Journal list menu
Export Citations
Download PDFs
Table of Contents
Polymers under Multiple Constraints: Restricted and Controlled Molecular Order and Mobility
- First Published: 03 July 2023
Recent Progress in Understanding Polymer Crystallization
- First Published: 09 February 2023
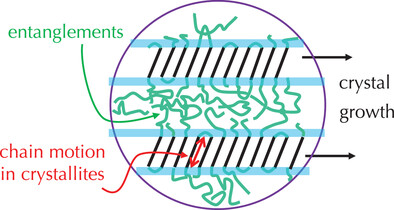
Recent insights into important factors that govern the semicrystalline morphology of crystallizable polymers, but have received less systematic attention, are reviewed. This concerns the influence of intracrystalline chain motion and the effect of entanglements in the amorphous phase. New perspectives on the thermodynamic driving forces underlying the structure formation are provided by computer simulations.
The Semicrystalline Morphology of Polybutylene Succinate Supports a General Scheme Based on Intracrystalline Dynamics
- First Published: 08 February 2023

Semicrystalline polymers with and without intracrystalline chain dynamics have been suggested to display different morphological characteristics. Combined nuclear magnetic resonance and small angle X-ray experiments measurements reveal that polybutylene succinate belongs to the class of crystal-fixed polymers without this dynamics and confirm the existence of the expected morphology.
Long Chain Polyamides: Influence of Methylene Sequence Length and External Forces on Structural Features
- First Published: 01 February 2023
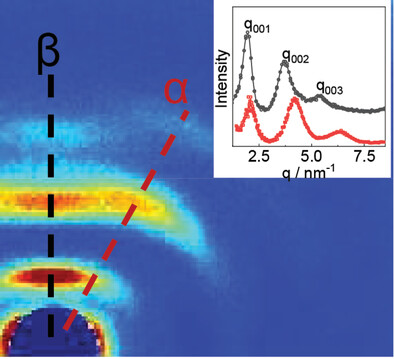
Crystallographic studies by X-ray diffraction are performed on a series of long chain polyamides. Different polymorphic states formed in bulk samples are identified in temperature-dependent measurements. Well oriented fibers are produced successfully by drawing of ram extruded filaments. Differences in the crystalline state between samples crystallized from bulk and under external forces are highlighted and discussed.
The Initial Molecular Interactions in the Course of Enthalpy Relaxation and Nucleation in Polyethylene Terephthalate (PET) as Monitored by Combined Nanocalorimetry and FTIR Spectroscopy
- First Published: 01 April 2023
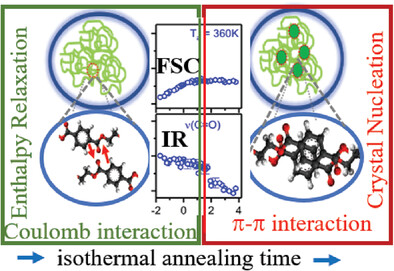
Combined fast scanning calorimetry (FSC) and IR spectroscopy reveal for the first time the molecular signatures of the initial stages of enthalpy relaxation and crystal nucleation occurring much earlier before the start of crystallization in polyethylene terephthalate. During enthalpy relaxation, the far-reaching Coulomb interactions between the polar carbonyl (C═O) moieties are active while crystal nucleation is dominated by π–π interactions.
On Thermodynamics and Kinetics of Interface-Induced Crystallization in Polymers
- First Published: 09 February 2023
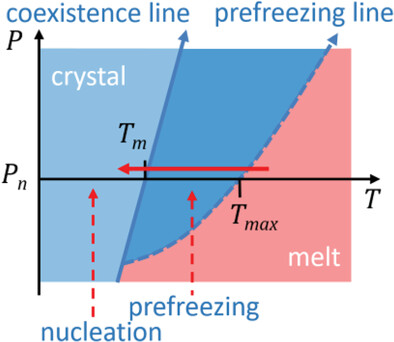
This perspective discusses recent achievements in the theoretical and experimental investigation of two phenomena of interface-induced crystallization in polymers – prefreezing and heterogeneous nucleation. Special attention is paid to the influence of substrate-material interactions on crystallization temperature, crystal orientation, and semicrystalline morphology.
Influence of Tacticity on the Structure Formation of Poly(methacrylic acid) in Langmuir/Langmuir–Blodgett and Thin Films
- First Published: 27 December 2022
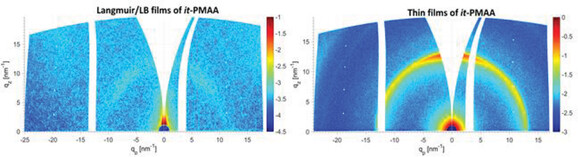
Structure formation of two different stereoisomers such as atactic (at)- and isotactic (it)- poly(methacrylic acid) (PMAA) in thin films prepared by Langmuir/LB and suspension-cast methods is demonstrated. While the at-PMAA dissolved in the water surface, the it-PMAA forms Langmuir/LB films with amorphous nano-entities during compression. The crystallization is only identified in it-PMAA in suspension-cast films.
Crystallizability of Free and Tethered Chains in Nanometer-Sized Droplets
- First Published: 10 June 2023
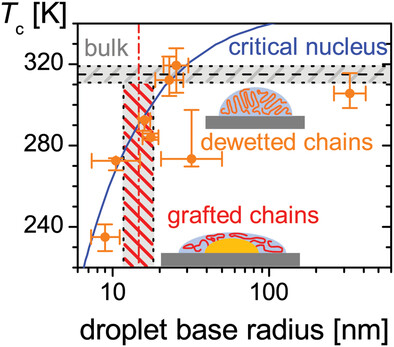
Crystallization in nanometer-sized polymer droplets is probed by a nano-dielectric spectroscopy setup. Spin-cast droplets down six chains each crystallize at reduced temperature according to classical nucleation theory. Aggregates, which are prepared via gold-nanoparticle deposition and a grafting-to reaction leading to a narrow volume distribution, consist of ten chains and exhibit no crystallization.
Monte Carlo Simulation of Long Hard-Sphere Polymer Chains in Two to Five Dimensions
- First Published: 18 March 2023
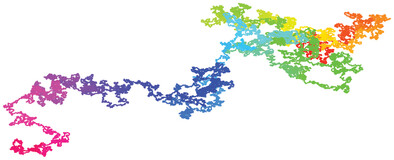
Using a recently devised binary-tree Monte Carlo method, simulations of long hard-sphere polymer chains with up to 107 repeat units subject to free and periodic boundary conditions are reported in two to five dimensions. Scaling analyses of end-to-end distance, radius of gyration, and their ratio provide strong support of universality. For measuring entropy and its derivatives new methods are introduced.
Dimerization of Polyglutamine within the PRIME20 Model using Stochastic Approximation Monte Carlo
- First Published: 30 May 2023
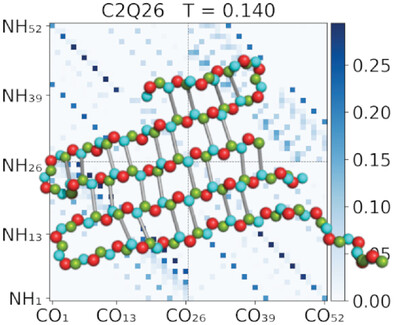
Stochastic Approximation Monte Carlo simulations are used in combination with the intermediate resolution protein model PRIME20 to simulate systems of two Polyglutamine chains of different chain lengths. Analysis of the specific heat and structural observables reveals an aggregation and folding transition around room temperature. The hydrogen bond contact probabilities reveal a chain length dependent aggregation route.
Atomistic MD Simulations of n-Alkanes in a Phospholipid Bilayer: CHARMM36 versus Slipids
- First Published: 12 March 2023
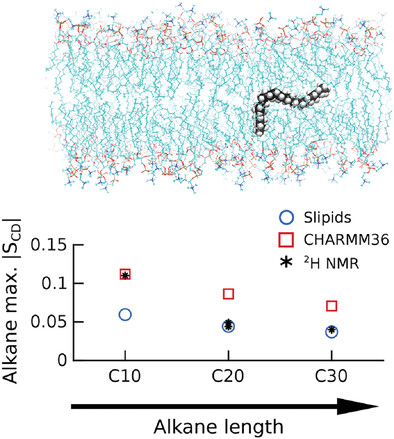
Molecular dynamics simulations using the Slipids and CHARMM36 force fields generate different NMR order parameters for alkanes incorporated in lipid bilayers. The predicted NMR order parameters are highly sensitive to the 1–4 electrostatic interactions and suggest that a reduction of the alkyl partial charges in CHARMM36 results in better performance for the longer alkyl chains.
Thermoplasmonic Manipulation for the Study of Single Polymers and Protein Aggregates
- First Published: 30 May 2023
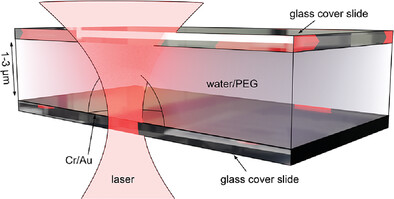
Thermoplasmonics, that is, the generation of heat with plasmonic nanostructures has recently found new applications in the manipulation of single macromolecules and protein aggregates. Recent developments are reviewed with a specific focus on thermophoretic trapping of single nano-objects and highlight the connection to temperature induced hydrodynamic effects providing new perspectives for the field.
How Single Site Mutations Can Help Understanding Structure Formation of Amyloid β1−40
- First Published: 16 February 2023
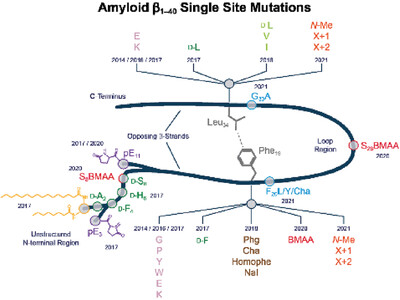
The contact between phenylalanine 19 and leucine 34 in Aβ molecules is highly conserved suggesting a particular significance for Aβ misfolding and, in turn, for its neurotoxicity. Point mutations in Aβ peptides at the contact F19−L34 help understanding this importance. The amyloid structure of Aβ is very robust against many modifications, but the toxicity highly depends on the F19−L34 contact.
Peptide Self-Assembly into Amyloid Fibrils at Hard and Soft Interfaces—From Corona Formation to Membrane Activity
- First Published: 22 February 2023
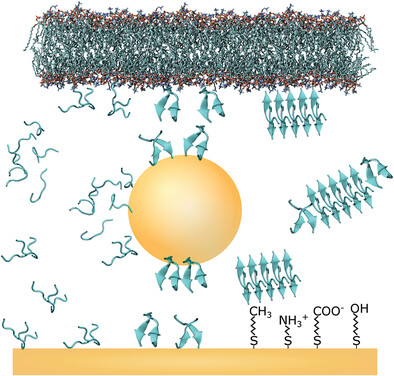
Nature is full of interfaces. Its physicochemical properties and biological activities control the function of biomolecules, such as peptides and proteins. In this review, the authors take a look at the influence of hard solid surfaces, nanoparticles, and soft matter interfaces, such as cell membranes, on peptide corona formation, peptide self-assembly and amyloid fibril formation.
Inhibition of the Fibrillation of Amyloid Aβ1-40 by Hybrid-Lipid-Polymer Vesicles
- First Published: 03 March 2023
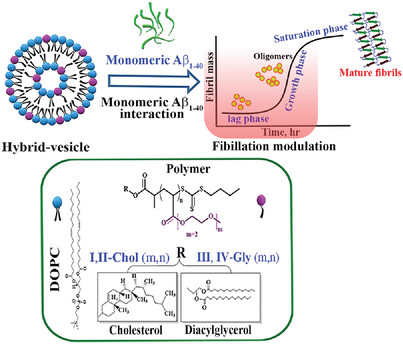
Hybrid-vesicles (≈100 nm), bearing cholesterol-/glycerol- anchored poly(di(ethylene glycol)macrylates)n embedded in a 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC) bilayer, inhibit amyloidic transformation of the Aβ1-40 peptide by prolonging lag- (tlag) and half times (t1/2) of Aβ1-40 fibrillation. The hybrid-vesicles promote the morphological transformation of Aβ1-40 fibrils to either amorphous or lead to a full inhibition of their formation.
Inherent Adaptivity of Alzheimer Peptides to Crowded Environments
- First Published: 17 April 2023
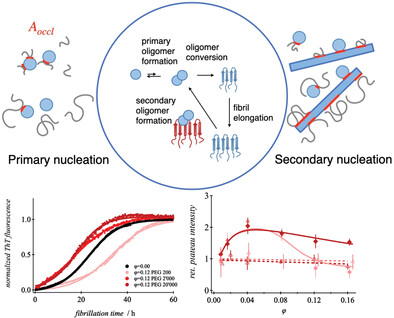
Macromolecular-crowded environments contrastively effect primary and secondary fibrillation pathways of the Alzheimer peptides. Occluded areas modify the rates of nucleation and growth revealing weak peptide-specific interactions of Aβ monomers and fibrils with the crowder molecules. Contrary, the entropic gain of the fibrillation process is mainly featured by primary nucleation processes.
A Competition of Secondary and Primary Nucleation Controls Amyloid Fibril Formation of the Parathyroid Hormone
- First Published: 22 February 2023
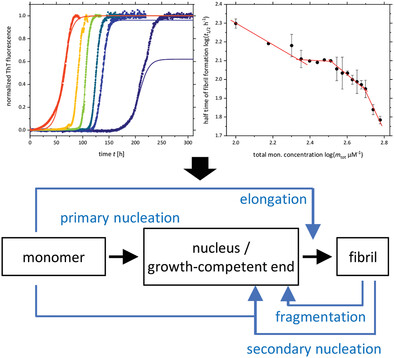
Kinetic analysis of amyloid formation by the parathyroid hormone PTH84 reveals an intriguing network of individual processes which depend on the peptide concentration. On the basis of various ThT detected fibrillation kinetics, a monomer-oligomer equilibrium is hypothesized to explain concentration dependent fibril formation and polymorphism, inhibition effects and the first case of a competition of primary with secondary nucleation.
Early Stage UV-B Induced Molecular Modifications of Human Eye Lens γD-Crystallin
- First Published: 21 February 2023
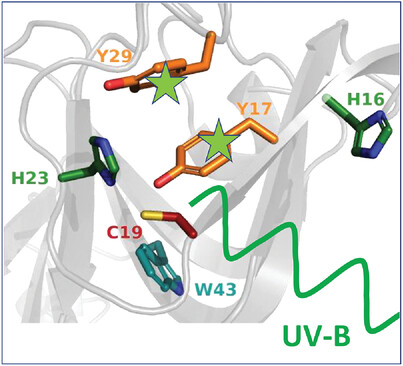
The eye lens gets continuously exposed to UV-B light during human lifespan. As early damage of still soluble and globular γD-crystallin, a local unfolding of the aromatic cluster in the N-terminal domain around tyrosine 17 and 29 can be identified by NMR spectroscopy. Eye lens extracts of cataract patients reveal some remaining photoprotective capacity.
Chain Sliding versus β-Sheet Formation upon Shearing Single α-Helical Coiled Coils
- First Published: 01 March 2023
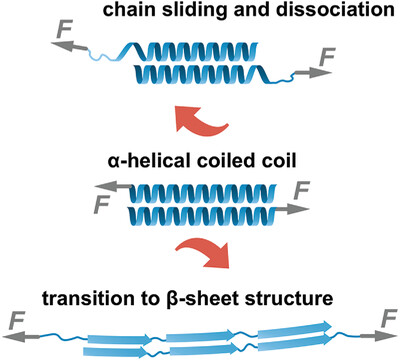
Coiled coils are key building blocks of self-assembled biogenic materials. Under load, coiled coils frequently undergo a structural transition from an α-helical structure to mechanically stronger β-sheets. This α-β transition is absent when shearing individual synthetic coiled coils with single-molecule force spectroscopy. Molecular dynamics simulations suggest that interchain sliding dominates over β-sheet formation on the chain separation pathway.
Secondary Structures in Synthetic Poly(Amino Acids): Homo- and Copolymers of Poly(Aib), Poly(Glu), and Poly(Asp)
- First Published: 15 November 2022
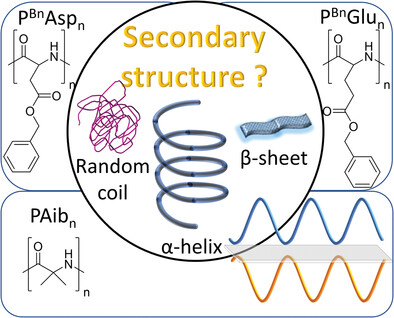
Poly(amino acids) allow to study the assembly and function of proteins. The folding and aggregation of oligo- and poly-glutamic acids, aspartic acids and α-aminoisobutyric acids and the impact of solvents and functional groups is reviewed. Embedded at the cross-section of protein fibrillation and supramolecular polymers, these molecules allow the future design of functional biomaterials, guiding further into amyloid fibrillation.






