Journal list menu
Export Citations
Download PDFs
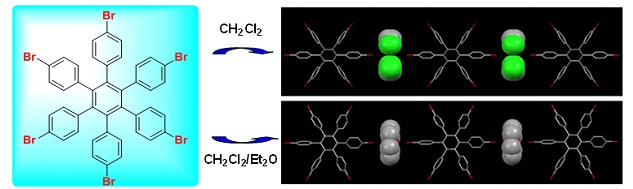
Cover Picture
Cover Picture: Bromine Bonding Induced Selective Recognition of Different Guests for Hexaphenylbenzene Bromides in the Solid State (Chin. J. Chem. 9/2015)
- Page: 993
- First Published: 17 September 2015

The cover picture shows the recognition of CH2Cl2 molecules for hexaphenylbenzene bromide HBH in solid state (Atom colors: C, gray; Br, red; Cl, green; H, white). With propeller-like shape, hexaphenylbenzene bromides display different recognition abilities for guest molecules in solid state. HBH can encapsulate CH2Cl2 or Et2O molecules selectively from different solvent systems. For DBH, the recognition pattern is dominated between themselves. In CH2Cl2/toluene/Et2O system, toluene molecule can be encapsulated selectively by MBH. In these supramolecular assemblies, bromine bonding plays an important role. More details are discussed in the article by Zhang et al. on page 1031–1036.
Contents
Review
Gold Nanoparticles for Cancer Theranostics
- Pages: 1001-1010
- First Published: 12 August 2015
Gold nanoparticles have seen unprecedented development in biomedical field, particularly for caner theranostics. This review introduces methods for preparing various shapes of gold nanoparticles and describes their current applications in the field of cancer treatment. Moreover, the review presents the development routes and current issues of gold nanoparticles in clinical theranostics.
Communications
Ruthenium Trichloride Catalyzed Highly Efficient Deoximation of Oximes to the Carbonyl Compounds and Nitriles without Acceptors
- Pages: 1011-1014
- First Published: 29 June 2015
Highly Efficient Synthesis of Arylpyrrole Derivatives via Rh(III)-Catalyzed Direct CH Arylation with Aryl Boronic Acids
- Pages: 1015-1018
- First Published: 12 August 2015
4-OH-TEMPO/TCQ/TBN/HCl: A Metal-Free Catalytic System for Aerobic Oxidation of Alcohols under Mild Conditions
- Pages: 1019-1023
- First Published: 12 August 2015
Full Papers
Synthesis of Hydroxyapatite Nanorods under Mild Conditions and Their Drug Release Properties
- Pages: 1024-1030
- First Published: 12 August 2015
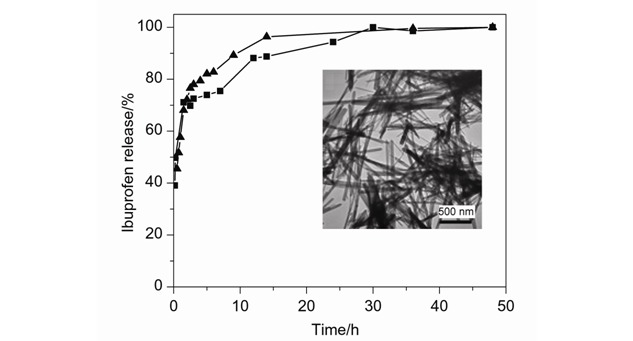
Hydroxyapatite (HAp) nanorods with high aspect ratio have been successfully synthesized via the hydrolysis of the precursor CaHPO4 at 60°C at atmospheric pressure. The as-prepared CaHPO4 sample using CTAB as surfactant is well crystallized at the first precipitation step. At the second hydrolysis step, the CTA+ and OH− act to control the formation and growth of HAp nanorods. The HAp nanorods modified with suitable surfactants are of great potential applications in drug delivery system.
Bromine Bonding Induced Selective Recognition of Different Guests for Hexaphenylbenzene Bromides in the Solid State
- Pages: 1031-1036
- First Published: 22 July 2015
Preparation and Intramolecular CC Coupling Reaction for Bis-benzimidazolium Salt
- Pages: 1037-1040
- First Published: 22 July 2015
Non-Flat Bisbenzylisoquinoline Alkaloid Fangchinoline As a Class of Potent G-Quadruplex Stabilizer with Anti-cancer Activity
- Pages: 1041-1048
- First Published: 29 June 2015
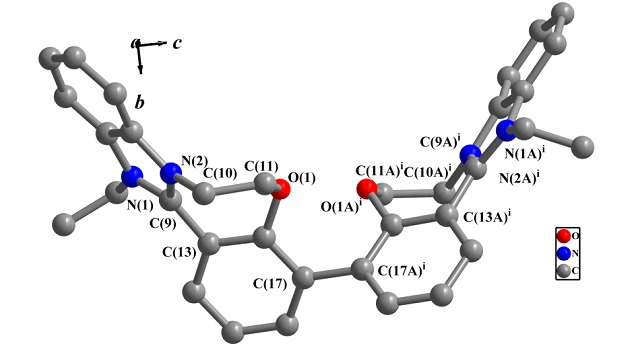
One-pot Two-Step Cycloaddition Reaction for Convenient Synthesis of Polycyclic Spirooxindole-fused [1,3]Oxazines
- Pages: 1049-1056
- First Published: 12 August 2015
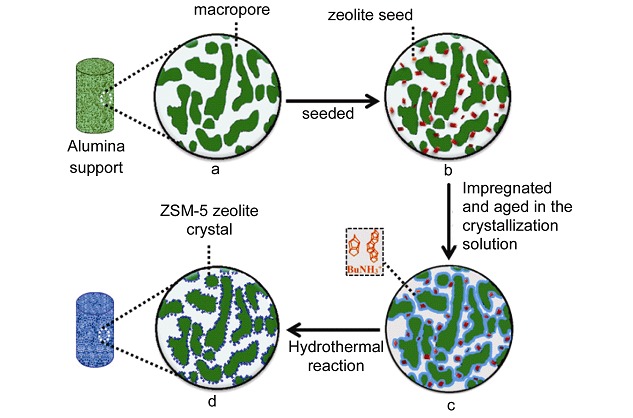
Preparation of ZSM-5 Coated on Monolithic Interconnected Macroporous Al2O3 Using Cheap n-Butylamine as Template
- Pages: 1057-1063
- First Published: 22 July 2015
A Fluorescent Probe for Hg2+ Based on Gold(I) Complex with An Aggregation-Induced Emission Feature
- Pages: 1064-1068
- First Published: 12 August 2015
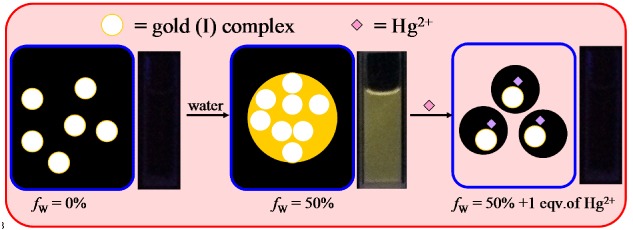
We designed and synthesized a gold(I) complex that exhibited aggregation-induced emission in acetonitrile-water mixtures. It showed high selectivity and sensitivity for Hg2+ in acetonitrile-water (1:1, V:V) solution. Dynamic light scattering measurements were conducted to verify that the addition of Hg2+ changed the particle size and induced fluorescence quenching.
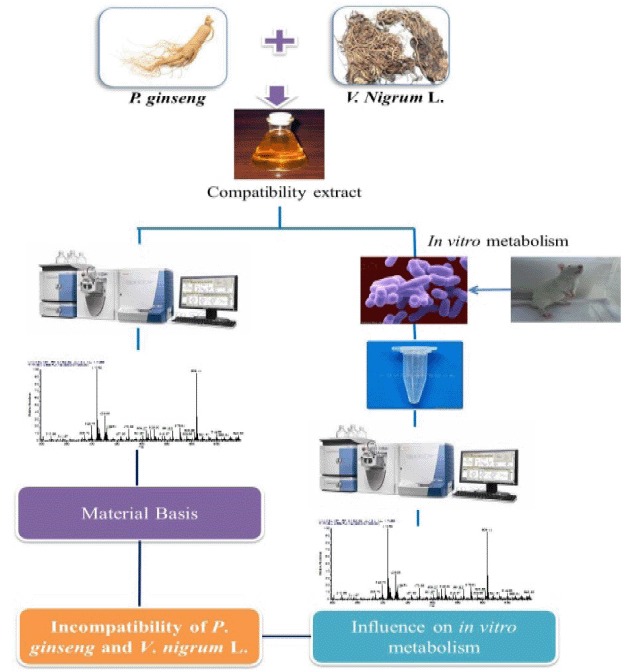
Mechanism of Incompatible Herb Pairs, Panax ginseng and Veratrum nigrum L.: Material Basis and Metabolic Profiles of Ginsenosides in Rat Intestinal Bacteria
- Pages: 1069-1076
- First Published: 12 August 2015
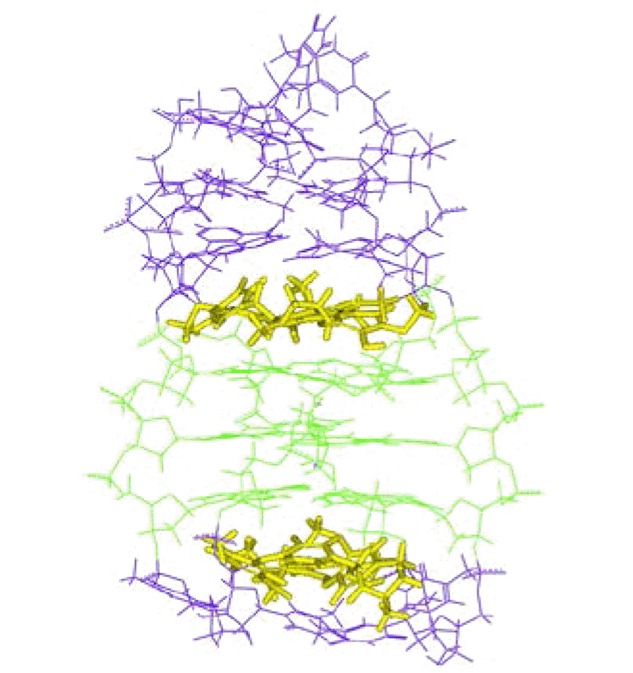
TMSCl Promoted Direct sp3 C-H Alkenylation to Construct (E)-2-Styryl-tetrahydrobenzo[d]thiazoles
- Pages: 1077-1083
- First Published: 22 July 2015
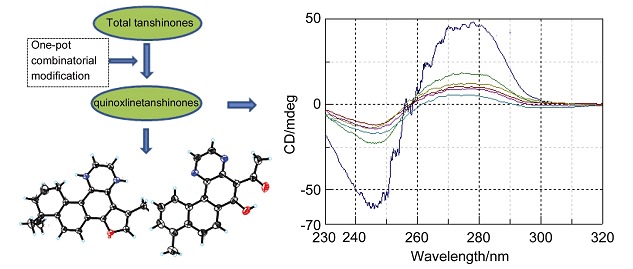
Enhancing the Structural Diversity and Bioactivity of Natural Products by Combinatorial Modification Exemplified by Total Tanshinones
- Pages: 1084-1088
- First Published: 22 July 2015
Note
Identification and Fibrinolytic Evaluation of an Isoindolone Derivative Isolated from a Rare Marine Fungus Stachybotrys longispora FG216
- Pages: 1089-1095
- First Published: 10 June 2015





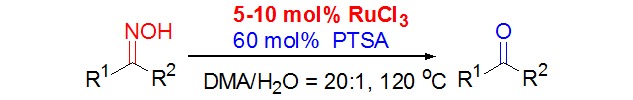
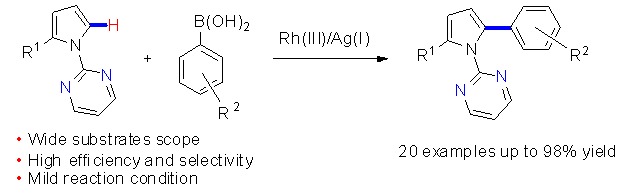

![One-pot Two-Step Cycloaddition Reaction for Convenient Synthesis of Polycyclic Spirooxindole-fused [1,3]Oxazines](/cms/asset/e9bced1f-5d1f-4682-9e0f-3998d8f0fd98/mcontent.jpg)
![TMSCl Promoted Direct sp3 C-H Alkenylation to Construct (E)-2-Styryl-tetrahydrobenzo[d]thiazoles](/cms/asset/8ac09418-41fb-459a-8e01-5c1e93b399ac/mcontent.jpg)