Journal list menu
Export Citations
Download PDFs
Cover Image
Cover Image, Volume 50, Issue 8
- Pages: i-ii
- First Published: 08 March 2012

Control of the helical nature of poly(quinoxaline-2,3-diyl)s by post-polymerization modification at the single polymer terminus is presented by Yuuya Nagata, Satoru Ohashi, and Michinori Suginome on page 1564. The cover picture depicts a molecule of left-handed helical poly(quinoxaline-2,3-diyl) bearing a chiral terminal boronate group, which is formed by post-polymerization esterification of a B(OH)2-terminated poly (quinoxaline-2,3-diyl) with a chiral 1,2-diol (shown right bottom of the picture). Removal of the terminal chiral diol by hydrolysis followed by esterification with an enantiomeric chiral 1,2-diol leads to the formation of right-handed helical poly(quinoxaline-2,3-diyl).
Inside Cover, Volume 50, Issue 8
- Pages: iii-iv
- First Published: 08 March 2012

Photoinduced depolymerization of poly(olefin sulfone)s mixed with a photo-base generating compound is investigated by Takeo Sasaki, Takayuki Kondo, Motoki Noro, Kazuya Saida, Hiroaki Yaguchi, and Yumiko Naka on page 1462. Irradiation of 254 nm UV light to films composed of a mixture of a photo-base generating compound and poly(olefin sulfone)s with volatile monomers causes photoinduced depolymerization and the irradiated part of the film is vaporized. The effect of the poly(olefin sulfone) structure on the photoinduced depolymerization process is investigated. The polymer systems examined in this study permit a wide variety of applications such as stereolithography without the need for development solvents, photo-detachable adhesives, and printable nanocircuit fabrication.
Rapid Communications
Synthesis of highly polymerizable 1,3-benzoxazine assisted by phenyl thio ether and hydroxyl moieties
- Pages: 1457-1461
- First Published: 28 January 2012
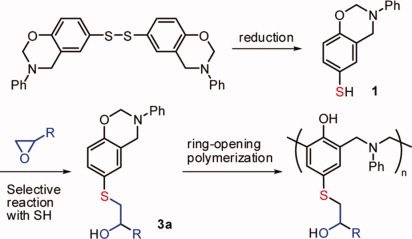
A benzoxazine bearing thiol moiety 1 was synthesized from its precursor, a bifunctional benzoxazine bearing disulfide linkage, through selective reduction of the disulfide. The thiol moiety thus formed readily reacted with glycidyl phenyl ether to give the corresponding benzoxazine 3a, which exhibited much higher polymerization ability than some referential monomers without sulfide moiety.
Articles
Photoinduced depolymerization in poly(olefin sulfone) films comprised of volatile monomers doped with a photobase generator
- Pages: 1462-1468
- First Published: 04 January 2012
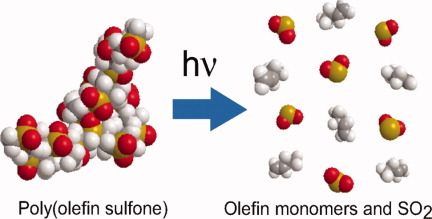
Photoinduced depolymerization of poly(olefin sulfone)s mixed with a photo-base generating compound was investigated. Irradiation of 254 nm UV light to films comprising a mixture of a photo-base generating compound and poly(olefin sulfone)s with volatile monomers caused photoinduced depolymerization and the irradiated part of the film vaporized. The effect of the poly(olefin sulfone) structure on the photoinduced depolymerization process was investigated.
Nanospace preparation by crosslinking helical syndiotactic-poly(methacrylic acid) in acetonitrile/water after stereocomplexation
- Pages: 1469-1476
- First Published: 01 February 2012
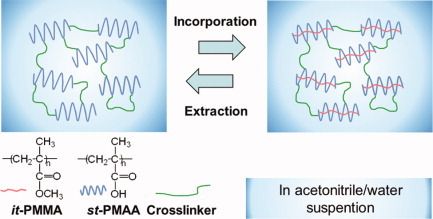
Various molecular weights of it-poly(methyl methacrylate) (PMMA) and st-poly(methacrylic acid) (PMAA) were used to form an it-PMMA/st-PMAA (1/2) stereocomplex in acetonitrile/water as the suspending solution. The stereocomplex was crosslinked with 1,11-diamino-3-6-9-trioxaundecane and water soluble carbodiimide at a 10 mol % concentration. The extraction of it-PMMA from the crosslinked stereocomplex and the successive re-incorporation of the it-PMMA were achieved at a moderate 20% value and was supported by FTIR/ATR and XRD analyses. The nanospace of the helical st-PMAA was available in the acetonitrile/water suspension state using the present crosslinking approach.
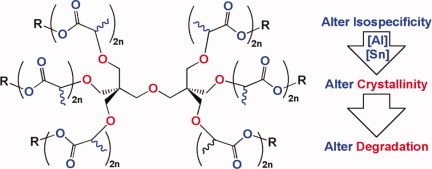
Control of thermal properties and hydrolytic degradation in poly(lactic acid) polymer stars through control of isospecificity of polymer arms
- Pages: 1477-1484
- First Published: 01 February 2012
Magnetite-polylactic acid core–shell nanoparticles by ring-opening polymerization under microwave irradiation
- Pages: 1485-1490
- First Published: 31 January 2012
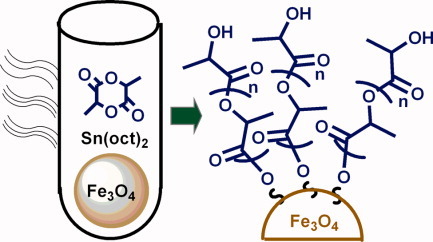
Sn(oct)2-catalyzed surface-initiated ROP of D,L-lactide in the presence of surface stabilized magnetite nanoparticles was successfully conducted by microwave irradiation for the first time. The rate accelerating effect of microwave irradiation allowed reducing the time of polymerization but also avoided application of solvents. Stable colloidal suspensions were obtained in water.
Synthesis of fluorescent, dansyl end-functionalized PMMA and poly(methyl methacrylate-b-phenanthren-1-yl-methacrylate) diblock copolymers, at ambient temperature
- Pages: 1491-1502
- First Published: 04 January 2012
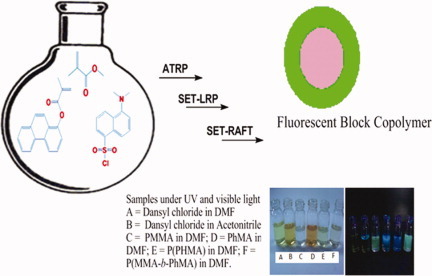
Controlled radical polymerization of methyl methacrylate, at ambient temperature, mediated by dansyl chloride, is successfully demonstrated via ATRP, SET, and SET-RAFT. For the three trithiocarbonate chain transfer agents (CTAs) used in SET-RAFT polymerizations, the R group in the CTA does not influence the propagation rate. Novel diblock copolymers P(MMA-b-PhMA) are synthesized from the PMMA macroinitiator. These block copolymers show two characteristic fluorescence signals from the dansyl end group and the phenanthren-1-yl methacrylate block.
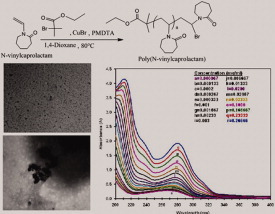
Synthesis of amphiphilic poly(N-vinylcaprolactam) using ATRP protocol and antibacterial study of its silver nanocomposite
- Pages: 1503-1514
- First Published: 23 January 2012
Polymer hydrogels of 2-hydroxyethyl acrylate and acrylic acid obtained by frontal polymerization
- Pages: 1515-1520
- First Published: 31 January 2012
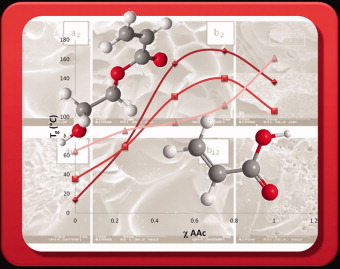
Homopolymers and copolymers of acrylic acid and 2-hydroxyethyl acrylate were prepared by the use of the frontal polymerization technique. The maximum temperature reached by the front and its velocity were found to be dependent on the monomer ratio. The corresponding hydrogels exhibit pH-responsive behavior at two critical values located at pH ≈ 6 and ≈ 11–13, respectively, depending on the composition.
Meta-linked and para-linked water-soluble poly(arylene ethynylene)s with amino acid side chains: Effects of different linkage on Hg2+ ion sensing properties in aqueous media
- Pages: 1521-1529
- First Published: 31 January 2012
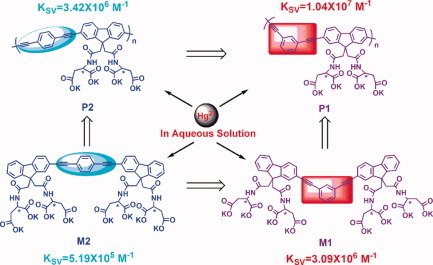
Water-soluble, meta- and para-linked poly(arylene ethynylene)s containing L-aspartic acid-functionalized fluorene units (P1 and P2) and their model compounds (M1 and M2) have been synthesized and they show the excellent selectivity and sensitivity to Hg2+ over other common metal ions in aqueous solution. Moreover, meta-linked P1 and M1 had better Hg2+-ion responsive sensitivity than para-linked P2 and M2 possibly due to the more flexible conformation of meta-linkage.
Synthesis and double doping behavior of a poly(p-phenylenevinylene)s bearing conjugated side chains
- Pages: 1530-1538
- First Published: 31 January 2012
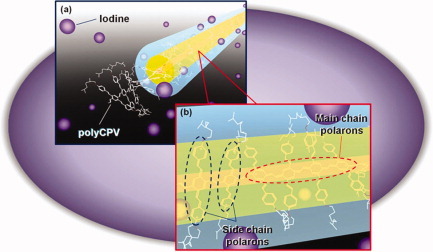
Double doping behavior of a poly(p-phenylenevinylene) bearing conjugated side chains is presented. Charge carriers are generated in both side chains and main chain of the polymer by vapor-phase doping of iodine. Two charge carriers (polarons in the main chain and polarons in the side chains) are considered to be delocalized over the conjugated side chains and the main chains. Restriction of iodine access to the conjugated main chain of polyCPV occurs, due to bulky side chain pseudo-layers during the early doping stage. The main chains were subsequently doped.
New polyelectrolytes based on 4-vinyl-1,2,3-triazole and 1-vinylimidazole
- Pages: 1539-1546
- First Published: 28 January 2012
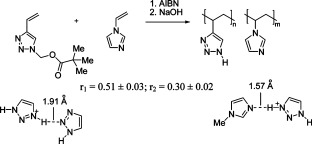
Copolymers of 4-vinyl-1,2,3-triazole and 1-vinylimidazole were obtained by radical copolymerization of (4-vinyl-1H-1,2,3-triazol-1-yl)methyl-2,2-dimethylpropanoate with 1-vinylimidazole followed by alkali hydrolysis. Hydrogen bonds between the protonated triazole cycle and the triazole or imidazole units were found to considerably influence the solubility and solution properties of the copolymers.
Synthesis of fluorine-containing star-shaped poly(vinyl ether)s via arm-linking reactions in living cationic polymerization
- Pages: 1547-1555
- First Published: 31 January 2012
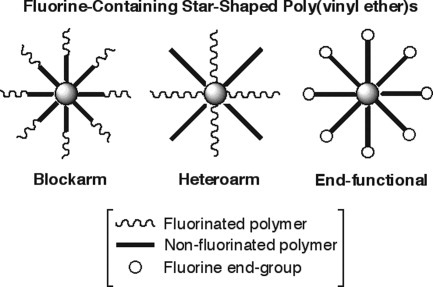
Various types of fluorine-containing star-shaped poly(vinyl ether)s, homoarm, block arm, heteroarm, and end-functionalized star polymers, were synthesized by crosslinking reactions of living polymers based on living cationic polymerization. The star polymers were characterized in terms of yield and molecular weight. Furthermore, the unique solubility characteristics (e.g., temperature-induced physical gelation) and physical properties were also examined.
Aminimide derived from benzoylformic acid ester as photolatent base/radical initiator
- Pages: 1556-1563
- First Published: 31 January 2012
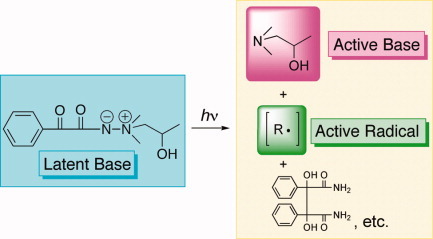
An aminimide possessing a benzoyl substituent, 1,1-dimethyl-1-(2-hydroxypropyl)amine benzoylformimide (BFI), proved to serve as an excellent photobase catalyst. BFI decomposes smoothly by the UV irradiation to give products containing the tertiary amines. BFI exhibits high photo/thermal dual- base activity in the polymerization of epoxide/thiol system. The photoinitiated free radical polymerization of vinyl monomers such as 2-hydroxylethyl methacrylate proceeds smoothly in the presence of BFI, indicating its high activity also as a photoradical initiator.
Control of helical chirality of poly(quinoxaline-2,3-diyl)s based on postpolymerization modification of the terminal group by small chiral molecules
- Pages: 1564-1571
- First Published: 01 February 2012
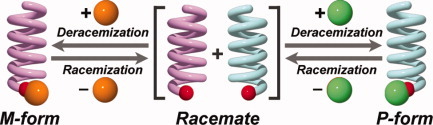
A synthesis of poly(quinoxaline-2,3-diyl)s having a terminal formyl or boronyl group was established by using organopalladium complexes bearing a protected formyl or boronyl group as initiators. The poly(quinoxaline)s were successfully deracemized by reacting them with small optically active molecules at their terminal formyl or boronyl group. The helix sense of the boronyl-terminated poly(quinoxaline) was reversibly controlled by attaching and removing the chiral group.
Click chemistry-based synthesis of azo polymers for second-order nonlinear optics
- Pages: 1572-1578
- First Published: 05 February 2012
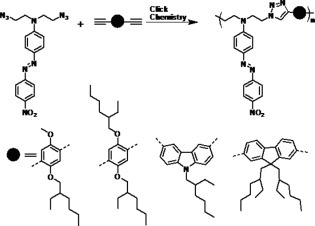
A series of polymers containing aromatic ring in the main chain and azo groups in side chain are synthesized by Click Chemistry route. The polymers have reasonably high Tg up to 140 °C. The AFM spectra have given clear presentation of noncentrosymmetrical arrangement after poling. The SHG intensity was found to remain stable up to about 100 °C. At room temperature, it was found to decrease by around 14% of initial value and remain stable up to 8 days of study.
Preparation of side-chain liquid crystalline Azopolymers by CuAAC postfunctionalization using bifunctional azides: Induction of chirality using circularly polarized light
- Pages: 1579-1590
- First Published: 03 February 2012
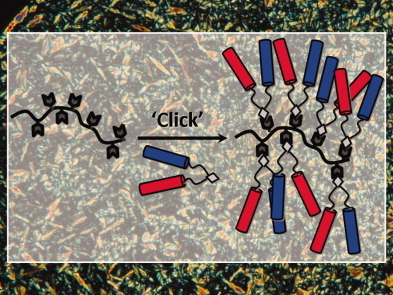
Liquid crystalline side-chain azopolymers have been prepared by ‘clicking’ bifunctional azides to poly(propargyl methacrylate). The incorporation of these bifuntional azides allows to increase the degree of functional moieties as well as to modulate the thermal transition temperatures and optical properties. For these polymers, a transfer of the chirality from circularly polarized light to material using the azobenzene units as chiral mediator has been induced.
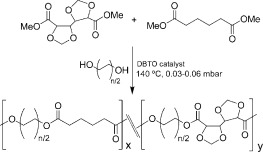
Carbohydrate-based copolyesters made from bicyclic acetalized galactaric acid
- Pages: 1591-1604
- First Published: 05 February 2012
Basic ionic liquid/FeCl3·6H2O as an efficient catalyst for AGET ATRP of methyl methacrylate
- Pages: 1605-1610
- First Published: 03 February 2012
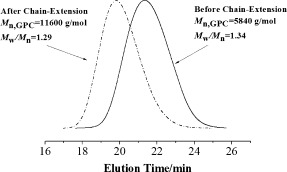
AGET ATRP of MMA using basic ionic liquid/FeCl3·6H2O as an efficient catalyst with cheap and commercially available tetrabutylammonium bromide or tetra-n-butylphosphonium bromide as the ligand could greatly enhance the polymerization rate and produce PMMA with controllable molecular weights and narrow molecular weight distributions.
Polynorbornene dicarboximide/amine functionalized graphene hybrids for potential oxygen barrier films
- Pages: 1611-1621
- First Published: 03 February 2012
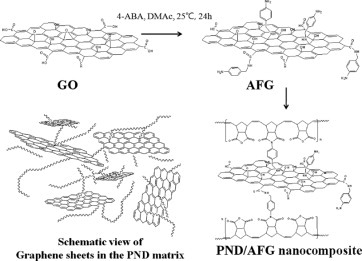
Novel polynorbornene dicarboximide (PND)/amine functionalized graphene (AFG) hybrids were prepared via modification of graphene oxide. The AFG sheets were well-dispersed in DMAc and randomly distributed throughout the PND matrix in the hybrid films, which enhanced the mechanical and oxygen barrier properties of the PND/AFG hybrid films. These hybrids have potential application for packaging materials and gas barrier films.
Influence of different copolymer sequences in low band gap polymers on their performance in organic solar cells
- Pages: 1622-1635
- First Published: 05 February 2012
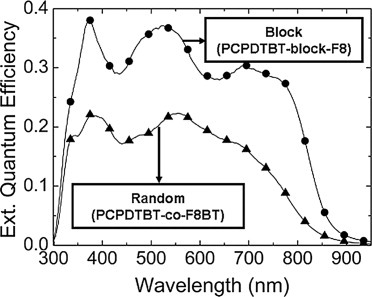
Two types of novel copolymers were synthesized consisting of PCPDTBT and F8BT (F8) sequences in which the chemical units were combined in a block (PCPDTBT-block-F8) or random (PCPDTBT-co-F8BT) fashion. Solar cells were prepared by blending the polymers with PC71BM where the block copolymer demonstrated higher power conversion efficiencies. Better performance for the block copolymer was supported by a unique thin film surface topography and a higher charge carrier mobility which resulted in a larger fill factor.
Styrene–vinyl pyridine diblock copolymers: Synthesis by RAFT polymerization and self-assembly in solution and in the bulk
- Pages: 1636-1644
- First Published: 10 February 2012
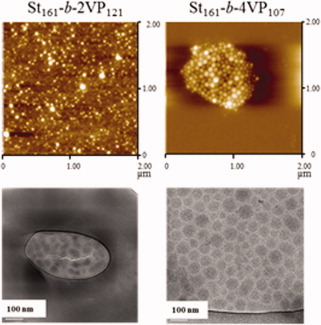
Step-wise reversible addition-fragmentation chain transfer polymerization was employed for the preparation of styrene (St) and 2-vinyl pyridine (2VP) or 4-vinyl pyridine (4VP) amphiphilic diblock copolymers of molecular weight about 25,000 g mol−1 and VP content around 30 mol %. The self-organization of the synthesized diblock copolymers was investigated both in toluene using atomic force microscopy and cryo-transmission electron microscopy (shown images) and in the bulk using transmission electron microscopy.
Bioreducible and core-crosslinked hybrid micelles from trimethoxysilyl-ended poly(ε-caprolactone)-S-S-poly(ethylene oxide) block copolymers: Thiol-ene click synthesis and properties
- Pages: 1645-1656
- First Published: 03 February 2012
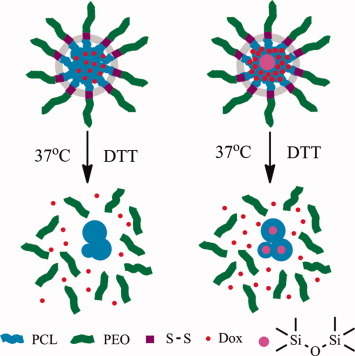
Bioreducible and core-crosslinkable block copolymers TMS-PCL-S-S-PEO were first synthesized by using thiol-ene chemistry and self-assembled into bioreducible and core-crosslinked hybrid micelles. The size of both uncrosslinked and core-crosslinked micelles changed dynamically in 10 mM DTT solution, demonstrating that PEO corona gradually shedded from PCL core. Notably, the core-crosslinked hybrid micelles showed about twofold drug-loading capacities and a half drug-release rate with a reduction-triggered profile compared with uncrosslinked counterparts, making them useful for cancer therapy.
Notes
Phosphites as alternative coreagents for the one-pot aminolysis/thiol-ene synthesis of maleimide-functionalized RAFT polymers
- Pages: 1657-1661
- First Published: 03 February 2012
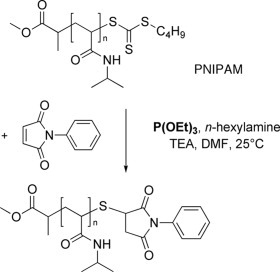
The one-pot aminolysis/thiol-ene chain-end modification between a well-defined trithiocarbonate-functionalized poly(N-isopropylacrylamide) (DP n = 13 and PDI = 1.06) and N-phenyl maleimide has been investigated using triethylphosphite (P(OEt)3) as a coreagent. A quantitative chain-end functionalization has been obtained as shown by 1H NMR spectroscopy and MALDI-TOF mass spectrometry.