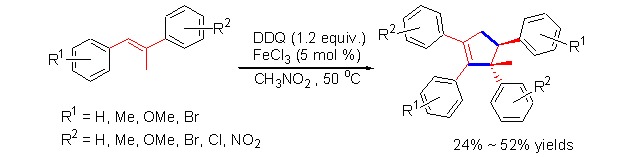Journal list menu
Export Citations
Download PDFs
Cover Picture
Cover Picture: Inhibitors of HIV-1 Integrase-Human LEDGF/p75 Interaction Identified from Natural Products via Virtual Screening (Chin. J. Chem. 12/2012)
- Page: 2729
- First Published: 27 December 2012

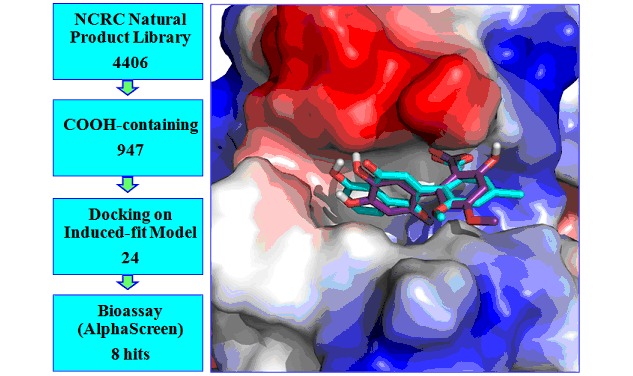
The cover picture shows the discovery of inhibitors targeting HIV-1 integrase-human LEDGF/p75 interaction via virtual screening. One of the most challenges for anti-HIV therapy is the rapid emergence of drug resistance. Protein-protein interaction inhibitors bring us hopes to overcome the viral resistance. We conducted a virtual screening combined with bioassays to search such inhibitors from natural product library. Several compounds were discovered to block integrase- LEDGF/p75 interaction with potent activities. These findings could be helpful for anti-HIV drug discovery. More details are discussed in the article by Tang et al. on page 2752–2758.
Editorial
Celebrating the 60th Anniversary of East China University of Science and Technology
- Page: 2731
- First Published: 27 December 2012
Contents
Communication
Gold-Catalyzed Direct Indolation of Tetrahydroisoquinolines
- Pages: 2741-2746
- First Published: 13 December 2012
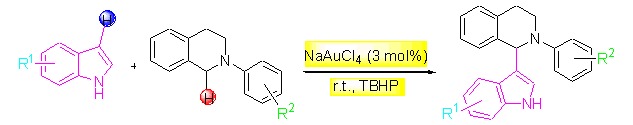
Nitrogen-containing heterocyclic compounds are important motifs of pharmaceuticals and functional materials, and there has been a growing interest in new synthetic methods for their preparations. In this paper, we report a direct cross-coupling reaction of indole with N-aryl tetrahydroisoquinolines in the presence of gold catalyst. The reaction is compatible with a wide range of substituted indoles, to enable the formation of various alkylated heteroarenes under very mild reaction conditions.
Full Papers
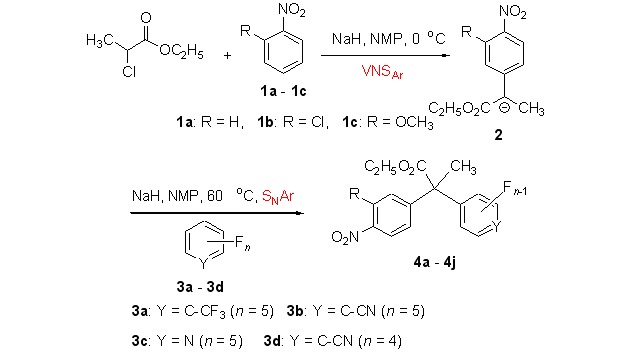
One-pot Three Component Synthesis of Polyfluoroarylated Arylacetates via VNSAr-SNAr Reaction
- Pages: 2747-2751
- First Published: 13 December 2012
Inhibitors of HIV-1 Integrase-Human LEDGF/p75 Interaction Identified from Natural Products via Virtual Screening
- Pages: 2752-2758
- First Published: 11 December 2012
Novel and Efficient Syntheses of Four Useful Shikimate-derived Epoxy Chiral Building Blocks via Cyclic Sulfite Intermediates
- Pages: 2759-2766
- First Published: 13 December 2012
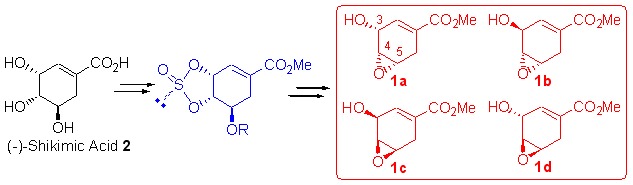
All of the four stereoisomers of methyl 4,5-epoxy-3-hydroxy-cyclohex-1-ene-carboxylate (1a–1d) are useful chiral building blocks. Novel and efficient syntheses of these four epoxy chiral building blocks from naturally abundant (−)-shikimic acid (2) via cyclic sulfite intermediates are described in this article. The compound (3R,4R,5S)-1a was synthesized via four steps from (−)-shikimic acid in 79% overall yield. The compounds (3S,4R,5S)-1b, (3S,4S,5R)-1c and (3R,4S,5R)-1d were synthesized via seven steps from (−)-shikimic acid in 56%, 64% and 65% overall yields, respectively.
Synthesis of 1,3,5-Trisubstituted [4-tert-Butyl 2-(5,5-difluoro-2,2-dimethyl-6-vinyl-1,3-dioxan-4-yl)acetate]pyrazoles via a Pd-Catalyzed CH Activation
- Pages: 2767-2773
- First Published: 13 December 2012
Fe3O4 Modified Up-Conversion Luminescent Nanocrystals for Biological Applications
- Pages: 2774-2778
- First Published: 30 October 2012
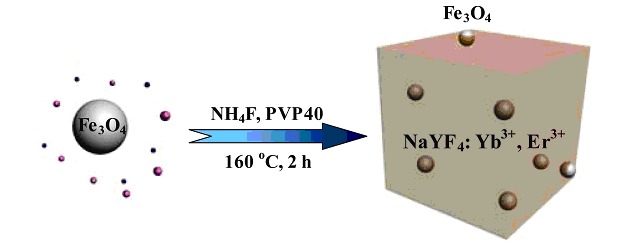
A new and facile two-step synthetic strategy was developed to prepare magnetic Fe3O4 modified single crystalline up-conversion luminescent NaYF4: Yb3+, Er3+ nanocrystals using Fe3O4 nanoparticles as the seeds. Acting as the "nuclei" in the reaction, the presence of Fe3O4 nanoparticles facilitates the formation of up-conversion NaYF4: Yb3+, Er3+ nanocrystals on their surfaces through heterogeneous nucleation process. The uptake and intracellular distribution of as-prepared multifunctional nanocrystals were also investigated in cell-labelling.
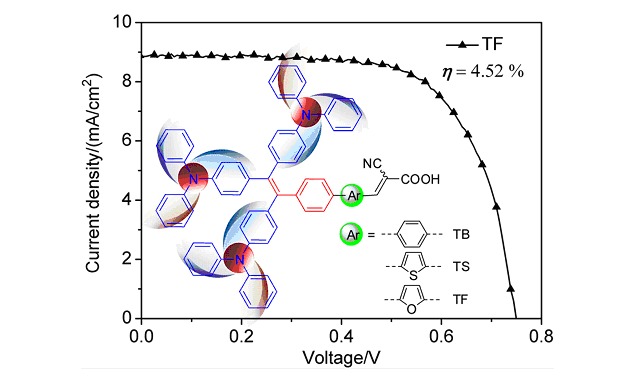
Novel Organic Dyes Based on Bulky Tri(triphenylamine)-Substituted Styrene for Dye-Sensitized Solar Cells
- Pages: 2779-2785
- First Published: 13 December 2012
Facile Synthesis of 4,5-Disubstituted 2H-1,2,3-Triazoles by Catalyst-free Cycloaddition between Substituted Vinyl Sulfones and Sodium Azide under Ambient Conditions
- Pages: 2786-2790
- First Published: 13 December 2012
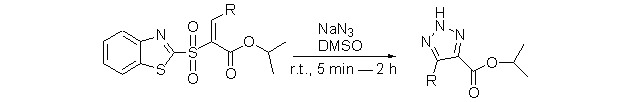
A highly efficient method for readily preparing 4,5-disubstituted 2H-1,2,3-triazoles was found. Under ambient conditions, a catalyst free cycloaddition between substituted vinyl sulfones and sodium azide could be completed in a very short time. In this cycloaddition process, sulfonyl group acts as a leaving group, while its ester group was retained.
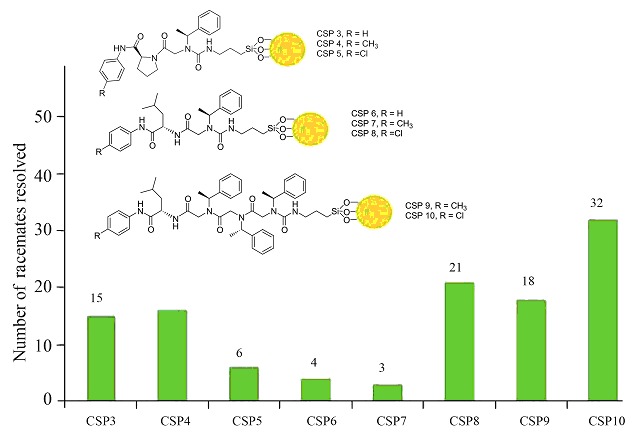
Investigation of Peptoid Chiral Stationary Phases Terminated with N′-Substituted Phenyl-L-proline/leucine Amide
- Pages: 2791-2797
- First Published: 13 December 2012
A New Synthetic Method of (Z)-4-Aryl-but-2-en-1-ols via Suzuki-Miyaura Cross-Coupling Reaction of 4-Substituted 1,2-Oxaborol-2(5H)-ols with Benzyl Bromides
- Pages: 2798-2804
- First Published: 17 December 2012
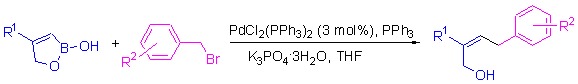
Z and E configuration 4-aryl-but-2-en-1-ols were isolated from several terrestrial plants, and (Z)-4-aryl-but-2-en-1-ols were found to have choleretic activity. Strategies have been reported to synthesize (E)-4-aryl-but-2-en-1-ols with high selectivity. However, there is no method to obtain (Z)-4-aryl-but-2-en-1-ols with high selectivity now. We developed a Suzuki-Miyaura cross-coupling reaction of 1,2-oxaborol-2(5H)-ols with benzyl bromides to synthesize (Z)-4-aryl-but-2-en-1-ols, the products were obtained in up to 94% isolated yield.
Preparation of Nanoporous Carbon/Graphene Composites and Its Application in Direct Methanol Fuel Cell
- Pages: 2805-2812
- First Published: 13 December 2012
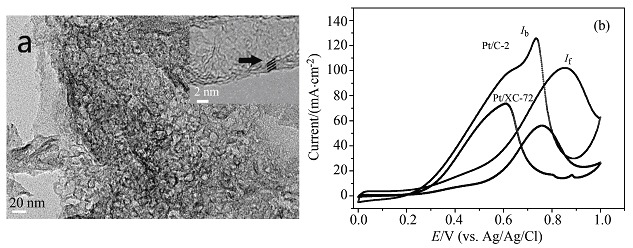
The nanoporous carbon/graphene composite (NCGC) has been prepared through a one pot hydrothermal method by using graphene oxide (GO) as raw material, phenol, formaldehyde as carbon resources and triblock copolymer F127 as template. Pt electrocatalyst taking NCGC as support shows better activity and stability than that with XC-72 as support toward methanol oxidation.
Synthesis and Pharmacological Properties of 5-Alkyl Substituted Nicotine Analogs
- Pages: 2813-2818
- First Published: 13 December 2012
Aqueous-Phase, Palladium-Catalyzed Heck Reaction: The Significant Role of CN-containing Counter Anion
- Pages: 2819-2822
- First Published: 30 October 2012
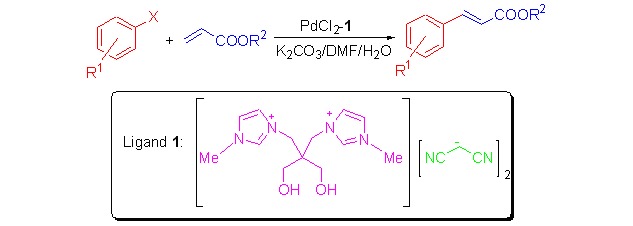
Water-soluble diol-functionalized imidazolium ionic liquid 1, prepared from 2,2-bis(1-methyl-methylimidazolium)propane-1,3-diol bromide, potassium hydroxide, and malononitrile, was used as an efficient phosphine-free ligand for palladium-catalyzed arylation of aryl halides with acrylates in aqueous phase under mild conditions.
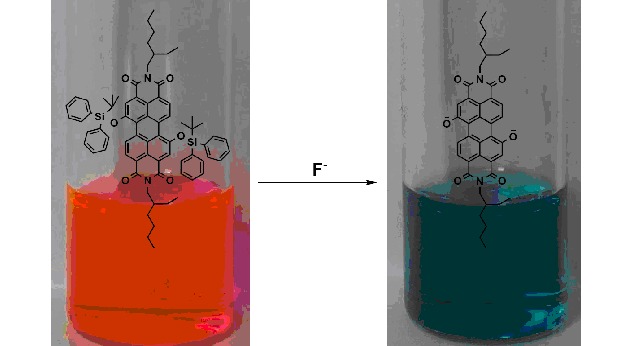
A Highly Sensitive and Selective Colorimetric Chemosensor for F− Detection Based on Perylene-3,4:9,10-tetracarboxylic Bisimide
- Pages: 2823-2826
- First Published: 17 December 2012
Ethylenediamine: A Highly Effective Catalyst for One-Pot Synthesis of Aryl Nitroalkenes via Henry Reaction and Dehydration
- Pages: 2827-2833
- First Published: 17 December 2012
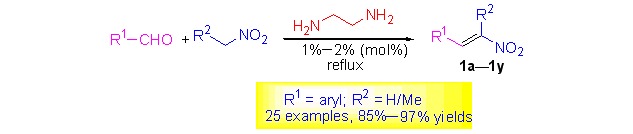
Ethylenediamine (H2NCH2CH2NH2) was found to be a highly effective catalyst for the condensation of aryl aldehydes with nitromethane (or nitroethane). When 1–2 mol% of ethylenediamine was used as the catalyst, the one-pot reaction of aryl aldehydes with nitromethane (or nitroethane) by refluxing for 3–10 h efficiently afforded various aryl nitroalkenes 1a–1y in 85%–97% yields.
Highly Substituted Cyclopentenes Formation via a Stereoselective Tandem CDC Reaction and Cyclization
- Pages: 2834-2838
- First Published: 17 December 2012
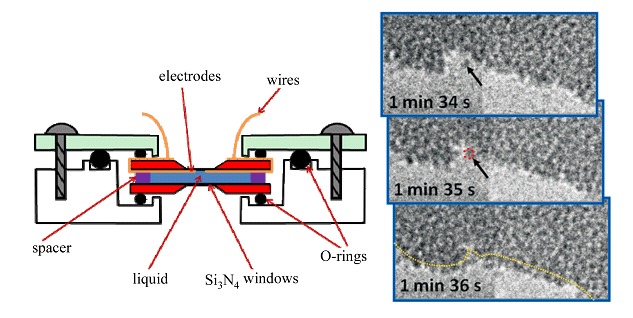
A Study of Nano Materials and Their Reactions in Liquid Using in situ Wet Cell TEM Technology
- Pages: 2839-2843
- First Published: 27 December 2012
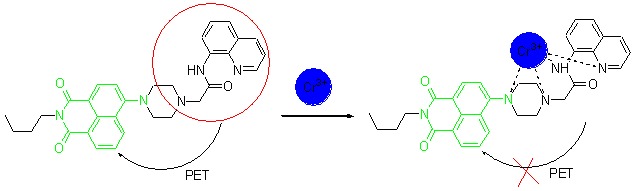
Highly Selective Fluorescence Turn-on Chemosensor Based on Naphthalimide Derivatives for Detection of Trivalent Chromium Ions
- Pages: 2844-2848
- First Published: 13 December 2012
Degradation of 2,4-DCP by the Immobilized Laccase on the Carrier of Fe3O4@SiO2-NH2
- Pages: 2849-2860
- First Published: 13 December 2012
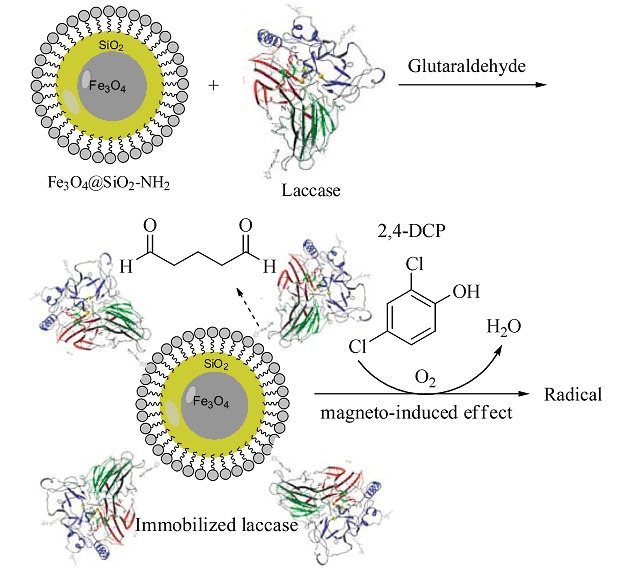
Fe3O4@SiO2-NH2 nano-carrier particles with chain structure were successfully prepared. Immobilization of laccase using Fe3O4@SiO2-NH2 as carrier through covalent attachment was carried out. The optimal conditions regarding degradation efficiency was discussed. Magnetic nanoparticles immobilized laccase was easy to operate, separate and recover from the reaction system. Moreover, because of its magnetic, catalytic, and other good characteristics, the immobilized laccase had a faster degradation rate than the free laccase by the "magneto-induced effect" on the laccase; the magnetic carrier Fe3O4@MSS-NH2 could promote the degradation reaction and the activity of immobilized laccase was kept almost unchanged during the course of repeated use.
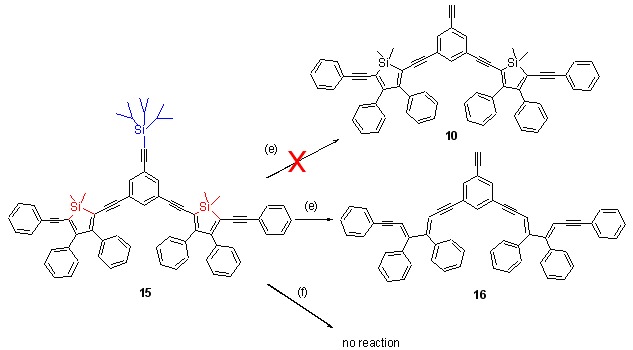
Synthesis of Conjugated Aryleneethynylenesiloles Dendron
- Pages: 2861-2868
- First Published: 17 December 2012
Nanoscale Interfacial Activity of the Natural Lipopeptide, [Asp1, Glu5] Surfactin-C16, and DMPC in Mixed Monolayer
- Pages: 2869-2873
- First Published: 27 September 2012
![Nanoscale Interfacial Activity of the Natural Lipopeptide, [Asp1, Glu5] Surfactin-C16, and DMPC in Mixed Monolayer](/cms/asset/7edea52e-d220-48c1-9cc7-5c32abb0360c/mcontent.jpg)
The monolayer technique combined with atomic force microscopy (AFM) was used to probe the interfacial behavior of DMPC and surfactin-C16 in the mixed monolayer. The two components are partially miscible in some cases. It is proposed that the miscibility is governed by hydrophobic interactions between surfactin-C16 and DMPC, and the ionization states and physical states of surfactin-C16 in the monolayer.
Note
Pyrene Excimer-based Bis-triazolyl Pyranoglycoligands as Specific Mercury Sensors
- Pages: 2874-2878
- First Published: 11 December 2012
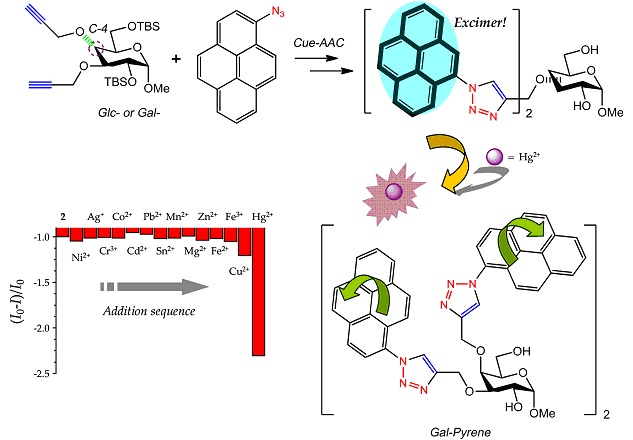
New C3,4-disubstituted bis-triazolyl glycoligands that feature a glucosyl or galactosyl scaffold incorporating dual pyrenyl groups were synthesized via the CuI-catalyzed azide-alkyne 1,3-dipolar cycloaddition reaction (Cue-AAC). These compounds exert a major emission band corresponding to that of pyrene excimer and respond specifically to mercury with a markedly quenched fluorescence. The epimeric nature of the pyranoglycosyl scaffold is determined influential toward the selectivity of the sensors.
Errata
Erratum: Immobilization of Glucose Oxidase on Ordered Macroporous Silicas Functionalized with Amino Group
- Page: 2879
- First Published: 27 December 2012
Erratum: Antimicrobial Aromatic Polyketides from Gorgonian-Associated Fungus, Penicillium commune 518
- Page: 2880
- First Published: 27 December 2012
Retraction
Retraction: A New Sesquiterpene from Caragana intermediia and Its Anti-Pyricularia oryzae P-2b Activity
- Page: 2881
- First Published: 27 December 2012





![Synthesis of 1,3,5-Trisubstituted [4-tert-Butyl 2-(5,5-difluoro-2,2-dimethyl-6-vinyl-1,3-dioxan-4-yl)acetate]pyrazoles via a Pd-Catalyzed C<span class='icomoon'></span>H Activation](/cms/asset/0192e957-09b5-4c5c-a96b-29316037de9f/mcontent.jpg)