (Figure S1). As all patients in this study had quiescent disease, these findings are consistent with observations that expansion of Tfh populations occurs during disease flares26, 37
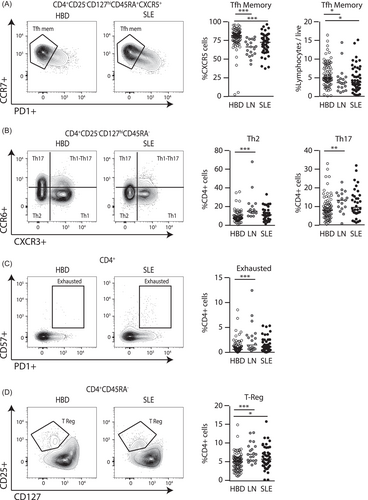
In non-Tfh helper lymphocytes, Th2 populations (CD4+CD25−CD127hiCD45RA−CXCR3−CCR6−) were expanded in LN patients consistent with prior reports,38 but not in SLE (Figure 3B,i). Patients with LN, but not SLE, also had significantly increased the non-Th1 non-Th17 (Th1-17) cells (CD4+CD25−CD127hiCD45RA−CCR6+/CXCR3+) (Figure 3B,ii). There were no significant differences seen in the Th1 or Th17 populations, which conflicts with observations made in other studies reporting an expansion in Th17 subsets in LN.39 Interestingly, likely as a result of chronic autoantigen stimulation, exhausted Th cells (CD4+CD57+PD1+) were also expanded in SLE and LN patients (Figure 3C).
Consistent with prior reports19 the proportion of T regulatory (Treg, CD4+CD25+CD127lo) cells were strikingly elevated in the LN group (Figure 3D). In our cohort, there was no significant differences in proportions of total CD8+ T cells, activated CD8+ T cells and activated CD4+ T cell populations between SLE, LN, and HBD groups. We did not observe any significant differences for total NK cells or other NK subtypes (Figure S1).
3.3 Myeloid and dendritic cells (DC)
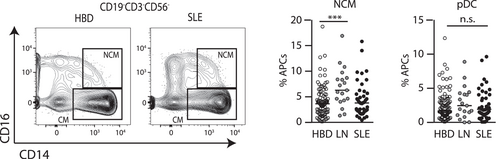
Similar to the repressive response seen in the CD4+ T cell populations, the increase in nonclassical monocytes in LN are suggestive of a state of chronic kidney inflammation (Figure 4). There were no significant differences in proportions of DC populations.
4 DISCUSSION
The immunopathogenesis of SLE is complex and involves dysregulation of both innate and adaptive immunity. Mechanisms influencing SLE end-organ involvement remains unclear. In this observational study of SLE and LN cohorts using flow cytometry analysis, we demonstrate distinct immunophenotypic features that are present in SLE patients, as well as features unique to those that develop LN.
4.1 Increased propensity for Tfh-independent MZB activation as a key SLE pathogenesis mechanism
In murine models of lupus, MZBs are increased and thought to progress to the follicle for maturation, suggesting antibody products of this process are more likely to be auto-reactive.40 We observed significantly decreased numbers of MZBs in both SLE and LN. MZBs are the first B cells to encounter foreign antigen and have lower activation thresholds than other B cells. This inherent reactivity, therefore, makes it unsurprising that MZB are closely linked to SLE. A key modulator of MZB tolerance is IgD which is essential to promoting tonic signaling supporting anergic responses and tolerising IgM responses in MZB cells.41 It has been shown that extrafollicular PB responses are associated with autoantibody production and hence this may be important in SLE pathogenesis.42
We observed no difference in Tfh effector cells but significantly decreased proportions of Tfh memory cells in both SLE and LN groups. This conflicts with previous studies that have shown an increase in circulating Tfh-like cells in individuals with SLE.37 Further, significantly decreased proportions of Bm and smB cells, in particular decreased IgA smB, were found in SLE patients. These reductions in the lymphocyte populations with essential roles in follicular responses, and lymphocytes arising from these responses, suggests overall follicular and GC responses are reduced in SLE patients. We hypothesize that these combined observations suggest that autoreactive extrafollicular responses derived from MZB cells are significantly more prominent SLE and LN patients and drive autoantibody development.
This is supported by previous findings that although the majority of IgG+ autoantibody-producing B cells in patients are somatically mutated—consistent with hyperactive germinal centre maturation—a proportion of IgG+ autoantibody-producing B cells in SLE patients lack somatic mutations, suggesting germinal centre-independent mechanisms exist.43 Furthermore, MZBs are known to undergo expansion in response to T-independent antigen stimulation and thus autoreactive MZBs may indeed play a significant role in generating autoreactive antibodies.44, 45 Overall our data suggests an increased propensity for Tfh-independent MZB activation as a key SLE pathogenesis mechanism.
We also observed increased CD21-lo/CD38+ transitional B cells (Trans-d) in SLE. It has previously been shown that transitional B cells in general are expanded in active SLE.46 Furthermore, Trans-d cells have been shown to represent a less mature subset of transitional B cells and produce relatively more autoantibodies.47 Therefore, Trans-d cells may play a significant, as yet undefined role in SLE pathogenesis.
4.2 Chronic autoantigen stimulation drives inflammation that overcomes Treg responses
We observed increased proportions of exhausted Th cells which reached significance in LN. This is consistent with existing data that suggests that T cell exhaustion is induced by chronic antigen stimulation25 reflecting ongoing T cell exposure to cognate self-antigen despite the presence or absence of overt clinical disease activity. Increased numbers of exhausted T cells are thought to reflect a mechanism for tolerance that helps to explain how SLE patients maintain remission despite having ongoing immune dysregulation.48
Interestingly, we also observed an increased proportion of T regulatory cells (Treg) in LN. Previous studies had contradicting results regarding numbers and function of SLE T regs,24, 49-51 but more recently T regs have been shown to be functional and expanded in SLE.49 These studies argue that the inability of expanded, functionally suppressive Treg cells to control SLE disease progression is likely secondary to the persistent inflammatory cytokine environment overcoming Treg function.52
Overall, our data suggests that chronic autoantigen stimulation likely drives both inflammation and the Treg response, but that Treg-mediated suppression is insufficient to contain clinical manifestations of autoimmunity.
4.3 Th1-17 cells in LN and SLE
Th1-17 cells are a subset of Th17 cells that retain the ability to produce IFN gamma and have previously been shown to be expanded in SLE patients with active disease.53 IL17 producing T cells have previously been observed in kidneys of patients with SLE suggestive of a role in propagating inflammation54 and IL-17 levels are reported to correlate with disease activity.18
Interestingly we observed a decreased proportion of Th1-17 cells which reached statistical significance in LN but not SLE. Furthermore, based on the current paradigm of SLE pathogenesis being polarized towards Th2 and Th17,1, 53 we expected to find an increase in Th17 and a decrease in Th1. However, we observed only a trend toward an increase in Th17, and a trend towards a decrease in Th1, which both did not reach statistical significance. These findings may reflect that the SLE/LN patients in our cohort did not have active disease or were in remission from treatment, leading to the absence of expected findings. We did not observe significant differences in proportions of R5-Th1, Tfh effector, Th1 and Th17 cell populations between SLE, LN and HBD groups.
We observed no significant differences in proportions of total CD8+ T cells, activated CD8+ T cells and activated CD4+ T cell populations between SLE, LN, and HBD groups. Although CD4+ T cells can play an important role in SLE pathogenesis,1 our data suggests that differences in subset differentiation are likely more important than expansion of total numbers. There were no significant differences in proportions of DC populations. This was surprising given the central role of plasmacytoid DCs in SLE pathogenesis1 and may reflect their activity in tissues rather than in the circulation. This may again reflect patients from the SLE/LN cohort having quiescent disease or being in remission from treatment. Alternatively, this may suggest that SLE pathogenesis is driven by aberrant function of DCs, as opposed to expansion of normal DCs.
The strengths of this study include the comprehensive and simultaneous analysis of all major lymphocyte populations, permitting greater interpretation of abnormalities in terms of altered immunological processes rather than isolated populations. Our study benefits from being a relatively large study of this kind.
Limitations to the study include a relative lack of control for disease activity amongst the SLE and LN cohorts and subclinical disease activity could not be excluded. Furthermore, cohorts were not matched for the treatment received or the dose or duration of therapy. This may have contributed to some of the unexpected findings in the prevalence of certain T cell subsets. These findings therefore are not readily generalizable.
In summary, this observational study highlights key immunopathogenetic features of SLE and provides some insights into the distinguishing cellular signatures specific to LN. A deeper understanding of differences in the peripheral immunophenotype and pathogenic mechanisms that underpin SLE and LN promises potential translational interventions in disease diagnosis and monitoring as well as personalised medicine interventions to improve disease outcomes.
AUTHOR CONTRIBUTIONS
Yi Tong V. Aw, Phillip J. Whiley and Simon H. Jiang had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Yi Tong V. Aw, Phillip J. Whiley and Simon H. Jiang study conception and design. Matthew Cook, Giles Walters, David A. Fulcher and Simon H. Jiang acquisition of clinical samples and data. Yi Tong V. Aw, Phillip J. Whiley, Ayla May Lorenzo, Tom Lea-Henry, Somasundhari Shanmuganandam, Maurice Stanley, Sonia N. Babu, Vicki Athanasopoulos, Jean Cappello, Julia I. Ellyard, Matthew Cook, Carola Vinuesa, Giles Walters, David A. Fulcher, Simon H. Jiang analysis and interpretation of data. All authors were involved in drafting the article or revising it, and all authors approved the final version to be published.
ACKNOWLEDGMENTS
We would like to thank Michael Devoy and Harpreet Vohra for all their help with flow experiments at the MCRF, JCSMR. We would like to think Ann-Maree Hatch with logistical support collecting all blood samples.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
ETHICS STATEMENT
The protocol was approved by the Sydney Local Health District Human Research Ethics Committee (HREC) at Concord Repatriation General Hospital, ACT Health HREC and Australian National University HREC. Our study was conducted as part of the Australian Point Mutation in Systemic Lupus Erythematosus study (APOSLE), the Centre for Personalized Immunology (CPI) program, the Healthy Blood Donors register and Genetic Mutation in Immune Mediated Kidney Disease study (The Canberra Hospital, Canberra, Australia). This study was carried out in accordance with the recommendations of the National Statement on Ethical Conduct in Human Research (2007), National Health and Medical Research Council with written informed consent from all subjects. All subjects gave written informed consent in accordance with the Declaration of Helsinki.
Open Research
DATA AVAILABILITY STATEMENT
The data set used in this study is not publicly available but shall be available from the corresponding author ([email protected]) upon reasonable request.