Info menu
Cancer Immunology
Research investigating the interface between tumor cells and the immune system arguably represent the fastest growing area of research in cancer over the past few years. For example, it is now established that cancer cells operate the immune checkpoint to “blind” T Cells to their “expansionist” intentions: essentially tricking T Cells that “see” tumor antigens presented on antigen presenting cells in the tumor’s neighborhood into “thinking” that the tumor is normal host tissue. It is no coincidence that immune checkpoint therapy is one of the most promising avenues of cancer research today. The way in which cancer evades the immune system is fundamental to understanding cancer from another important perspective: that of an abnormal cellular state in which tumor cells become ever more dissimilar from the normal host cells: a process of “cancer evolution” that some researchers liken to the emergence of a new, parasitic, “species” within the host. This clearly takes us increasingly into the realm of self/non-self discrimination, the ancient principle of all immune systems. As well as manipulating the immune system and evading immune recognition, tumor cells have a tendency to manipulate other cells in their vicinity to gain advantage: for example, perfectly “normal” fibroblasts, which become so-called cancer-associated fibroblasts (CAFs), a state that, in many respects, represents a type of symbiosis, because both cell types benefit from the “relationship”. This is a situation in which the immune system – policing the body as it does for abnormal cellular interactions – would ordinarily intervene; however, cancer cells can very effectively stop that. A more holistic view of cancer as a “cellular behavior” in which interactions with surrounding cells form a type of "ecosystem” in which competition, manipulation, parasitism, but also symbiosis, are manifest, will likely bring us closer to more effective treatments and cures for many cancers. We hope that you enjoy reading through this Special Collection of articles from the European Journal of Immunology, Advanced Biosystems, BioEssays, and WIREs Nanomedicine and Nanobiotechnology, which will take you from the latest research findings through novel insights and hypotheses that await testing.
Andrew Moore, PhD
Editor-in-Chief, BioEssays
Why the Outcome of Anti‐Tumor Immune Responses is Heterogeneous: A Novel Idea in the Context of Immunological Heterogeneity in Cancers
Abstract
The question as to why some hosts can eradicate their tumors while others succumb to tumor-progression remains unanswered. Here, a provocative concept is proposed that intrinsic differences in the T cell receptor (TCR) repertoire of individuals may influence the outcome of anti-tumor immunity by affecting the frequency and/or variety of tumor-reactive CD8 and/or CD4 tumor-infiltrating lymphocytes. This idea implicates that the TCR repertoire in a given patient might not provide sufficiently different TCR clones that can recognize tumor antigens, namely, “a hole in the TCR repertoire” might exist. This idea may provide a novel perspective to further dissect the mechanisms underlying heterogeneous anti-tumor immune responses in different hosts. Besides tumor-intrinsic heterogeneity and host microbiome, the various factors that may constantly shape the dynamic TCR repertoire are also discussed. Elucidating mechanistic differences in different individuals’ immune systems will allow to better harness immune system to design new personalized cancer immunotherapy.
Graphical Abstract
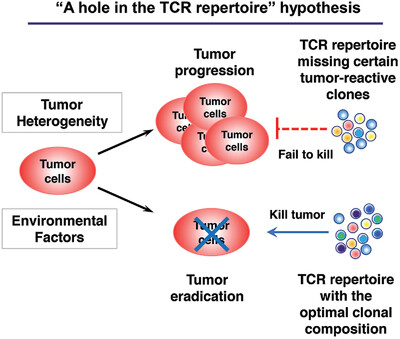
Underlying mechanisms that dictate the immunological heterogeneity in cancers remain elusive. Besides tumor-intrinsic heterogeneity and environmental factors, intrinsic differences in the T cell receptor (TCR) repertoire may play a role. “A hole in the TCR repertoire” hypothesis is proposed to explain heterogeneity of anti-tumor responses that may significantly impact cancer immunotherapy.
Targeting the tumor microenvironment and T cell metabolism for effective cancer immunotherapy
Abstract
The successful implementation of immunotherapies has provided new impetus in the fight against cancer. Antibody-mediated blockade of immune checkpoint molecules PD-1/PD-L1 and CTLA-4 has had a dramatic impact upon the treatment of previously intractable cancers such as malignant melanoma, while adoptive cell therapies using chimeric antigen receptor-bearing T cells have proven highly efficacious in B cell leukemia. Furthermore, significant progress has been made in understanding the mechanisms by which tumors evade or become resistant to these immunotherapies. In this regard, approaches to broaden the applicability and enhance the efficacy of immunotherapies increasingly include modulation of tumor and immune cell metabolism. In this mini-review, we highlight the most recent studies describing novel approaches and targets for the manipulation of the tumor microenvironment and T cell metabolism and describe how these approaches are being combined with current immunotherapies in preclinical studies.
Graphical Abstract
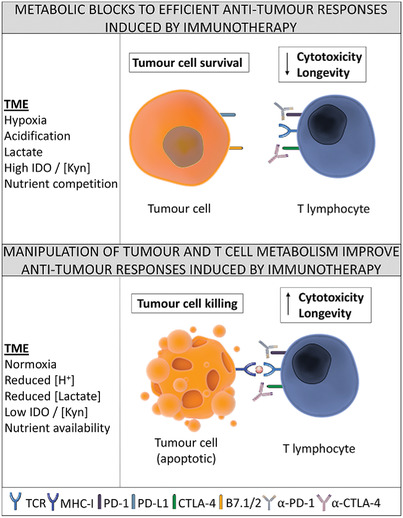
The efficacy of cancer immunotherapies, such as checkpoint inhibitors and adoptive T cell transfer, can be influenced by the presence of a nutrient-depleted and challenging tumor microenvironment, resulting in immune cell dysfunction. We discuss preclinical studies showing how targeting cancer and immune cell metabolism can improve antitumor immunotherapeutic responses.
Targeting Treg cells in cancer immunotherapy
Abstract
Foxp3-expressing regulatory T (Treg) cells, which are indispensable for preventing autoimmunity, also suppress effective tumor immunity. Treg cells abundantly infiltrate into tumor tissues, which is often associated with poor prognosis in cancer patients. Removal of Treg cells enhances anti-tumor immune responses but may also elicit autoimmunity. A key issue in devising Treg-targeting cancer immunotherapy is, therefore, how to specifically deplete Treg cells infiltrating into tumor tissues without affecting tumor-reactive effector T cells, while suppressing autoimmunity. This can be achieved by differentially controlling Treg and effector T cells by various ways. In this review, we discuss how tumor-infiltrating Foxp3+ Treg cells hamper effective anti-tumor immune responses especially in tumor tissues and how they can be specifically targeted for augmenting tumor immunity but not autoimmunity.
Graphical Abstract
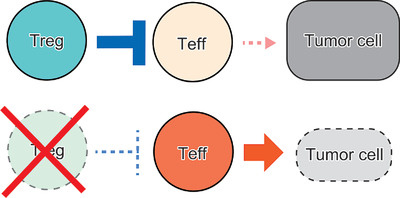
Regulatory T (Treg) cells highly infiltrate into various tumor tissues and hinder immune responses against tumor cells by effector T (Teff) cells. To effectively enhance anti-tumor immunity, it is essential to selectively remove tumor-infiltrating Treg cells while preserving tumor-reactive Teff cells.
Exposure to anti‐PD‐1 causes functional differences in tumor‐infiltrating lymphocytes in rare solid tumors
Abstract
The pervasive use of therapeutic antibodies targeting programmed cell death protein 1 (PD-1) to boost anti-tumor immunity has positioned this approach to become the standard-of-care for some solid tumor malignancies. However, little is known as to how blockade of PD-1 may alter the function or phenotype of tumor-infiltrating lymphocytes (TIL). We used our ongoing Phase II clinical trial of pembrolizumab for patients with rare solid tumors from various types (NCT02721732) with matched core biopsies taken at baseline and after initial dose of anti-PD-1 (15–21 days post-dose) to elucidate this question. One fresh core needle biopsy was used to propagate TIL ex vivo to analyze phenotype and function using flow cytometry in both CD8+ and CD4+ TIL populations. An enriched CTLA-4 expression in the CD4+ TIL population was observed in TIL expanded from the on-treatment samples compared to TIL expanded from the matched baseline (n = 22, p = 0.0007) but was not observed in patients who experienced tumor regression. Impact on functionality was evaluated by measuring secretion of 65 soluble factors by expanded TIL from 26 patients at baseline and on-treatment. The CD8+ TIL population demonstrated a diminished cytokine secretion profile post-pembrolizumab. Overall, our study assesses the ramifications of one dose of anti-PD-1 on TIL in rare solid tumor types.
Graphical Abstract
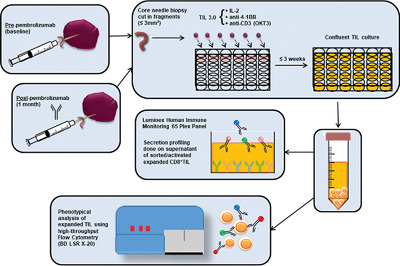
Utilizing core needle biopsies from rare tumors pre- and post-exposure to anti-PD-1, we investigate phenotypic and functional differences in expanded tumor-infiltrating lymphocytes (TIL). This research is pertinent to understanding the impact of checkpoint blockade on TIL to design improved therapeutics for rare tumors.
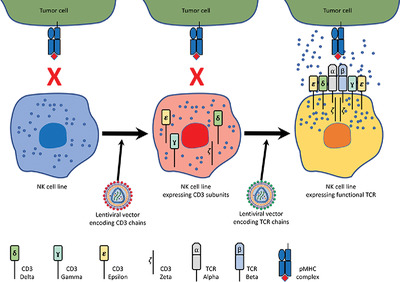
Engineering antigen‐specific NK cell lines against the melanoma‐associated antigen tyrosinase via TCR gene transfer
Abstract
Introduction of Chimeric Antigen Receptors to NK cells has so far been the main practical method for targeting NK cells to specific surface antigens. In contrast, T cell receptor (TCR) gene delivery can supply large populations of cytotoxic T-lymphocytes (CTL) targeted against intracellular antigens. However, a major barrier in the development of safe CTL-TCR therapies exists, wherein the mispairing of endogenous and genetically transferred TCR subunits leads to formation of TCRs with off-target specificity. To overcome this and enable specific intracellular antigen targeting, we have tested the use of NK cells for TCR gene transfer to human cells. Our results show that ectopic expression of TCR α/β chains, along with CD3 subunits, enables the functional expression of an antigen-specific TCR complex on NK cell lines NK-92 and YTS, demonstrated by using a TCR against the HLA-A2-restricted tyrosinase-derived melanoma epitope, Tyr368-377. Most importantly, the introduction of a TCR complex to NK cell lines enables MHC-restricted, antigen-specific killing of tumor cells both in vitro and in vivo. Targeting of NK cells via TCR gene delivery stands out as a novel tool in the field of adoptive immunotherapy which can also overcome the major hurdle of “mispairing” in TCR gene therapy.
Poly(I:C) stimulation is superior than Imiquimod to induce the antitumoral functional profile of tumor‐conditioned macrophages
Abstract
Macrophage plasticity is the ability of mononuclear phagocytes to change phenotype, function, and genetic reprogramming upon encounter of specific local stimuli. In the tumor microenvironment, Tumor-Associated Macrophages (TAMs) acquire an immune-suppressive and tumor-promoting phenotype. With the aim to re-educate TAMs to antitumor effectors, in this study, we used two immunestimulatory compounds: the TLR7 agonist Imiquimod (IMQ) and the TLR3 agonist Poly(I:C). To better mimic in vitro the response of TAMs, we used Tumor-Conditioned Macrophages (TC-Mϕ) differentiated in the presence of tumor cell supernatants. Our results show that TC-Mϕ respond differently from conventional M2-polarized macrophages. Upon stimulation with IMQ, TC-Mϕ did not upregulate major histocompatibility complex (MHC II) molecules and unexpectedly expressed increased CD206. With both compounds, TC-Mϕ produced higher levels of inflammatory cytokines than M2 macrophages. IMQ and Poly(I:C) differed in the types of regulated genes and secreted mediators. Reflecting their signaling pathways, only IMQ significantly induced IL-1β and IL-6, while only Poly(I:C) stimulated CXCL10, and both upregulated CCL5. Of note, using a novel cytotoxicity assay, Poly(I:C), but not IMQ, was effective in triggering the cytotoxic activity of TC-Mϕ against cancer cells. Overall, the results demonstrate that Poly(I:C) stimulation of TC-Mϕ is superior than IMQ in terms of macrophage re-education toward antitumor effectors.
Graphical Abstract
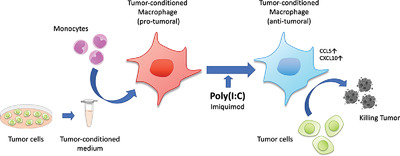
TC-Mϕ displays a protumoral phenotype. Treatment with the toll-like receptor (TLR)-agonist Poly(I:C) effectively repolarized their functional profile into antitumor cytotoxic macrophages. Another TLR-agonist (Imiquimod) was much less effective. The results designate Poly(I:C) as a promising drug for immune-stimulation therapies directed against TAMs.
ER‐aminopeptidase 1 determines the processing and presentation of an immunotherapy‐relevant melanoma epitope
Abstract
Dissecting the different steps of the processing and presentation of tumor-associated antigens is a key aspect of immunotherapies enabling to tackle the immune response evasion attempts of cancer cells. The immunodominant glycoprotein gp100209-217 epitope, which is liberated from the melanoma differentiation antigen gp100PMEL17, is part of immunotherapy trials. By analyzing different human melanoma cell lines, we here demonstrate that a pool of N-terminal extended peptides sharing the common minimal epitope is generated by melanoma proteasome subtypes. In vitro and in cellulo experiments indicate that ER-resident aminopeptidase 1 (ERAP1)—but not ERAP2—defines the processing of this peptide pool thereby modulating the T-cell recognition of melanoma cells. By combining the outcomes of our studies and others, we can sketch the complex processing and endogenous presentation pathway of the gp100209-217-containing epitope/peptides, which are produced by proteasomes and are translocated to the vesicular compartment through different pathways, where the precursor peptides that reach the endoplasmic reticulum are further processed by ERAP1. The latter step enhances the activation of epitope-specific T lymphocytes, which might be a target to improve the efficiency of anti-melanoma immunotherapy.
Graphical Abstract
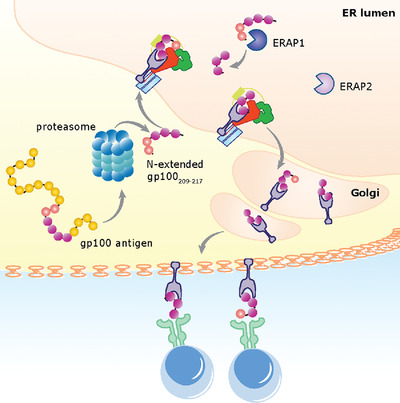
The gp100209-217 CD8+ T cell epitope derived from melanoma antigen gp100PMEL17 is a potential target in immunotherapy. This study demonstrates that melanoma proteasomes generate only N-terminal extended epitope precursors, whereas the minimal gp100209-217 epitope is exclusively generated by ER-aminopeptidase 1 (ERAP1), suggesting strategies enhancing ERAP1 activity could improve epitope-specific immunotherapies.
The multifaceted Foxp3fgfp allele enhances spontaneous and therapeutic immune surveillance of cancer in mice
Abstract
It is well established that therapeutic impairment of Foxp3+ Treg in mice and humans favors immune rejection of solid tumors. Less explored is the impact Foxp3 allelic variants may have on tumor incidence, progression and therapy. In this work, we tested and demonstrate that the Foxp3fgfp reporter allele, found previously to either enhance or reduce Treg function in specific autoimmunity settings, confers increased anti-tumor immunity. Our conclusions stem out of the analysis of three tumor models of different tissue origin, in two murine genetic backgrounds. When compared to wild type animals, mice carrying the Foxp3fgfp allele spontaneously delay, reduce or prevent primary tumor growth, decrease metastasis growth, and potentiate the response to anti-CTLA4 monotherapy. These findings suggest allelic variances at the Foxp3 locus may serve as predictive indicators for personalized therapy and prognostics, and point at possible new therapeutic targets.
Graphical Abstract
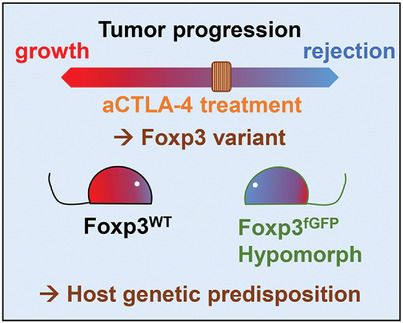
Genetic alterations at the Foxp3 locus are most commonly associated with autoimmunity and inflammatory manifestations. In this work we evidence that a Foxp3 variant known to affect regulatory T cell functions acts as an hypomorph in a tumor context and enhances the effectiveness of anti-CTLA4 immunotherapy.
Liposomal Delivery Enhances Immune Activation by STING Agonists for Cancer Immunotherapy
Abstract
Overcoming the immunosuppressive tumor microenvironment (TME) is critical to realizing the potential of cancer immunotherapy strategies. Agonists of stimulator of interferon genes (STING), a cytosolic immune adaptor protein, have been shown to induce potent antitumor activity when delivered into the TME. However, the anionic properties of STING agonists make them poorly membrane permeable, and limit their ability to engage STING in the cytosol of responding cells. In this study, cationic liposomes with varying surface polyethylene glycol levels are used to encapsulate 2′3′ cyclic guanosine monophosphate-adenosine monophosphate (cGAMP) to facilitate its cytosolic delivery. In vitro studies with antigen-presenting cells (APCs) revealed that liposomal formulations substantially improve the cellular uptake of cGAMP and proinflammatory gene induction relative to free drug. Liposomal encapsulation allows cGAMP delivery to metastatic melanoma tumors in the lung, leading to antitumor activity, whereas free drug produces no effect at the same dose. Injection of liposomal cGAMP into orthotopic melanoma tumors shows retention of cGAMP at the tumor site and colocalization with tumor-associated APCs. Liposomal delivery induces regression of injected tumors and produces immunological memory that protects previously treated mice from rechallenge with tumor cells. These results show that liposomal delivery improves STING agonist activity, and could improve their utility in clinical oncology.
Graphical Abstract
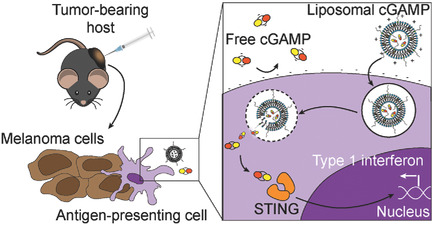
Immune activation is enhanced by stimulator of interferon genes (STING) agonist delivery via liposomes. Cationic liposomes show an improved ability to stimulate the STING pathway in immune cells in vitro relative to free drug. Liposomes allow increased activity of the drug in metastatic melanoma in the lungs and enhance immune memory formation when injected into skin melanoma tumors.
Improving Cancer Vaccine Efficiency by Nanomedicine
Abstract
Cancer vaccines, which have been widely investigated in the past few decades, are one of the most attractive strategies for cancer immunotherapy. Through the precise delivery of antigens and adjuvants to lymphoid organs or lymphocytes via nanotechnology, innate and adaptive immunity can be boosted to prevent the growth and relapse of malignant tumors. Indeed, nanomedicine offers great opportunities to improve the efficiency of vaccines. Various functional platforms are used to deliver small molecules, peptides, nucleic acids, and even whole cell antigens to the target area of interest, achieving enhanced antitumor immunity and durable therapeutic benefits. Herein, the recent progress in cancer vaccines based on nanotechnology is summarized. Novel platforms used for delivering tumor antigens, promoting adjuvant functions, and combining other therapeutic strategies are discussed. Moreover, possible striving directions and major challenges of nanomedicine for vaccination are also reviewed.
Graphical Abstract
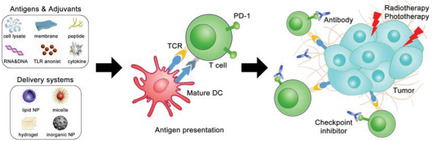
Cancer vaccines are broadly investigated to initiate robust immune responses for cancer immunotherapy. Immune stimulators delivered by nanotechnology can further enhance vaccination efficacy. Herein, recent studies for improving cancer vaccine efficiency by nanomedicine are reviewed. Novel vaccine vehicles used for promoting antigen and adjuvant functions as well as for combination therapy are throughly discussed.
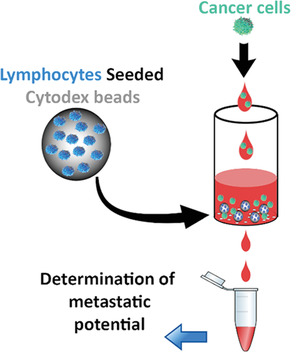
Development of 3D Lymph Node Mimetic for Studying Prostate Cancer Metastasis
Abstract
Lymph node (LN) metastasis causes poor prognosis for patients with prostate cancer (PCa). Although LN-cells and cellular responses play a pivotal role in cancer metastasis, the interplay between LN-cells and PCa cells is undetermined due to the small size and widespread distribution of LNs. To identify factors responsible for LN metastasis, a 3D cell culture biosystem is fabricated to simulate LN responses during metastasis. First, it is determined that LN explants previously exposed to high metastatic PCa release substantially more chemotactic factors to promote metastatic PCa migration than those exposed to low-metastatic PCa. Furthermore, T-lymphocytes are found to produce chemotactic factors in LNs, among which, CXCL12, CCL21, and IL-10 are identified to have the most chemotactic effect. To mimic the LN microenvironment, Cytodex beads are seeded with T cells to produce a LN-mimetic biosystem in both static and flow conditions. As expected, the flow condition permits prolonged cellular responses. Interestingly, when PCa cells with varying metastatic potentials are introduced into the system, it produces PCa-specific chemokines accordingly. These results support that the LN mimetic helps in analyzing the processes underlying metastasized LNs and for testing various treatments to reduce cancer LN metastasis.
Progress in Tumor‐Associated Macrophages: From Bench to Bedside
Abstract
Tumor-associated macrophages (TAMs) are of great interest in cancer immunology as they play an important role in the tumor microenvironment as cancer stromal cells recruited from circulating monocytes. TAMs are closely associated with tumor progression, including initiation, trophic growth, metabolism, angiogenesis, and metastasis; moreover, in clinical practice, their quantity can be related to poor prognosis. Fundamental and translational studies imply that TAMs are one of the most promising targets in tumor therapy. Herein, the biological origination and classification of TAMs, which correspond to their functions and differentiations, are reviewed in detail. In addition, recent basic research and clinical preprocess of TAMs in tumor immunotherapy are also discussed. Finally, the advances in the use of nanotechnology and TAMs for tumor therapy are discussed. This review focuses on the background and status of basic research and clinical significance of TAMs, points out the potential of TAMs in tumor immunological therapy, and clarifies the possibility of translation TAM-targeting therapies in medicine.
Graphical Abstract
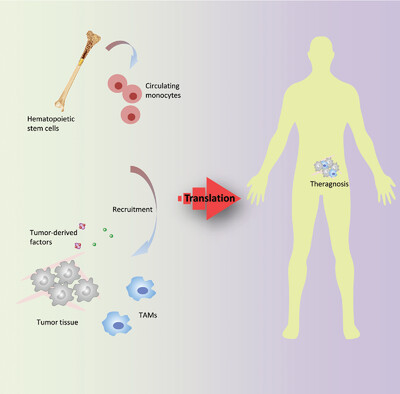
Owing to their contribution to tumor progression, microenvironment regulation, and prognosis, targeting macrophages is a promising strategy in clinical cancer treatment. Much effort has been devoted to elucidate the mechanisms and outcomes behind macrophage-targeting therapeutics. Herein, the background and progresses of tumor-associated macrophages, which present a perspective of macrophage-targeting therapeutics, are reviewed.
Biomimetic Nanoparticle Vaccines for Cancer Therapy
Abstract
It is currently understood that in order for a tumor to successfully grow, it must evolve means of evading immune surveillance. In the past several decades, researchers have leveraged increases in the knowledge of tumor immunology to develop therapies capable of augmenting endogenous immunity and eliciting strong antitumor responses. In particular, the goal of anticancer vaccination is to train the immune system to properly utilize its own resources in the fight against cancer. Although attractive in principle, there are currently only limited examples of anticancer vaccines that are successfully translated to the clinic. Recently, there has been a significant push toward the use of nanotechnology for designing vaccine candidates that exhibit enhanced potency and specificity. Here, the recent developments in the field of anticancer nanovaccines are discussed. By taking advantage of the flexibility offered by nanomedicine to purposefully program immune responses, this new generation of vaccines has the potential to address many of the hurdles facing traditional platforms. A specific emphasis is placed on the emergence of cell membrane–coated nanoparticles, a novel biomimetic platform that can be used to generate personalized nanovaccines that elicit strong, multiantigenic antitumor responses.
Graphical Abstract
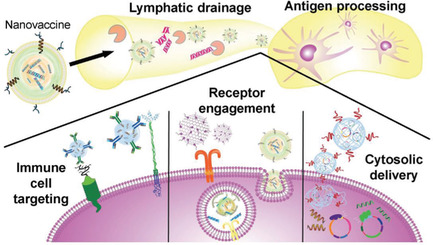
Vaccination represents an attractive approach for the treatment of cancer. By leveraging nanotechnology, it is possible to design nanovaccines with improved potency, specificity, and durability. The recent emergence of cell membrane coating technology has enabled the facile synthesis of biomimetic nanoparticles with enhanced function and antigenicity, potentially paving the way for the future creation of effective and personalized anticancer vaccines.
Cas9 Ribonucleoprotein Delivery via Microfluidic Cell‐Deformation Chip for Human T‐Cell Genome Editing and Immunotherapy
Graphical Abstract
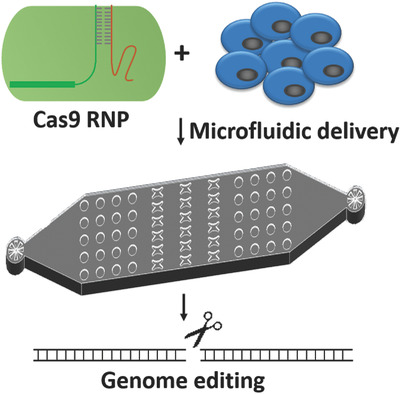
This study reports a microfluidic cell deformation-based method to deliver the Cas9 ribonucleoprotein (RNP) complexes to different cell types for efficient genome editing, including hard-to-transfect human primary CD4+ T cells. The RNP based CRISPR-Cas9 system has great advantage in shortening reaction time and reducing off-target problems, which holds great potential in future gene therapy applications.
Mesoporous Caged‐γ‐AlOOH‐Double‐Stranded RNA Analog Complexes for Cancer Immunotherapy
Abstract
Alum exhibits the superior biocompatibility in the history of human healthcare, as the only FDA-approved inorganic adjuvant in most human vaccines. However, up to now, there are few reports about the in-depth research between its nanostructure and carrier properties in vitro and in vivo. In this study, the caged architecture of uniform mesoporous γ-AlOOH nanorods (MANRs) comprises realigned pores and open windows at the edges, 1D tubular tunnels, and oxyhydroxide layer that can serve as carriers for cancer immunotherapy. Compared with commercial alum, the MANRs exhibit a higher loading amount of a model cancer antigen and a stronger intracellular uptake by THP-1-differentiated macrophage-like cells in vitro. Compared with commercial alum and soluble ovalbumin, the MANRs loaded with a model cancer antigen, ovalbumi, and an immunopotentiator, double-stranded RNA analog poly(I:C) markedly increase the anticancer immunity and CD4+ and CD8+ T cell populations in mouse splenocytes in vivo. The MANRs are promising as an adjuvant substance in cancer immunotherapy for clinical use.
Graphical Abstract
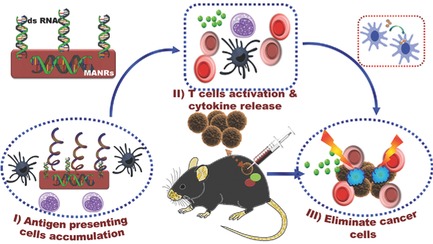
The caged architecture of uniform mesoporous γ-AlOOH nanorods is successfully fabricated as carriers for cancer immunotherapy. Compared with commercial alum, the MANRs exhibit a high loading amount of a model cancer antigen, a strong intracellular uptake by antigen presenting cells, and a remarkable increase in the anticancer immunity and CD4+ and CD8+ T-cell populations in mouse splenocytes in vivo.
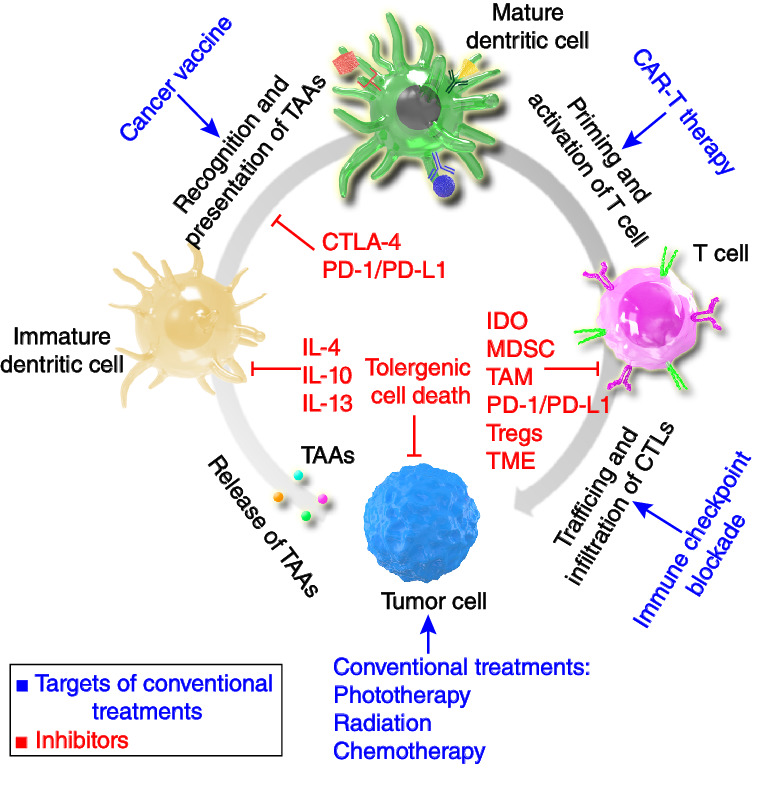
Understanding Immune Tolerance of Cancer: Re‐Purposing Insights from Fetal Allografts and Microbes
Abstract
Cancer cells seem to exploit mechanisms that evolve as part of physiological tolerance, which is a complementary and often beneficial form of defense. The study of physiological systems of tolerance can therefore provide insights into the development of a state of host tolerance of cancer, and how to break it. Analysis of these models has the potential to improve our understanding of existing immunological therapeutic targets, and help to identify future targets and rational therapeutic combinations. The treatment of cancer with immune checkpoint inhibitors aims to reverse the progression to tolerance of cancer, and achieve an immunogenic, rather than tolerogenic, homeostasis. Broadening the efficacy and durability of checkpoint inhibitors focuses on reversing tolerance and stimulating immunogenicity in the cancer, host, and environment. Two examples of important physiological states of tolerance that may inform tolerance of cancer are microbial infection and placental reproduction. These states of tolerance result from bilateral shaping of host and non-self, akin to immunoediting in cancer, and offer reliable models to study the immune tolerance paradigm.
Graphical Abstract
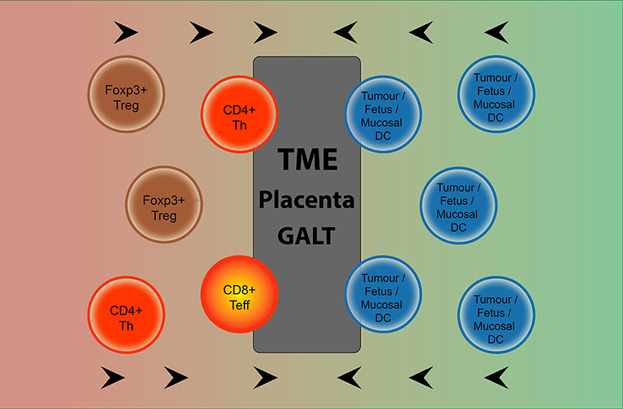
Tolerance of cancer results from repurposing of physiological tolerance that has evolved under positive selective pressure. Two examples of physiological states dependent on immune tolerance are placental reproduction and the gut microbiome. Analysis of physiological tolerance can provide insight into tolerance of cancer, and how to break it.
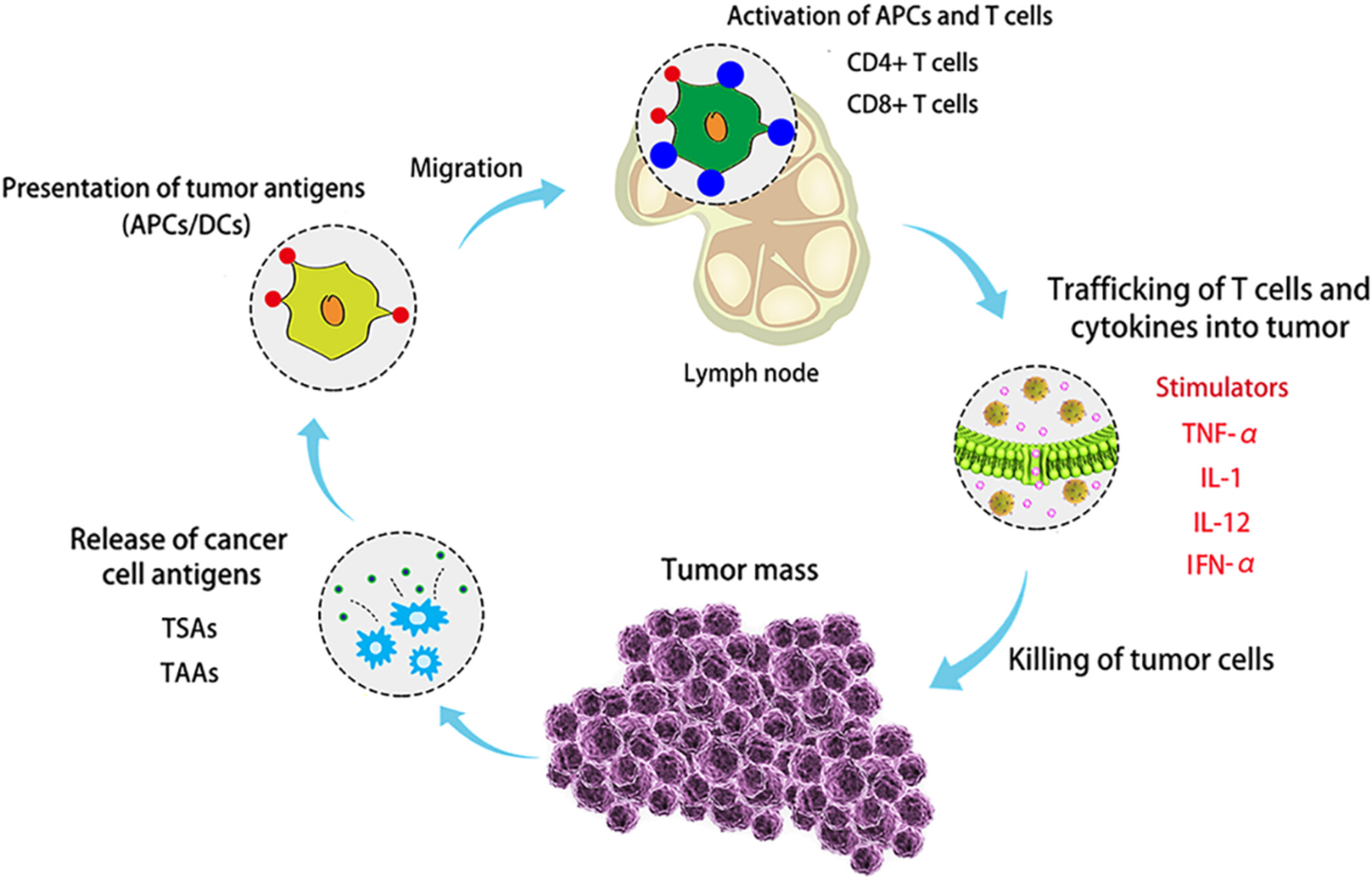
Missed Druggable Cancer Hallmark: Cancer–Stroma Symbiotic Crosstalk as Paradigm and Hypothesis for Cancer Therapy
Abstract
During tumor evolution, cancer cells use the tumor-stroma crosstalk to reorganize the microenvironment for maximum robustness of the tumor. The success of immune checkpoint therapy foretells a new cancer therapy paradigm: an effective cancer treatment should not aim to influence the individual components of super complex intracellular interactomes (molecular targeting), but try to disrupt the intercellular interactions between cancer and stromal cells, thus breaking the tumor as a whole. Arguments are provided in favor of a hypothesis that such interactions include formation of synapse-like structures (interfaces) where the interacting cells are located at a distance of ∼10–15 nm. Within these interfaces, molecules initiating and strengthening the interaction are organized, and allow optimum cross-signaling; a very confined intercellular space facilitates the concentration of secreted cytokines, enhancing the paracrine cross-communication. These features of tumors form a druggable cancer hallmark the tumor-stroma symbiotic crosstalk—which represents a new target for efficient cancer drug discovery.
Graphical Abstract
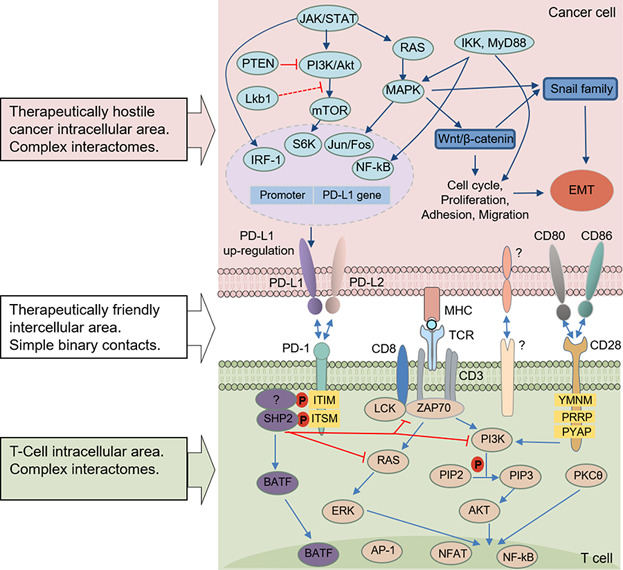
Immune checkpoint therapy creates a new cancer treatment paradigm. Effective treatment should not aim at intracellular interactome components, but instead at intercellular interactions between cancer and stromal cells, breaking the tumor as a whole. Within these interfaces, synapse-like structures facilitate paracrine symbiotic crosstalk, a cancer hallmark, promising new cancer drug discovery.
Surveillance of Retroelement Expression and Nucleic‐Acid Immunity by Histone Methyltransferase SETDB1
Abstract
In human cancers, histone methyltransferase SETDB1 (SET domain, bifurcated 1) is frequently overexpressed but its significance in carcinogenesis remains elusive. A recent study shows that SETDB1 downregulation induces de-repression of retroelements and innate immunity in cancer cells. The possibility of SETDB1 functioning as a surveillant of retroelement expression is discussed in this study: the cytoplasmic presence of retroelement-derived nucleic acids (RdNAs) drives SETDB1 into the nucleus by the RNA-interference route, rendering the corresponding retroelement transcriptionally inert. These RdNAs could, therefore, be signals of genome instability sent out for SETDB1 present in the cytoplasm to maintain genome integrity.
Graphical Abstract
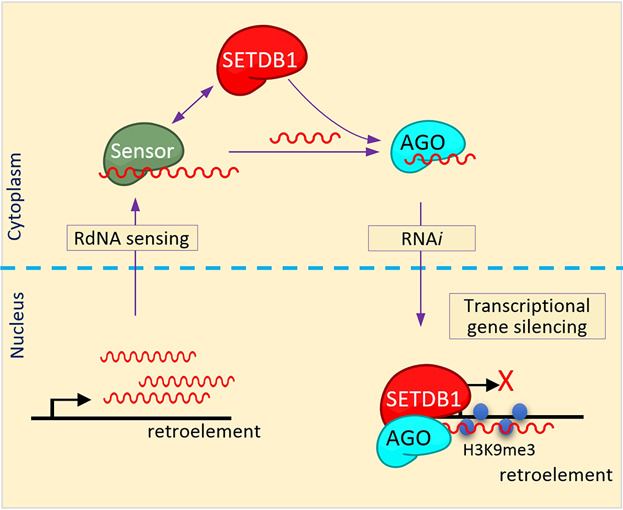
The cytoplasmic presence of retroelement-derived nucleic acids (RdNAs) hypothetically drives SETDB1 into the nucleus by the RNAi route, rendering the corresponding retroelement transcriptionally inert. This scenario of negative feedback regulation of retroelements by SETDB1 exploiting the RNAi-TGS mechanism can be an example of an inter-compartmental, trans-functional RNAi phenomenon.
Cancer: Towards a general theory of the target
Abstract
General theories (GT) are reductionist explications of apparently independent facts. Here, in reviewing the literature, I develop a GT to simplify the cluttered landscape of cancer therapy targets by revealing they cluster parsimoniously according to only a few underlying principles. The first principle is that targets can be only exploited by either or both of two fundamentally different approaches: causality-inhibition, and ‘acausal’ recognition of some marker or signature. Nonetheless, each approach must achieve both of two separate goals, efficacy (reduction in cancer burden) and selectivity (sparing of normal cells); if the mechanisms are known, this provides a definition of rational treatment. The second principle is target fragmentation, whereby the target may perform up to three categoric functions (cytoreduction, modulation, cytoprotection), potentially mediated by physically different target molecules, even on different cell types, or circulating freely. This GT remains incomplete until the minimal requirements for cure, or alternatively, proof that cure is impossible, become predictable.
Graphical Abstract
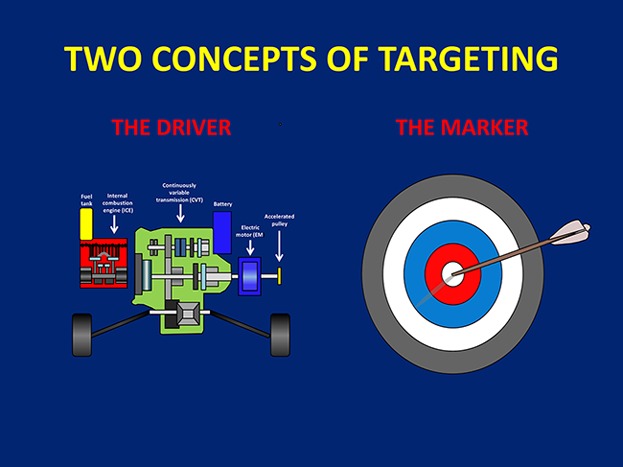
A reductionist General Theory of Cancer Targets is presented, proposing that all good targets are based either on causality-inhibition, or recognition; essential tasks (efficacy and selectivity) emerge differently in each case. Sophisticated hybrids may provide additional benefit. General theory analysis, including target fragmentation concepts, provides deeper insights into therapeutic mechanisms.
Nanovaccines for cancer immunotherapy
Abstract
The past few decades have witnessed the booming field of cancer immunotherapy. Cancer therapeutic vaccines, either alone or in combination with other immunotherapies such as adoptive cell therapy or immune checkpoint blockade therapy, are an attractive class of cancer immunotherapeutics. However, cancer vaccines have thus far shown suboptimal efficacy in the clinic. Nanomedicines offer unique opportunities to improve the efficacy of these vaccines. A variety of nanoplatforms have been investigated to deliver molecular or cellular or subcellular vaccines to target lymphoid tissues and cells, thereby promoting the potency and durability of anti-tumor immunity while reducing adverse side effects. In this article, we reviewed the key parameters and features of nanovaccines for cancer immunotherapy; we highlighted recent advances in the development of cancer nanovaccines based on synthetic nanocarriers, biogenic nanocarriers, as well as semi-biogenic nanocarriers; and we summarized newly emerging types of nanovaccines, such as those based on stimulator of interferon genes agonists, cancer neoantigens, mRNA vaccines, as well as artificial antigen-presenting cells.
This article is categorized under:
- Therapeutic Approaches and Drug Discovery > Nanomedicine for Oncologic Disease
Graphical Abstract
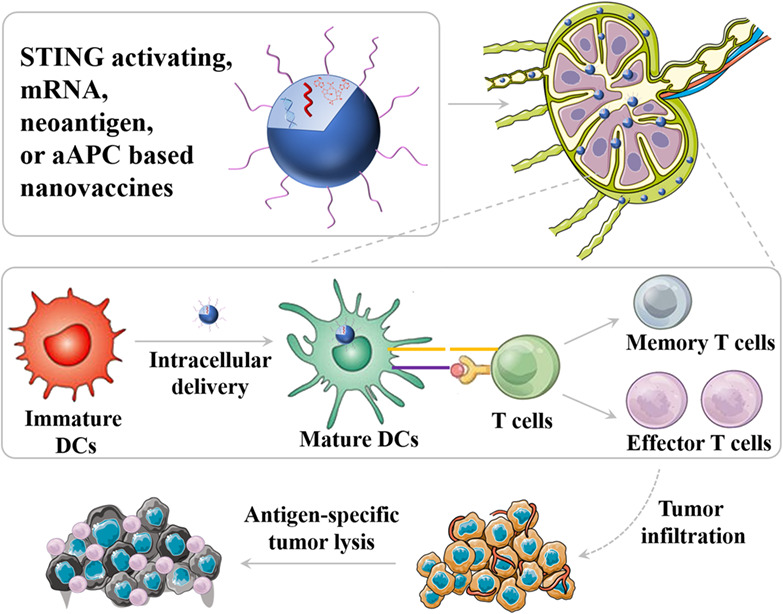
Nanovaccines not only can enable efficient tissue and cell delivery of vaccines but also permit co-delivery of antigens and adjuvants. And more importantly, nanovaccines allow multivalent antigens and/or adjuvants to potentiate immunomodulation. Nanotechnology has the potential to significantly improve the therapeutic efficacy compared with traditional formulations and alter the landscape of cancer therapeutic vaccines.
Nanotechnology platforms for cancer immunotherapy
Abstract
Various cancer therapies have advanced remarkably over the past decade. Unlike the direct therapeutic targeting of tumor cells, cancer immunotherapy is a new strategy that boosts the host's immune system to detect specific cancer cells for efficient elimination. Nanoparticles incorporating immunomodulatory agents can activate immune cells and modulate the tumor microenvironment to enhance antitumor immunity. Such nanoparticle-based cancer immunotherapies have received considerable attention and have been extensively studied in recent years. This review thus focuses on nanoparticle-based platforms (especially naturally derived nanoparticles and synthetic nanoparticles) utilized in recent advances; summarizes delivery systems that incorporate various immune-modulating agents, including peptides and nucleic acids, immune checkpoint inhibitors, and other small immunostimulating agents; and introduces combinational cancer immunotherapy with nanoparticles, especially nanoparticle-based photo-immunotherapy and nanoparticle-based chemo-immunotherapy. Undoubtedly, the recent studies introduced in this review prove that nanoparticle-incorporated cancer immunotherapy is a highly promising treatment modality for patients with cancer. Nonetheless further research is needed to solve safety concerns and improve efficacy of nanoplatform-based cancer immunotherapy for future clinical application.
This article is categorized under:
- Therapeutic Approaches and Drug Discovery > Nanomedicine for Oncologic Disease
Regulation of cancer‐immunity cycle and tumor microenvironment by nanobiomaterials to enhance tumor immunotherapy
Abstract
In the past decade, we have witnessed the revolution in cancer therapy, especially in the rapid development of cancer immunotherapy. In particular, the introduction of nanomedicine has achieved great improvement in breaking the limitations of and immunological tolerance caused by clinic-approved immunotherapies (cancer vaccine, CAR-T, and immune checkpoint blockade) to enhance immunogenicity, antigen presentation and T lymphocyte infiltration for eradicating the primary tumors and distant metastases simultaneously. However, some fundamental but significant issues still need to be thoroughly clarified before the combination of nanomedicine and immunotherapy moves toward clinical translation such as biological safety and synergistic mechanisms of nanomaterials in the systematic immune responses. Therefore, in this review, the role of nanomaterials in cancer immunotherapy is summarized, mainly focusing on the effective activation and long-term stimulation of both the innate and the adaptive immune responses and regulation of or remodeling the tumor microenvironment, especially the tumor immunosuppressive microenvironment. Also, we elaborate on the targets and challenges of nanomaterials in the cancer-immunity cycle, summarize several main strategies to convert the cold tumor immune microenvironment to the hot one, and illustrate the progress in regulation of tumor immune microenvironment by targeting specific immunosuppressive cells. Finally, we prospect the nano-combined immunotherapy strategies in tumor-targeting, normalization of tumor immune environment and modification of macrophages.
This article is characterized under:
Therapeutic Approaches and Drug Discovery > Emerging Technologies
Diagnostic Tools > In Vivo Nanodiagnostics and Imaging
Nanotechnology Approaches to Biology > Nanoscale Systems in Biology
Therapeutic Approaches and Drug Discovery > Nanomedicine for Oncologic Disease
Applications of molecular engineering in T‐cell‐based immunotherapies
Abstract
Harnessing an individual's immune cells to mediate antitumor and antiviral responses is a life-saving option for some patients with otherwise intractable forms of cancer and infectious disease. In particular, T-cell-based engineered immune cells are a powerful new class of therapeutics with remarkable efficacy. Clinical experience has helped to define some of the major challenges for reliable, safe, and effective deployment of T-cells against a broad range of diseases. While poised to revolutionize immunotherapy, scalable manufacturing, safety, specificity, and the development of resistance are potential roadblocks in their widespread usage. The development of molecular engineering tools to allow for the direct or indirect engineering of T-cells to enable one to troubleshoot delivery issues, amplify immunomodulatory effects, integrate the synergistic effects of different molecules, and home to the target cells in vivo. In this review, we will analyze thus-far developed cell- and material-based tools for enhancing T-cell therapies, including methods to improve safety and specificity, enhancing efficacy, and overcoming limitations in scalable manufacturing. We summarize the potential of T-cells as immune modulating therapies and the potential future directions for enabling their adoption for a broad range of diseases.
This article is categorized under:
- Nanotechnology Approaches to Biology > Nanoscale Systems in Biology
- Therapeutic Approaches and Drug Discovery > Nanomedicine for Oncologic Disease
- Nanotechnology Approaches to Biology > Cells at the Nanoscale
Graphical Abstract
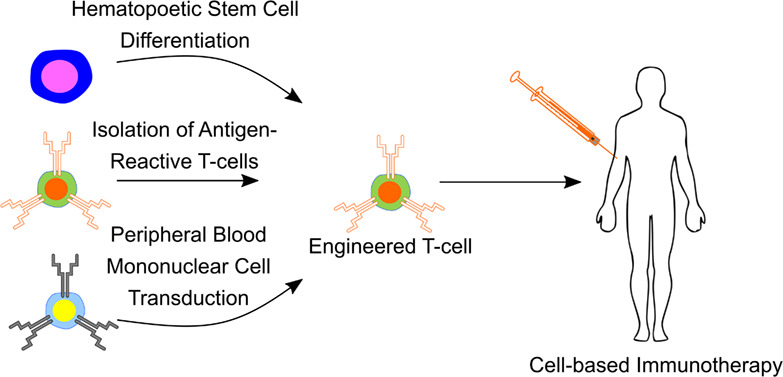
T-cell-based immunotherapies are manufactured from patient- or donor-isolated T-cells or hematopoietic stem and progenitor cells. These cells are subsequently modified using molecular engineering techniques to confer antigen-specific effector function and infused into patients as a cell-based therapy.
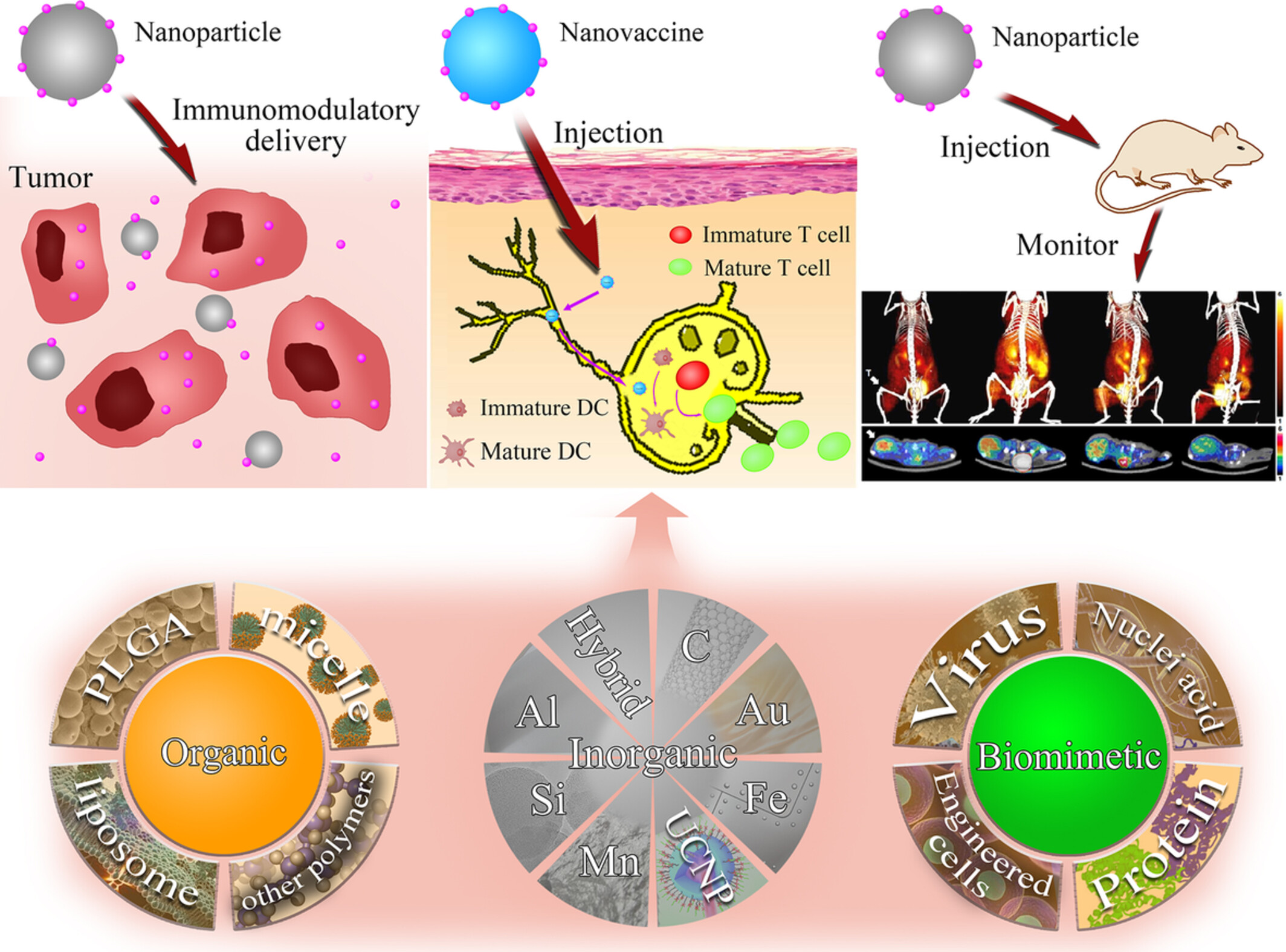
Advances of functional nanomaterials for cancer immunotherapeutic applications
Abstract
Immunotherapy has made great progress by modulating the body's own immune system to fight against cancer cells. However, the low response rates of related drugs limit the development of immunotherapy strategies. Fortunately, the advantages of nanotechnology can just make up for this shortcoming. Nanocarriers of diverse systems are utilized to co-deliver antigens and adjuvants, combined with drugs for immunomodulatory, such as chemotherapy, radiotherapy, and photodynamic. Here we review recent studies on immunotherapy with biomimetic, organic, and inorganic nanomaterials. They are going to potentially overcome the drawbacks in cancer immunotherapy with delivering immunomodulatory drugs, delivering cancer vaccine, and monitoring the immune systems.
This article is characterized under:
- Therapeutic Approaches and Drug Discovery > Nanomedicine for Oncologic Disease