Peptide Self-Assembly into Amyloid Fibrils at Hard and Soft Interfaces—From Corona Formation to Membrane Activity
Abstract
Peptides and proteins are exposed to a variety of interfaces in a physiological environment, such as cell membranes, protein nanoparticles (NPs), or viruses. These interfaces have a significant impact on the interaction, self-assembly, and aggregation mechanisms of biomolecular systems. Peptide self-assembly, particularly amyloid fibril formation, is associated with a wide range of functions; however, there is a link with neurodegenerative diseases, such as Alzheimer's disease. This review highlights how interfaces affect peptide structure and the kinetics of aggregation leading to fibril formation. In nature, many surfaces are nanostructures, such as liposomes, viruses, or synthetic NPs. Once exposed to a biological medium, nanostructures are coated with a corona, which then determines their activity. Both accelerating and inhibiting effects on peptide self-assembly have been observed. When amyloid peptides adsorb to a surface, they typically concentrate locally, which promotes aggregation into insoluble fibrils. Starting from a combined experimental and theoretical approach, models that allow for a better understanding of peptide self-assembly near hard and soft matter interfaces are introduced and reviewed. Research results from recent years are presented and relationships between biological interfaces, such as membranes and viruses, and amyloid fibril formation are proposed.
1 Introduction to Peptides, Amyloid Fibrils, and Self-Assembly at Interfaces
Peptides and proteins form the basis for fundamental biological functions in organisms, such as building blocks of the muscles,[1] antigens and antibodies of the immune system,[2] or enzymes to catalyze chemical reactions.[3] They self-assemble and fold into three-dimensional structures to optimize their energy.[4, 5] The characteristic structure of a peptide or protein typically determines its function.[6] While the primary structure is defined as the sequence of its building blocks, the amino acids, the secondary structure (e.g., α-helices and β-sheets) is formed by backbone hydrogen bonding,[7] determined by the amino acid side chains’ physicochemical properties (polarity, hydrophobicity, charge).[8, 9] Van der Waals and hydrophobic interactions facilitate further packaging into the protein tertiary structure.[10] Quaternary structures describe the assembly of multiple polypeptides into functional complexes.
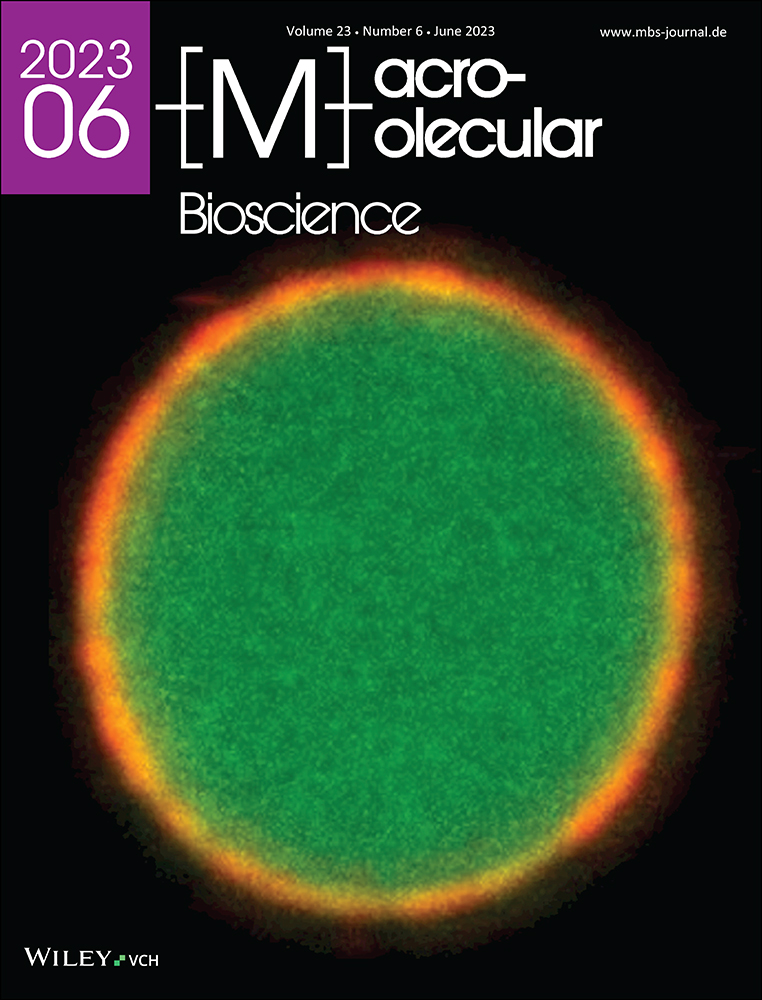
While peptides and proteins are natively soluble in the aqueous cell environment, some “misfold” into insoluble fibrils called amyloid fibrils.[11] The term amyloid originally described extracellular deposits of abnormally folded protein aggregates associated with amyloidosis diseases.[12] Biophysicists now use the term to describe any peptide or protein that forms cross-β-sheet fibrils (Figure 1), independent of any pathological link.[13-15] In a cross-β-sheet structures, the β-sheets are parallel-oriented and β-strands perpendicular to the fibril axis.[13] They are called cross-β-sheets because of the characteristic X-ray diffraction patterns with distinct spacings of about 4.8 Å (inter-strand spacing) and 10 Å (inter-sheet spacing).[16, 17] Amyloid fibrils are typically 7–12 nm in diameter and several 100 nm in length.[18, 19] Several alternative fibril morphologies have been identified (polymorphism), including α-helical fibril structures.[20-24] It has been reported that the formation of β-sheet rich fibrils involves an α-helical peptide conformation as transient intermediate on or off the pathway to β-sheets.[25-28]
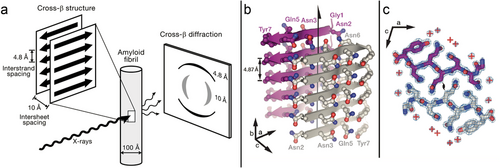
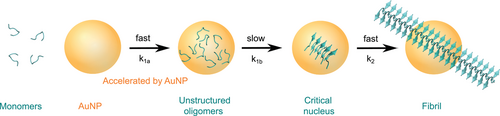
Peptide self-assembly into amyloid fibrils follows a two-step nucleation-polymerization mechanism (Figure 2).[30-33] First, peptide monomers self-assemble into unstructured oligomers that slowly transform into critically sized nuclei likely rich in β-sheet conformation (nucleation phase), followed by the rapid growth of protofibrils and their elongation into mature fibrils (growth phase) (nucleated conformational conversion model).[30, 31, 34-36] The detailed kinetics and mechanism of protein aggregation were reviewed by Morris et al.,[37] Cohen et al.,[38] and John and Gladytz et al.[39] The aggregation kinetics depend on the intrinsic aggregation propensity of the peptide,[40, 41] its environment (e.g., solvent),[25, 42, 43] the temperature,[44] and the peptide concentration.[25, 45] For example, it was demonstrated that the organic solvent 2,2,2-trifluoroethanol (TFE) stabilizes peptides in an α-helical conformation, which impacts peptide self-assembly into amyloid fibrils.[25, 46-48] The α-helical conformation of a peptide might accelerate the formation of small oligomers during the lag phase of aggregation through intermolecular helix–helix interactions.[26, 27, 49, 50] Regions of a peptide could adopt the α-helical conformation while other regions of the peptide transition into β-sheets and grow into fibrils.[51, 52] Computational studies identified an increase in entropy as the driving force for the α-helix to β-sheet transition, and a random coil transition state was observed.[53] The morphology and aggregation kinetics of peptides can be altered by small changes in the amino acid sequence.[25, 54, 55] Hydrophobic residues have been identified as important driving factors for amyloid fibril formation.[25, 54, 56-58] This knowledge has been incorporated into theoretical prediction algorithms developed to calculate the aggregation propensity and solubility of a peptide, and to identify aggregation-prone regions within larger peptides and proteins.[59-63]
Amyloidogenic peptides and their self-assembly into fibrils and plaques have been linked to several ageing-related diseases,[11, 64] such as Alzheimer's disease (amyloid-β, Aβ peptide),[65, 66] Parkinson's disease (α-synuclein),[67-69] and type 2 diabetes (islet amyloid polypeptide, IAPP).[70-72] However, the trigger that initiates amyloid peptide aggregation in organisms is not yet fully understood, and its causal association with disease is controversial.[73, 74] While the insoluble fibrils were originally thought to be the toxic species,[75] many researchers now consider the soluble oligomers as the causative agent of disease.[76-79] Although peptide aggregation processes have been known for many years, a complete picture of the molecular mechanisms and their implications for disease development is still lacking.[11, 80, 81] In addition to the pathological link to ageing and neurodegenerative diseases, amyloid fibrils have also been identified as having a functional role (e.g., curli fibers in bacteria)[82-86] and have been exploited as bionanomaterials.[87-90] The high surface area allows the use of fibrillar structures as catalyst supports[90] and the high strength of fibrillar materials, such as spider silk, has been explored for developing tougher materials.[91, 92]
Amyloid-forming peptides and proteins vary in size, but are typically rich in hydrophobic residues.[59, 60] In many cases, short peptide sequences from the full-length amyloid proteins have been identified as models for peptide aggregation, representing the sequences responsible for aggregation.[93] These model peptides are important aggregation motifs in amyloid proteins.[13, 29] Some examples are GNNQQNY (from Sup35 yeast protein), NNFGAIL (from amylin, IAPP), VEALYL (from insulin), and VQIVYK (from tau).[15, 94] While proteins are >100 amino acids in length, smaller peptides are not only easier to study experimentally but also more accessible for computer simulations than full-length proteins.[95] Typical experimental characterization methods[96] include imaging techniques to study fibril morphologies (e.g., electron microscopy and atomic force microscopy [AFM]),[81, 97] spectroscopic techniques to understand fibril structure and interactions (e.g., fluorescence of thioflavin T,[98] CD,[99] FTIR,[100] and NMR[101]), and mass spectrometry.[102] The experimental methods used to understand the molecular mechanism of peptide adsorption, conformational transitions, and the initial steps of aggregation are often complemented by computer simulations, such as molecular dynamics (MD) simulations, to obtain molecular mechanistic insights.[103, 104] Alternative computational approaches to simulate full-length proteins include coarse-grained methods in which amino acids are represented by beads rather than individual functional groups, allowing longer time scales to be achieved. However, they are limited in their validity compared to more representative united-atom or fully atomistic simulations accessing shorter time frames.[105, 106]
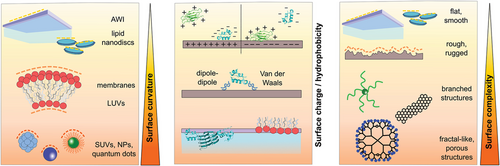
Nature is full of interfaces, such as cell membranes and implanted materials.[107, 108] Once a peptide or protein comes into contact with such interfaces, its conformation and activity may adapt depending on the interface properties.[109-111] Interfaces are characterized by their specific charge, hydrophilicity, polarity, roughness, and curvature.[112-115] These properties can be tailored, such as by designing implanted materials with highly biocompatible surfaces to reduce the adverse effects of a foreign body reaction upon contact with the material.[116, 117] Surfaces can also be coated with polyethylene glycol (PEG) or anti-adhesive proteins (e.g., lubricin [LUB]) to increase its repellent character toward peptide or protein adsorption.[118-123] These properties are particularly relevant to the kinetics of amyloid peptide aggregation.[25, 39, 124, 125] By influencing a peptide's conformation or local concentration through surface adsorption and alignment, its aggregation kinetics can be impacted.[25, 39, 112]
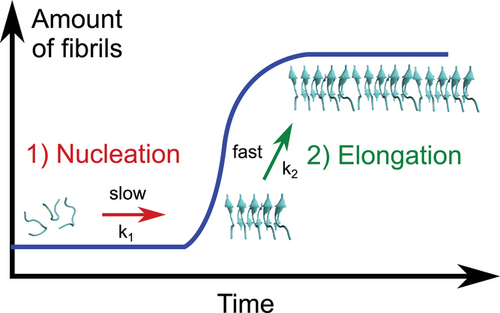
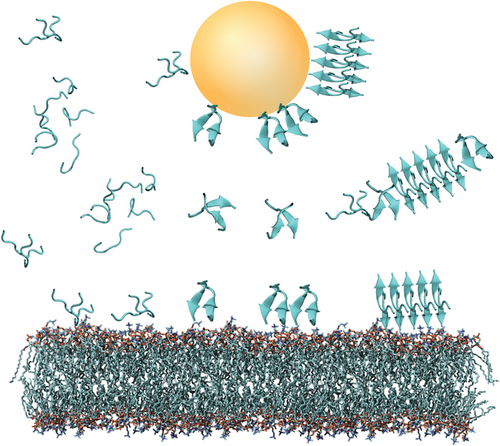
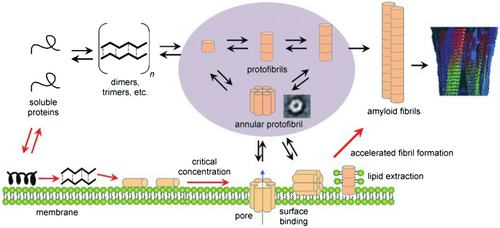
Nanostructured surfaces are particularly relevant for peptide self-assembly as they are abundant as natural liposomes and as synthetic particles, such as air-pollutants, components of cosmetics, and nanoparticles (NPs) used in nanomedicine.[39, 126-131] Due to their small size, nanostructured interfaces have a relatively large surface-to-volume ratio, making them highly relevant for organisms.[39, 132, 133] A recent review by John and Gladytz et al. provides a detailed overview of the observed effects of NPs on amyloid peptide aggregation.[39] In addition to inorganic surfaces and NPs, biological matter, such as membranes, liposomes, and viruses, can contribute and impact peptide self-assembly (Figure 3).[25, 134-137] Peptide–membrane interactions are of particular interest as amyloid toxicity has been associated with the activity of the soluble peptide oligomers toward membranes,[124, 138-140] suggesting links between amyloid-forming and membrane-active peptides.[54, 141-143]
In this review, we present a perspective on the role of biologically relevant interfaces at which amyloid peptide aggregation occurs.[25, 39] As an important parameter, the role of surface chemistry and its functionalization to tailor surface properties for low-peptide binding or high biocompatibility is reviewed.[112, 117] Both inorganic surfaces[39, 104, 112, 117] and biological membranes[25, 54, 147] are discussed as biologically relevant interfaces. Models and mechanisms of the influence of hard and soft matter interfaces are presented to elucidate the accelerating[104, 127, 148] and inhibiting[149-152] effects of particularly nanostructured interfaces on peptide self-assembly.[153] The research highlighted here is based on biophysical studies (in vitro) and computer simulations (in silico).
2 Interface Properties and Functionalization for Tailored Surfaces
Interfaces can be characterized by a range of specific properties, for example, roughness, polarity, charge, curvature, and chemical composition (Figure 4).[154] This diversity of interface properties can be exploited when new materials are surface-functionalized to obtain new properties.[155] Such tailored surfaces are highly relevant for the development of coatings for implants, where high biocompatibility of these materials is required for organisms to prevent unwanted aggregation processes, as part of the foreign body rejection reaction.[116, 117, 155, 156] Polar surfaces, such as PEG coatings have been identified as a viable candidate as they presented very low surface–adsorbate interactions.[123, 157] To develop materials with high performance, design principles often mimic nature, although a comprehensive understanding of the complexity of life and its human-made imitation are limited.[158-160]
To elucidate the role of different functional groups in the adsorption and self-assembly of amyloid peptides, studies using functionalized thiols immobilized on gold substrates were undertaken by John et al.[112] and Hajiraissi et al.[161] Thiols spontaneously chemisorb on gold, making these systems suitable for the formation of high-quality self-assembled monolayers.[162, 163] Surfaces were functionalized with methyl, amino, carboxyl, and hydroxyl terminal groups to form relatively flat and functionally homogeneous surfaces (Figure 5).[112, 162] While methyl-functionalized surfaces were more hydrophobic than unmodified gold surfaces, amino-, hydroxyl-, and carboxyl-modified surfaces were more hydrophilic.[112] These tailored surfaces varied in functionality, polarity, charge, hydrophilicity, and their effects on peptide adsorption.[112, 161] To investigate nanogram binding events to such modified surfaces, a quartz crystal microbalance with dissipation monitoring is a suitable tool.[164] Peptides self-assemble into amyloid fibrils under physiological conditions at low concentrations;[165] thus, studies were performed at peptide concentrations where fibril formation in an aqueous environment would not be expected.[148] Surface adsorption is an important catalytic factor as peptides are locally concentrated, allowing self-assembly into amyloid fibrils.
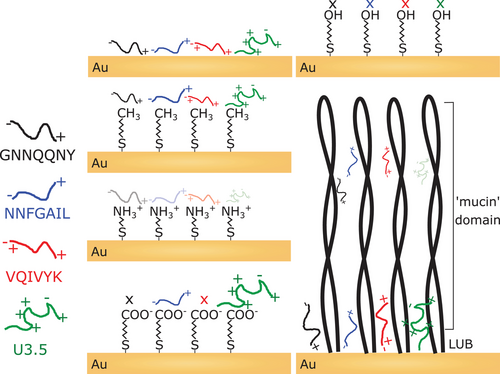
John et al. studied the amyloid-forming peptides GNNQQNY, NNFGAIL, VQIVYK, and Uperin 3.5 (U3.5).[112] Electrostatic interactions (charge attraction and repulsion) as well as the hydrophilicity or polarity of the peptide and the surface determined the surface adsorption affinity of the peptides. The mostly hydrophobic peptides adsorbed most strongly to hydrophobic (methyl and unmodified gold) and countercharged surfaces (U3.5 on carboxyl). The hydroxyl surface was the only surface that inhibited any peptide adsorption, likely due to the combination of neutral charge and high polarity.[112] Hajiraissi et al. studied the positively charged full-length human IAPP (hIAPP) peptide and followed its aggregation to amyloid, as a consequence of the adsorption.[161] They reported that fibril formation was retarded by methyl surfaces, whereas carboxyl-terminated surfaces led to fibril formation due to attractive electrostatic interactions.[161] Hydroxyl and amino surfaces were suggested to influence fibril formation via hydrogen bonding. The strong adsorption of hIAPP to methyl surfaces might have retarded peptide aggregation, while the more hydrophilic surfaces resulted in weaker binding, allowing for peptide self-assembly and conformational transitions.[39]
The glycoprotein LUB (proteoglycan 4), known as a joint lubricant in our body, was previously identified as an anti-adhesive protein.[121, 166, 167] Although the LUB protein is known as an anti-adhesive protein, recent studies reported its size-selective transport properties.[168] John et al. observed similar mass binding to the LUB-coated surface compared to unmodified gold, albeit accompanied by structural changes in the adsorbed peptide-LUB layer.[112] The grafting density of LUB on the gold surface was not sufficiently dense to prevent the diffusion of small peptides through its structure. This must be taken into account when surfaces are tailored to properties desired for applications. While LUB coatings offer an excellent anti-adhesive toward larger biomolecules such as proteins, densely packed and flat coatings of polar groups, for example, in PEG, are more favorable to prevent the adsorption of smaller biomolecules.[112]
The availability of surfaces with tailored properties is crucial for the design of biocompatible materials and nanointerfaces in medical devices, drug delivery applications, and diagnostics.[132, 169-171] For materials for use in implants, it is crucial that cell adhesion is promoted.[117] Pagel et al. designed a multifunctional peptide coating that has a high adhesion affinity for titanium surfaces as well as a high cell binding affinity. The peptide contained l-3,4-dihydroxyphenylalanine, a post-translationally modified amino acid (hydroxylated tyrosine) found in mussel glue proteins (Mytilus edulis) with high binding affinity to many surfaces. The mussel-derived peptide was modified with a cyclic integrin-binding peptide (RGD, c[RGDfK]) and a heparin binding peptide (FHRRIKA), both to enhance cell growth and viability on the peptide coating.[117] The cooperative use of strategies in a multifunctional coating increases its success. In addition to bioactive properties (e.g., cell binding), coatings should also have antifouling properties that render surfaces inert or “stealth”-like (surface passivation; e.g. block recognition by the immune system) and prevent nonspecific adsorption and formation of a protein coating.[155] Established approaches to preparing antifouling surfaces include the use of hydrophilic polysaccharide coatings, surface assembled monolayers, or PEGylation.[155, 169] Bioactivity is achieved by the incorporation of cell binding motifs that reproduce integrins and other receptors, that are abundant in external membranes, as presented by Pagel et al.[117, 155]
The focus of research on peptide fibrils has recently been broadened to their functional roles,[87] such as to engineer surface properties.[88, 172] Peptide-based biomaterials have the advantage that a few simple building blocks can be used to build complex structures in a hierarchical manner, as known for other biological systems such as DNA, although the cost are still high.[87, 173] The immobilization of peptide fibrils for the design of tailored surfaces has been studied by John et al.[174] The NNFGAIL peptide (IAPP21–27) was biotinylated at its N-terminus and the resulting fibrils were immobilized on streptavidin-coated supports. The biotin-(strept)avidin system is known for its high binding affinity and has been used for many biochemical applications.[175, 176] This proof-of-concept study provided the basis for fabricating amyloid microstructures on surfaces.
3 Influence of Nanostructures and Nanoparticles on Amyloid Fibril Formation
Nanostructured surfaces and NPs are ubiquitous in nature and have been developed for applications in cosmetic products, materials, and nanomedicine.[90, 130, 177] NPs are small with diameters between 1 and 100 nm (Figure 6),[178] resulting in specific properties, such as a high surface-to-volume ratio, the color of metal NPs (dipole plasmon resonance), or the superparamagnetic character of magnetic NPs.[179, 180] The potential risk and cytotoxicity of nanostructures for organisms has been recognized.[177, 181] John and Gladytz et al. have studied and reviewed the impact of NPs on amyloid peptide aggregation in particular.[39] Although NP–peptide interactions have been previously studied in a number of publications, contradictory effects and mechanisms have been reported.[39, 132] Both accelerating and inhibiting effects have been observed.[104, 127, 148, 149, 182] This conflicting knowledge was the starting point for further investigation.
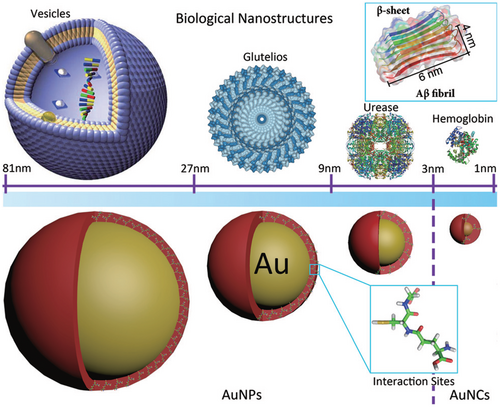
Once a peptide or protein comes into contact with a NP, the NP surface is covered with a so-called “corona” (Figure 7).[126, 183] The peptide or protein corona governs the biological properties and activity of NPs, and thus also their potential risks, as it is the entity that further interacts with molecules in organisms.[127, 184, 185] The formation of the corona depends on the surface coating and size of the NP, the properties of the peptides in solution, as well as the attractive or repulsive interaction between the NP and peptides.[39, 152, 153, 186-188] It has been shown that a balance between peptide–NP and peptide–peptide interaction leads to an acceleration of peptide self-assembly into amyloid fibrils, with the NP corona serving as a nucleation seed.[39, 104] Attractive interactions between peptide and NP lead to a local up-concentration of peptide and to rapid formation of unstructured oligomers on the NP surface. When the interaction with the NP surface is in a range that enables conformational transition into β-sheets, the NPs accelerate fibril formation by increasing the peptide concentration while still enabling mobility (condensation-ordering mechanism, Figure 8).[39, 104, 189] The β-sheet rich peptide corona has a similar aggregation-accelerating property as critical-size peptide oligomers in the absence of NPs (Figure 9).[39, 104] Accelerating effects on peptide aggregation were also observed when the peptide corona was formed from a different but similarly amyloid-forming peptide than the one aggregating (cross-seeding effect).[148, 190]
When peptide–NP attraction is weak, NPs do not influence peptide aggregation, while when the interactions are strong, peptides are prevented from conformational changes and cannot form amyloid fibrils.[39] When NPs were coated with a layer of PEG, amyloid peptide aggregation was unaffected or even inhibited.[148] This is attributed to the repulsive properties of PEG and weak NP–peptide interactions, as outlined above. Similar inhibitory effects on fibril formation were observed for NPs modified with specific amino acids or peptides.[149, 191, 192] This led to the development of functional NPs for medicine and the potential treatment of Alzheimer's disease.[119, 131, 151]
To better understand the effects of NPs on amyloid peptide aggregation, MD simulations have been employed.[39, 104, 153, 193-196] The availability of a force field that reliably represents experimentally observed peptide–NP interactions is crucial for such computational studies.[197-199] The GolP-OPLS/AA (Gold-Protein-Optimized Potentials for Liquid Simulations/All Atom) force field combines the GolP force field for gold by Corni et al. and the OPLS/AA force field for peptides and proteins and has been used in several studies.[198-200] A recently developed alternative force field is the polarizable Lennard–Jones potential by Heinz et al. that was reported to represent experimental results very accurately.[201] MD studies can identify the initial molecular interactions between peptides and surfaces.[39, 104] This has been applied to investigate the effects of citrate-coated gold surfaces on peptide structure and amyloid aggregation.[39, 104] A citrate coating is one approach used to stabilize otherwise unstable gold NPs (AuNPs) in solution.[39, 202] The simulations revealed that the studied peptides bound to the citrate-coated gold layer first with their N-terminus or positively charged side chains (e.g., lysine).[39, 104] Weakly surface-bound peptide monomers formed β-sheet rich dimers within the simulation time of 200 ns.
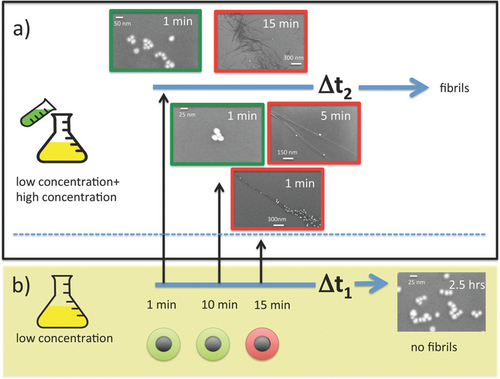
In a recent combined experimental and computational study, the peptides Aβ40, GNNQQNY, VQIVYK, and NNFGAIL all rapidly bound to citrate-coated AuNPs; however, different effects on peptide aggregation into amyloid were observed using fluorescence spectroscopy, electron microscopy, AFM, and dynamic light scattering (DLS).[153, 203] While GNNQQNY and VQIVYK aggregation were retarded or even inhibited by the presence of AuNPs, NNFGAIL aggregation was accelerated. The contrary influence of the same NPs toward different peptides can be attributed to different intrinsic aggregation propensities of the peptides in combination with varying peptide–NP interaction strengths.[153] Peptides with high aggregation propensity, such as NNFGAIL, aggregated faster in the presence of NPs, while peptides with lower aggregation propensity, such as GNNQQNY, aggregated more slowly when NPs were present. The peptide sequences were unstructured in the aqueous environment, and the effects of NPs resulted from a combination of aggregation propensities and peptide–surface interactions.[195] Correlations between aggregation propensity and NP effects have been suggested previously, although they linked high protein stability with a lower aggregation propensity and thus an acceleration of aggregation by NPs.[186]
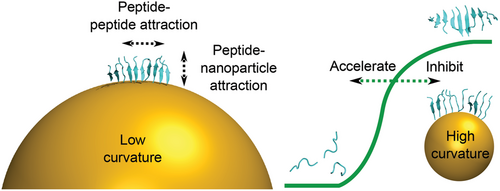
Besides the differential influence on different peptides, the diameter of the AuNPs was varied and it was found that smaller NPs (5 nm) had a greater inhibitory effect (Aβ40, GNNQQNY, VQIVYK), while the larger NPs (20 nm) had a stronger accelerating effect (NNFGAIL).[153] The size dependence of the NP impact on amyloid peptide aggregation was previously reported by Gao et al.[152] for the Aβ40 peptide. Smaller NPs inhibited Aβ40 aggregation and larger NPs accelerated this process. While the larger NPs (20 nm) appeared as nearly flat surfaces to the peptide monomers, the smaller NPs (5 nm) were in a similar size range to the fibril structures formed. Thus, the smaller NPs not only provided a surface but also interfered with fibril formation.[39, 153] MD simulations were used to understand the stability of β-sheet oligomers on curved surfaces.[153, 203] The larger NPs (20 nm) stabilized the oligomers, while the smaller NPs (5 nm) destabilized the structures (Figure 10). It has previously been shown that the free energy of adsorption decreases while the charge density and interaction potential increase with decreasing radius at convex surfaces.[204, 205] While the small NPs strongly interfere with the fibril structure, the larger NPs with lower surface potential provide a seed and accelerate fibril formation.[153]
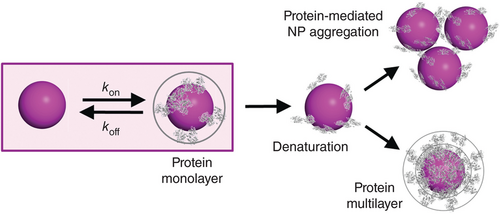
Wang et al. recently discussed and investigated the biomolecular corona (Figure 11).[206] They emphasize that studying the biomolecular corona is a challenging endeavor due to the enormous diversity of engineered NPs in terms of their physicochemical properties and the large number of biomolecules present in biofluids that may bind to NPs with widely different strengths. They also point out that depending on the interaction strengths, coronas can be dynamic (reversible) or static (irreversible) on a chosen experimental time scale. Static coronas are simpler to handle because they can be analyzed after separation of NPs and immersion fluid. Dynamic coronas, however, require in situ studies of NPs in the immersion fluid (e.g., protein solutions or blood), for which only a few methods exist. They critically discuss the in situ biomolecular corona characterization using diffusion-based NP size experiments, fluorescence correlation spectroscopy and DLS, both of which directly determine the hydrodynamic radius of NPs in a fluid via the Stokes–Einstein relation.[206] Understanding the dynamic corona is important because this is the biologically active species in organisms; thus impacting cellular internalization, biodistribution, and toxicity.[207, 208] Mohammad-Beigi et al. reported that the presence of a corona of human serum proteins on NPs changes the influence of the NP on fibril formation. The protein corona blocked the NP surface and thus reduced the NP effect.[209] For applications in nanomedicine, a balance between NP stealth properties to prevent corona formation and targeting properties is required.[210]
4 Membrane Interaction of Amyloid-Forming Peptides
Biological interfaces such as membranes or tissues can influence biochemical processes, including amyloid fibril formation.[25, 39, 112, 211] Membranes are complex structures composed of phospholipids, sterols, proteins, glycolipids, and sphingolipids that serve as barriers for the compartmentalization of cells.[107, 212-214] The precise composition affects the membrane stability, physicochemical properties, and interactions with molecules and ions.[213] For biophysical studies, simplified model systems representing the characteristic properties of membranes have been used.[54, 147] Phospholipids are the main component of membranes and consist of a polar, hydrophilic phosphate head group and an apolar, hydrophobic carbon tail. A typical representative for modeling eukaryotic membranes is the use of the zwitterionic phospholipids DMPC or POPC.[54, 215] Unlike fully saturated DMPC, the POPC lipid is monounsaturated.[144] Mammalian membranes are mimicked by a mixture of DMPC/POPC and cholesterol, which makes the membrane stiffer.[107, 213, 216] The anionic character of bacterial membranes is modeled by a mixture of DMPC/POPC and DMPG/POPG.[25, 54, 215] Depending on the membrane composition, different effects on peptide self-assembly were observed (Figure 12).[25, 135, 217-220]
We studied the role of different membrane components on the aggregation kinetics of U3.5 peptide, namely the zwitterionic phospholipid DMPC, anionic DMPG, and cholesterol.[25] Membranes, particularly sphingolipids and bacterial anionic lipids, have previously been identified to accelerate amyloid peptide aggregation.[217, 221-225] It has been suggested that electrostatic interactions, rather than the headgroup chemistry, are most important for the effect of the membrane component on peptide aggregation.[217] The lipid influence on peptide aggregation was investigated with different amounts of lipid present. When excess lipid was present (peptide:lipid ratio of 1:9), U3.5 aggregation was inhibited by anionic DMPG liposomes, retarded by DMPC and no influence was observed when cholesterol was present. The inhibition or retardation of peptide aggregation was caused by a stabilization of the α-helical peptide conformation. When lower amounts of lipids were present, U3.5 aggregation was accelerated by DMPG and DMPC, consistent with a weak stabilization of the α-helical peptide conformation.[25] When the α-helical conformation of a peptide was highly stabilized, the peptide was locked in that conformation, thus preventing amyloid aggregation (Figure 13).[25, 27, 226] The targeted stabilization of a peptide in its α-helical conformation could thus be a strategy to prevent peptide aggregation and possible related diseases.[227]
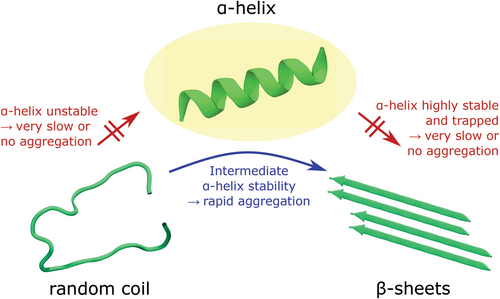
The α-helical conformation of a peptide is known to be the major peptide species for antimicrobial peptides such as U3.5 when they come into contact with membranes.[6, 228] In addition to membranes, fibril formation of U3.5 with different amounts of TFE present has been investigated.[25, 229] TFE is a protic organic solvent known to enhance helical secondary structure in peptides and proteins.[46, 229, 230] U3.5 was unstructured in water with up to 8% TFE present and α-helical with above 14% TFE in the mixture. Intermediate structures were formed in between, partially unstructured (random coil) and partially α-helical.[25] With 20% TFE present, U3.5 formed β-sheet rich structures and immediately aggregated. At higher TFE contents (40%), the α-helical conformation was highly stabilized, and the peptide did not aggregate for at least 5 days.[25] These results suggest that a weakly stabilized α-helix results in faster aggregation kinetics, while strong stabilization locks the peptide in the α-helical conformation and prevents any fibril formation.[25, 48]
These observations may have implications for the development and progression of neurodegenerative diseases.[11] Cellular changes lead to changes in the physiological environment and thus likely to changes in the most favored peptide or protein secondary structure.[25] Aging is often associated with defective mechanisms[231] to combat oxidative stress, which has also been shown to accelerate the aggregation of amyloidogenic proteins.[136, 232] The interaction of amyloid-forming peptides with membranes has been studied bidirectionally, that is, the role of membranes was considered not only to trigger an increased peptide aggregation rate,[25, 54, 233-235] but also as a target of amyloid peptide activity. It is unclear whether amyloid-forming peptides first initiate pathogenic processes in membranes and change their structures, or whether changes in membranes are the cause of peptide aggregation.[236-238] Since the processes of membrane binding and peptide aggregation occur simultaneously in a complex environment, it is challenging to probe these processes independently, leading in many cases to ambiguous conclusions of experimental data.[239] Thus, the use of complementary methods and computational approaches is important to correctly interpret data from such complex processes.
When the membrane activity of peptides is studied in vitro, a mixture of monomeric and oligomeric peptide species is usually present. It is much more challenging to identify the membrane-active species of a peptide or protein that is present when bound to a membrane.[54] Martin et al. used an approach in which freshly prepared, incubated, and centrifuged (supernatant, pellet residue) U3.5 peptide sample species were prepared. It was suggested that the freshly prepared peptide solution contained mainly monomeric species, whereas the incubated sample would contain a mixture of mature fibrils, protofibrils, smaller oligomers, and monomers. The supernatant of the centrifuged sample would contain mostly soluble oligomeric and monomeric species, while the pellet residue would contain the mature fibrils. Although all samples presented the same membrane-activity, the kinetics varied.[54] Since U3.5 is an amphipathic antimicrobial peptide,[240] it can be assumed that its α-helical species is the membrane-active species.[228, 241] One hypothesis could be that the freshly prepared samples contained peptide species that formed the membrane-active α-helical oligomers, whereas the β-sheet rich fibrils were not membrane-active. Further, the incubated peptide sample, particularly the soluble portion after centrifugation, likely contained more aged and larger oligomeric species that appeared to be less membrane-active than the monomers and smaller oligomers in the fresh samples.
To understand the interaction of amyloid-forming peptides and proteins with lipids and cell membranes, it is crucial to understand their toxicity and pathological relevance in the context of neurodegenerative diseases.[135] Oligomeric associations of peptides have been identified as membrane-binding and toxic species.[239, 242-245] It has been suggested that these oligomers form pores in lipid membranes, resembling the pore-forming properties of antimicrobial peptides.[142, 143, 246-250] Antimicrobial peptides are abundant in many organisms as host-defense peptides and are part of the innate immune system.[251-253] The formation of membrane pores disrupts the barrier function of cell membranes and could explain the toxicity of amyloidogenic peptides.[254] Other hypotheses postulated the formation of reactive oxygen species, interactions with cell receptors or metals, or the direct disruption of cell membranes by peptide oligomers as toxic species.[124, 140, 255-257]
When comparing antimicrobial and amyloid-forming peptides, both form amphipathic structures with hydrophobic and hydrophilic domains.[124, 141, 229, 258] They adapt an α-helical conformation upon contact with membranes and are particularly attracted to anionic membranes.[25, 26, 259] Torrent et al. suggested that antimicrobial peptides may have emerged from aggregation-prone peptide regions by mutations of hydrophobic to charged residues.[260] Martin et al. studied the antimicrobial and amyloid-forming peptide U3.5 from the Australian toadlet Uperoleia mjobergii.[54, 229, 240, 261] The 17 amino acid U3.5 peptide was membrane-active, while its R7A variant was not. Instead, U3.5 R7A formed amyloid fibrils much faster and to a larger extent than the U3.5 wildtype,[54] in agreement with the proposed model by Torrent et al.[260] Electrostatic interactions were important for membrane activity and disruption of bacterial mimetic membranes (DMPC/DMPG) by U3.5.[54] The eukaryotic mimetic membrane (DMPC, zwitterionic) was also disrupted by U3.5 peptide, while the mammalian mimetic membrane (DMPC/cholesterol) presented a binding and pore formation mechanism.[54] These two distinct mechanisms could explain the antibacterial activity toward bacteria and cytotoxicity toward mammalian cells.[228]
5 Summary and Outlook on the Road Ahead
Peptides and proteins are exposed to many interfaces in nature. In this review, the role of biologically relevant interfaces in peptide adsorption and self-assembly into amyloid fibrils has been illustrated for functionalized and nanostructured surfaces, particularly for NPs and biomimetic membranes. The properties of these interfaces have been shown to define and influence the interaction structure, behavior, and ultimately the function of a peptide or protein. Polar surfaces rich in hydroxyl groups were almost always inert to peptide adsorption, while hydrophobic or charged surfaces attracted peptides with similar hydrophobic character or a counter charge, leading to adsorption to the surface.[112] The adsorption strength on a surface determined the effect on peptide self-assembly. Peptide adsorption to a surface requires monomers to concentrate locally, increasing their propensity to aggregate into amyloid fibrils.
When NPs encounter peptides, a corona forms as the first layer. It has previously been shown that this peptide corona can seed aggregation similar to small peptide oligomers.[104] It has been observed that NPs both accelerate and inhibit amyloid peptide aggregation, depending on the interaction strength between the NP and the peptide and the intrinsic propensity for peptide aggregation.[39, 104] While a low peptide–surface attraction had no effect on the aggregation, high peptide–surface attraction resulted in surface binding that prevented the peptides from aggregation. Intermediate peptide–surface attraction could locally concentrate peptides at the surface while allowing conformational conversion into β-sheet rich fibrils.[39, 104] High surface curvature destabilized prefibrillar structures, leading to greater inhibition of peptide aggregation by smaller NPs.[153] In a physiological environment, NPs are immediately covered with a biofilm formed from the biomolecules that are abundant in biological fluids. This biofilm then determines the activity of the coated NPs. Thus, in order to make firm conclusions about the in vivo effects of NPs, studies should be performed where the NPs are first coated with biologically relevant biofilms before conducting toxicity studies.
Nano-sized objects are not only introduced artificially by humans, but also occur in nature in the form of liposomes and viruses (Figure 14). The question of whether a biological membrane can accelerate or inhibit peptide aggregation depends on its composition and peptide-to-lipid ratio.[219, 235] This finding has important consequences in terms of a physiological environment. When an organism becomes infected with a bacterium containing anionic phospholipids or a membrane becomes oxidized and not repaired, for example, through aging, these factors could induce peptide aggregation and cause the onset of neurodegenerative diseases in vivo.[237] The α-helical peptide conformation appears to be a crucial intermediate on the path to amyloid fibrils; however, α-helical conformations were not observed for short amyloid model peptides.[25, 104]
The mechanism behind the genesis of neurodegenerative diseases is still under investigation and it is still unclear whether the amyloid fibrils, oligomers or another (intermediate) species are responsible. Amyloid peptides likely have a native function in organisms, and some have already been recognized.[83, 87] One hypothesis is that amyloidogenic peptides are antimicrobial peptides and thus are part of the native immune system of organisms or have an evolutionary relationship with this function.[54, 260, 263] Both classes of peptides demonstrate similar membrane activity and lead to the disruption of bacterial membranes.[54] The toxicity of amyloidogenic peptides has recently been attributed to the soluble oligomers.[78, 138] Since peptides adopt an α-helical conformation on membrane surfaces, these toxic oligomers could be α-helical peptide bundles. Knowing the identity of the toxic species for amyloid-related diseases is essential to understand and interpret the impact of environmental factors on disease development and progression. Whether interfaces are harmful or beneficial to an organism depends on whether the amount of toxic species present is increased or reduced and whether and how peptide self-assembly is involved at all.
It was recently reported that the viral protein corona also affects amyloid aggregation (Figure 14).[262] Thus, amyloid-related diseases could be associated with viral infections, which has been suggested for herpes simplex virus.[264] The conclusions reported here on the effect of NP–peptide interaction strength, NP size, and membrane composition could serve as a basis to interpret the effects of viruses of similar size. This theory also supports the hypothesis that amyloid-forming peptides are functional parts of the immune system[265] or are evolutionarily related to antimicrobial peptides through mutations of charged to hydrophobic amino acids.[260] In another context, amyloid fibrils have been identified in semen that bind HIV virus particles and promote their attachment to cells (semen-derived enhancer of virus infection), providing a model for the high HIV infection rate through semen.[266] This can be used for applications where viruses need to be concentrated and delivered.[267]
The processes that contribute to amyloid fibril formation are complex, especially when studied in a physiological context. Experimental methods usually capture observables arising directly or indirectly from many single parameters in a complex environment, for example, via bound dyes, and thus primary processes are not easily accessible. Computer simulations or theoretical approaches can be used to explain such macroscopic experimental results or to predict peptide self-assembly behavior provided that the algorithms or MD force fields are well parametrized for the system under study. Simulations, however, are limited in their time scales, system sizes, or accuracy due to limited computing capacities. At present, experiments can probe much longer time scales and larger systems, while simulations are best suited to understand the primary molecular processes. Further research is needed to identify the disease-causing mechanisms and the natural function of amyloid-forming peptides in organisms so that effective treatments can be efficiently developed.
Acknowledgements
The authors acknowledge generous support from the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation, project number 189853844, SFB-TRR 102, B1, “Polymers under multiple constraints: restricted and controlled molecular order and mobility”). T.J. thanks the Friedrich-Ebert-Stiftung for a Ph.D. fellowship, and the Australian Government, Department of Education and Training, and Scope Global for the support through an Endeavour Research Fellowship. The authors also thank Dr. Anika Gladytz (Leipzig, Germany), Prof. Dr. Herre Jelger Risselada (Dortmund, Germany), and Prof. Dr. Daniel Huster (Leipzig, Germany) for discussions and cooperation on the research topic. The contents of the literature review in this article are partially based on the Ph.D. thesis “Interaction and aggregation mechanisms of peptides at biologically relevant interfaces” completed by T.J. in 2020.
Open access funding enabled and organized by Projekt DEAL.
Conflict of Interest
The authors declare no conflict of interest.
Biographies

Torsten John is a postdoc in the Department of Biological Engineering at MIT (USA). He received his Ph.D. in chemistry summa cum laude from Leipzig University (Germany), where he investigated the aggregation of peptides near nanoparticle and membrane surfaces. His current research focuses on the self-assembly of DNA nanomaterials. He was a visiting scientist at the Leibniz Institute of Surface Engineering (Germany), Monash University, the University of Queensland, and RMIT University (Australia). He was recognized as a CAS Future Leader, Young Scientist at the Lindau Nobel Laureate Meeting, and Feodor Lynen Research Fellow of the Alexander von Humboldt Foundation.

Lisa Martin studied chemistry at Monash University and received her Ph.D. from The Australian National University on the magnetism and electrochemistry of encapsulated metal ions. Postdoctoral stays in Switzerland, Germany (Alexander von Humboldt Fellowship), and the USA (Fulbright Fellowship) shifted her interest to bioinspired research. She was an academic at Flinders University before moving to Monash University in 2003. Her current research is at the interface of chemical biology and medicinal chemistry, pioneering methods for studying membrane proteins and peptides at biomimetic membranes . She is a fellow of the Royal Society of Chemistry (UK) and the Royal Australian Chemical Institute.

Bernd Abel received his Ph.D. in chemistry from the University of Goettingen (Germany). After a postdoctoral stay at MIT (USA), he became associate professor at the University of Goettingen and the MPI for Biophysical Chemistry (Germany). In 2008, Bernd Abel received a full professor position (W3) at the Wilhelm-Ostwald-Institute for Physical and Theoretical Chemistry at Leipzig University (Germany) and in 2012 became head of the Department Functional Surfaces at the Leibniz Institute of Surface Engineering. Since 2022, Bernd Abel is professor of chemical technology at Leipzig University. The Abel group focuses on materials/ methods for sensing and energy applications.