Cas9 Ribonucleoprotein Delivery via Microfluidic Cell-Deformation Chip for Human T-Cell Genome Editing and Immunotherapy
Graphical Abstract
This study reports a microfluidic cell deformation-based method to deliver the Cas9 ribonucleoprotein (RNP) complexes to different cell types for efficient genome editing, including hard-to-transfect human primary CD4+ T cells. The RNP based CRISPR-Cas9 system has great advantage in shortening reaction time and reducing off-target problems, which holds great potential in future gene therapy applications.
The clustered regularly interspaced short palindromic repeats (CRISPR)-Cas9 bacterial immunity system has been discovered and engineered for use as an efficient genome editing tool in a range of organisms and applications.1 In the two-component system, a single-guide RNA (sgRNA) directs the Cas9 nuclease to generate site-specific double-strand breaks (DSBs) for targeted gene inactivation or modification.[1] The ability to perturb and edit the genome in a precise and targeted manner is crucial to understand genetic contributions to biology and disease.[1, 2] The system has been used to correct disease-causing mutations in a flexible and affordable manner and enables new avenues of gene therapy in human therapeutics.2, 3
T-cell genome editing holds great promise for immunotherapies for cancer, human immunodeficiency virus (HIV), primary immune deficiencies, and autoimmune diseases, but genetic manipulation of human T cells with high efficiency has been challenging. The CRISPR-Cas9 technology facilitates genome editing in many cell types, but its efficiency has been limited in difficult-to-transfect cells such as primary human T cells. Delivery poses a great challenge when utilizing a CRISPR-Cas9 based gene editing strategy, especially in human primary T cells.4 Hence, improved tools are highly needed to efficiently target and edit genes to modulate T-cell function and correct disease-associated mutations.
The appropriate alternative of delivery system is critical to guarantee efficient genome editing in the targeted cells or organisms. Cas9 and sgRNA can be encoded within the plasmid DNA of viral or nonviral vectors for delivery into cells. However, plasmid-mediated delivery can result in the uncontrolled integration of the DNA sequence into the host genome and unwanted immune responses.5 Furthermore, the prolonged and excess expression of Cas9 and sgRNA from plasmid DNA can worsen off-target cleavage effects.6 Using the Cas9/sgRNA ribonucleoprotein (RNP) complex for delivery provides an alternative means of reducing off-target effects and avoiding unwanted plasmid integration. As well, the electroporation of Cas9 RNPs has been achieved for efficient genome editing in different cell types.7 However, this method can induce cell damage with a high impact on cell viability. Mediators, such as lipid nanoparticles,8 cell-penetrating peptides,9 and self-assembled DNA nanoclews,10 have been reported to assist in the delivery of Cas9 and sgRNA for gene disruption. In these methods, the delivery efficiency is often dependent on the cell type, and the endosome escape mechanism most of these methods rely on is often inefficient, which do not serve for primary T cells. Therefore, the development of new Cas9 RNP delivery methods with high delivery efficiency and cell viability in human T cells remains highly desirable.
Our lab has developed a microfluidic cell deformation delivery method that uses physical constriction to deform and shear cells for delivery.11 Rapid mechanical deformation of cells can produce transient membrane disruptions or holes that facilitate passive diffusion of materials into the cytosol.12 Membrane deformation-based microfluidic devices have the advantage of high-throughput delivery of almost any macromolecule into almost any cell type.11, 12 With the advantages of broad applicability across different cell types, this method has the potential to serve as a broad-based universal delivery platform with macroscale throughput and precise control over treatment conditions at the single-cell level. Previously, we have successfully delivered plasmids encoding Cas9 and sgRNA into different cell types and achieved efficient genome editing.11 Here, we optimized the delivery chip and improved the delivery efficiency of Cas9 RNPs into different cell types including human primary CD4+ T cells (Figure 1A). Sequence and biochemical analyses demonstrated that Cas9 RNP delivery using our device achieved highly efficient genome editing and reduced off-target effects related to plasmid transfection. Furthermore, knock-in genome modifications in primary T cells were achieved in programmed cell death protein 1 (PD-1 or PDCD-1), a validated target for tumor immunotherapy. Thus, our delivery method could facilitate Cas9 RNP-directed genome editing and therapeutic genome engineering applications.
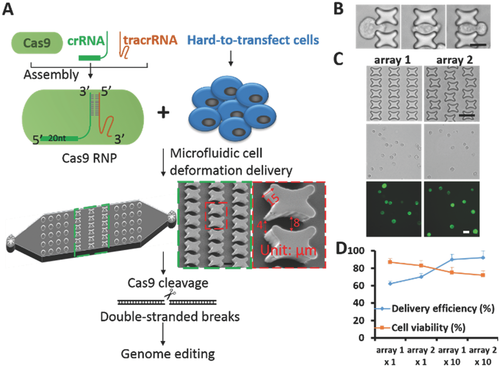
To improve efficiency, we first optimized the delivery chip design, which was based on the principle that rapid mechanical deformation of the cell causes transient membrane disruption or holes that facilitate the passive diffusion of material into the cell cytosol.12 We previously demonstrated that the constriction design and the dimensions were key factors that affect delivery efficiency. We modified the constriction shape to a curved tunnel in which cell deformation occurred to an enhanced degree over an extended time (Figure 1B). Flow velocity simulation was applied to the curved tunnel through which the cells passed (Figure S1, Supporting Information). The devices were fabricated with standard polydimethylsiloxane (PDMS) microfluidics technology and each chip consisted of 10 structure arrays that formed microconstrictions. We first compared chip performance of the new design versus our previous design. Fluorescein isothiocyanate (FITC)-labeled 70 kDa dextran molecules were delivered into human luminal-like SK-BR-3 breast cancer cells through the two chips and the delivery efficiency and cell viability were calculated (Figure S2, Supporting Information). The new chip showed nearly identical cell viability with much higher delivery efficiency. To further optimize the chip performance, we also rearranged the structures horizontally and vertically to form various curved tunnels (Figure 1C; Figure S3, Supporting Information). Suspended cells were applied to the chip through Tygon tubing connected to the inlet, and fluid flow was controlled by a syringe pump. We did a series of test deliveries of FITC-labeled 70 kDa dextran molecules into human luminal-like SK-BR-3 breast cancer cells. Parameters including constriction width and fluid flow rates were chosen based on our previous experiences. For the initial test, the constriction width of the curved tunnel varied from 4 to 8 µm and the flow rate was set at 150 µL min−1. The delivery efficiency and cell viability were calculated for different structure arrangements, forming various curved tunnel cell passages in arrays 1–4 (Figure 1D). The delivery efficiency of 70 kDa dextran varied from about 60%–70% in the array 1–4 designs. To achieve higher delivery efficiency, we increased the identical cell deformation zones to 10 repeats in one chip to attain greater than 90% delivery efficiency of 70 kDa dextran (Figure 1D). The cell recovery rates after delivery exceeded 90% for all of the designs (Figure S4, Supporting Information). However, higher delivery efficiency was often accompanied by lower cell viability using this mechanical delivery method, which could influence the future application of the method for different purposes.
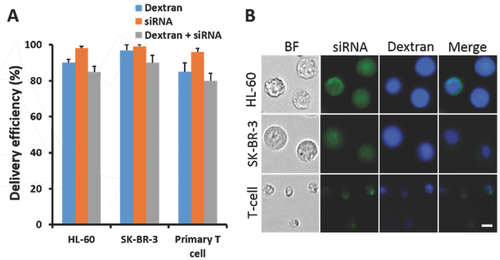
To investigate the adaptability of this method in different cell types, we selected fluorescent labeled dextran molecules and short interfering RNAs (siRNA) for codelivery tests. Adherent SK-BR-3 and nonadherent neutrophil-like HL-60 cells were initially chosen to assess the delivery ability of the chip across different cell types. FITC-labeled 70 kDa dextran molecules or siRNAs were delivered separately into the two cell lines with an efficiency greater than 90% (Figure 2A; Figures S5 and S6, Supporting Information). Further, the efficiency of the co delivery of Cascade Blue-labeled dextran and FITC-labeled siRNA in these cells was greater than 80% (Figure 2). One of the biggest advantages of our delivery chip is that it has the potential to solve problems related to the use of cells that are difficult to transfect. We chose primary T cells, the well-known, hard-to-transfect cells, for our delivery tests. Because primary T cells are less than 10 µm in size, we reduced the constriction width of the curved tunnel which varied from 2 to 4 µm. Even in the hard-to-transfect primary T cells, we achieved greater than 80% delivery efficiency for dextran and 90% for siRNA (Figure 2A; Figures S5 and S6, Supporting Information). The efficiency of the codelivery of dextran and siRNA was greater than 70% (Figure 2), suggesting the potential application of our delivery method in the hard-to-transfect cells. In future applications, the devices and delivery parameters could be optimized further to improve the delivery performance in other specific cell types.
After the initial dextran and siRNA delivery tests, we began to use the Alt-R CRISPR transactivating RNA (tracrRNA) and crRNA system for Cas9 RNP assembly and delivery (Figure 1A). The native bacterial CRISPR system in Streptococcus pyogenes requires a sequence-specific crRNA and a conserved tracrRNA, which interact through partial homology to form a crRNA:tracrRNA complex.13 The crRNA:tracrRNA complex guides and activates Cas9 to cleave double-stranded DNA targets.14 The crRNA was designed to target a sequence within enhanced green fluorescent protein (EGFP), and the recombinant Cas9 protein was fused with nuclear-localization signals and purified following overexpression in Escherichia coli. We first confirmed that the resulting Cas9/tracrRNA/crRNA complex was active in vitro by cleavage of a plasmid encoding EGFP (Figure S7, Supporting Information). SK-BR-3 cells stably expressing EGFP were then used to demonstrate the Cas9 RNP delivery and genome editing ability of our delivery chip. The Cas9 RNP complex was delivered into cells for 3 d and flow cytometric analyses were used to determine the knockout efficiency of EGFP (Figure 3A). The delivery of Cas9 RNP-induced EGFP expression was abolished in a protein and RNA concentration-dependent manner. When the concentration of Cas9 RNP delivered to the cells increased from 0.25 to 2 × 10−6 m, the number of EGFP positive cells decreased from 67.4% to 14.2% (Figure 3B; Figure S8, Supporting Information). As expected, EGFP expression was not affected in the negative control cells, to which only Cas9 nuclease was delivered. These data indicate that we successfully delivered Cas9 RNPs using our chip and achieved highly efficient genome editing.
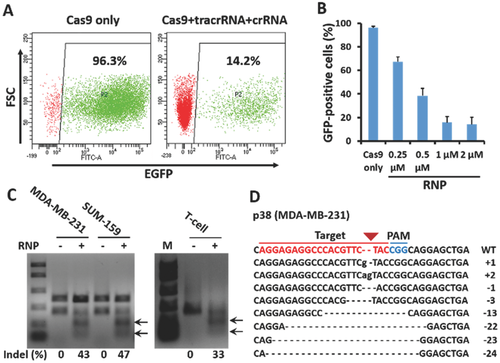
To determine whether our delivery platform was suitable for gene disruption and function analyses, we delivered Cas9 RNPs targeting p38 MAPKs (p38 mitogen-activated protein kinases), a class of kinases that participate in a signaling cascade controlling cellular responses to cytokines and stress,15 into human basal-like MDA-MB-231 and SUM-159 breast cancer cells. The cells were allowed to recover in culture for 3 d after delivery, followed by polymerase chain reaction (PCR) amplification of the specific sgRNA target region. A surveyor mutation detection assay revealed substantial cleavage at the p38 MAPK locus, with a mutation frequency of about 43% in MDA-MB-231 cells and 47% in SUM-159 cells (Figure 3C). To compare gene editing frequencies among different cell types, Cas9 RNPs targeting p38 MAPKs were also delivered into primary human T cells. We achieved a mutation frequency of about 33% in human T-cells, suggesting the adaptability of our method (Figure 3C). Small insertions and deletions (indels), characteristic of error-prone DSB repair via nonhomologous end joining were observed at the target site through the sequencing of the PCR amplicons (Figure 3D). Western blotting analysis revealed that endogenous p38 expression was abolished compared with the control in both cell lines (Figure S9, Supporting Information), which indicated our delivery platform was effective for gene disruption and function analysis.
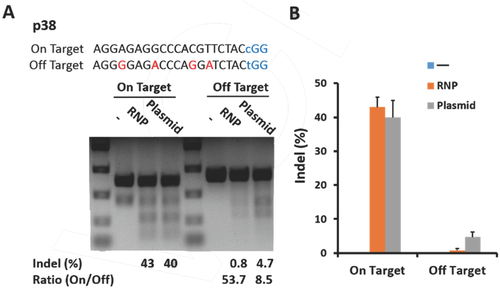
The CRISPR-Cas9 system can induce off-target mutations at sites that are highly homologous to on-target sites6 and cause unwanted chromosomal rearrangements such as translocations.[6] However, Cas9 RNP delivery has the advantage to reduce off-target effects compared with plasmid transfection for genome editing.[7, 9] Thus, we evaluated the off-target activities following the targeted delivery of Cas9 RNP to the p38 MAPK locus using our chip (Figure 4A). As expected, the off-target mutation frequencies in cells in which the Cas9 RNP was delivered with our chip were drastically lower than those treated with plasmid transfection, whereas the on-target mutation frequencies were comparable between the two groups. The ratio of on- to off-target activities was 6.3-fold (53.7/8.5) higher for p38 RNP delivery by chip versus plasmid transfection, which indicated our chip-mediated Cas9 RNP delivery was an efficient and precise genome editing method (Figure 4). Importantly, RNP delivery did not sacrifice genome-editing activities at on-target sites, while reducing off-target effects, which would greatly benefit CRISPR technology for therapeutic genome editing in future.
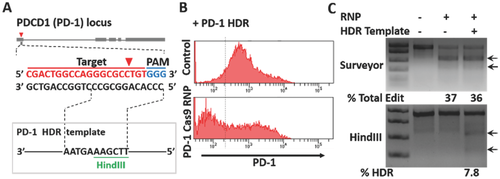
CRISPR-Cas9 technology for genome editing has been limited in primary human T cells.16 The ability to ablate key targets and correct pathogenic genome sequence in human T cells would have direct therapeutic applications, eventually allowing T cells to be edited ex vivo and then reintroduced into patients.[16] Hence, we chose human CD4+ T cells to investigate whether our chip-mediated Cas9 RNP delivery could improve genome editing in these hard-to-transfect cells. Cas9 RNP and homologous donor DNA were codelivered into human CD4+ T cells using our delivery chip to test the knock-in genome modification frequency. The crRNA and homology-directed repair (HDR) template containing a novel HindIII restriction enzyme cleavage site was designed to target the PD-1 (PDCD-1) locus (Figure 5A). PD-1 is a trans-membrane receptor found on the surface of T cells that negatively regulates immune responses by preventing the activation of T cells.17 PD-1 inhibitors are approved for the treatment of metastatic melanoma, and genetic deletion of PD-1 might prove useful in engineering T cells for cell-based cancer immunotherapies.18 PD-1 Cas9 RNPs codelivered with the HDR template using our chip significantly reduced the percentage of cells with high PD-1 cell-surface expression relative to controls (Figure 5B). To assess the variability of the delivery efficiency, we repeated the experiments for three times and the results showed reliability of our delivery method (Figure S10, Supporting Information). We likewise assessed the specificity of the HDR templates for targeted nucleotide replacement. As we expected, we observed efficient PD-1 editing by PD-1 Cas9 RNPs regardless of whether or not they were delivered using the PD-1 HDR template. By contrast, the HindIII site was only incorporated into PD-1 in the presence of both PD-1 Cas9 RNP and the PD-1 HDR templates, which indicated HDR-mediated knock-in genome modifications were achieved in human primary CD4+ T cells using our delivery method (Figure 5C). RNP based CRISPR-Cas9 system has great advantage in shortening reaction time and reducing off-target problems. The new system has much greater potential in future gene therapy applications. In particular, with the new capability, we successfully edited a validated target PD-1 for tumor immunotherapy, which may benefit the T-cell based cancer immunotherapy filed. Together, our microfluidic cell deformation-based Cas9 RNP delivery provided efficient and precise genome editing in primary T cells, which holds great promise for therapeutic T-cell engineering and applications such as the treatment of infection, autoimmunity, and cancer.
Acknowledgements
This study was funded by NIH-R01 DA035868, R01 CA180083, R56 AG049714, and R21 CA191179.