Understanding Immune Tolerance of Cancer: Re-Purposing Insights from Fetal Allografts and Microbes
Abstract
Cancer cells seem to exploit mechanisms that evolve as part of physiological tolerance, which is a complementary and often beneficial form of defense. The study of physiological systems of tolerance can therefore provide insights into the development of a state of host tolerance of cancer, and how to break it. Analysis of these models has the potential to improve our understanding of existing immunological therapeutic targets, and help to identify future targets and rational therapeutic combinations. The treatment of cancer with immune checkpoint inhibitors aims to reverse the progression to tolerance of cancer, and achieve an immunogenic, rather than tolerogenic, homeostasis. Broadening the efficacy and durability of checkpoint inhibitors focuses on reversing tolerance and stimulating immunogenicity in the cancer, host, and environment. Two examples of important physiological states of tolerance that may inform tolerance of cancer are microbial infection and placental reproduction. These states of tolerance result from bilateral shaping of host and non-self, akin to immunoediting in cancer, and offer reliable models to study the immune tolerance paradigm.
1 Introduction
Immune checkpoint inhibitors are one pillar of the drug treatment of solid organ cancers alongside targeted therapy, chemotherapy, and hormonal therapy. The quest to improve the efficacy and durability of immune checkpoint inhibitors centers on understanding the mechanisms for establishing a state of immunological tolerance, and how to break it. This paper reviews the different physiological states of tolerance, and how understanding these mechanisms may help us to break the state of immunological tolerance to cancer.
Immunological tolerance is seen in four major physiological settings, and displays distinct but overlapping mechanisms in each: tolerance to self; tolerance to ingested, inhaled, or topically applied molecules; tolerance to microbes; and maternal tolerance to “altered-self” in the fetal allograft.
Immunological tolerance to self-molecules ensures that immune reactions are not triggered by self-peptides activating T cell receptors, self-carbohydrates activating surface antibody on B cells, or self-nucleic acids activating intracellular receptors. The evolutionary pressure that selects for tolerance to self results from the unfavorable consequences of autoimmune or autoinflammatory disease.
Immunological tolerance to ingested, inhaled, or topically applied molecules is relevant in atopy and allergic disease. Its significance is highlighted by the increasing prevalence of peanut allergy, a consequence of cutaneous before intestinal peanut exposure, which bypasses the hypo-responsiveness induced by initial oral administration.1
Immunological tolerance to microbes is relevant for the existence of the gut microbiome and other commensals. Immunology arose from the field of microbiology, with a focus on immune reactions for resisting infection. Subsequent harnessing of resistance mechanisms revolutionized public health through immunization. The harnessing of microbial tolerance mechanisms, to the gut microbiome and select systemic infections, may also lead to breakthroughs.
Maternal tolerance to altered self in the form of allogeneic (fetal) cells is critical for live birth. Without tolerance, the implantation of a fetus, half of whose genes are paternally derived, would usually elicit immune rejection. The fetus, however, is protected from maternal attack during gestation thanks to a state of immunological tolerance in the mother. Maternal-fetal tolerance is present in many animals, having evolved independently in multiple species of fish, reptiles, and mammals. These observations support the notion that there is a fitness benefit of this tolerance-dependent form of reproduction.
Cancer cells exploit mechanisms that evolved for one or more of these four physiological states of tolerance, hence promoting a tolerogenic homeostasis that is ultimately harmful − i.e., it allows the development and progression of cancer (Figure 1). Analysis of physiological states of tolerance can broaden our understanding of the mechanisms of tolerance, identify changes leading to maladaptation in cancer, and expose inherent vulnerabilities. This review explores physiological tolerance from the perspective of two evolutionary “drivers”: microbes and placental reproduction. We explore the concept that these two examples of tolerance share mechanisms with cancer tolerance and therefore can be used to identify potential treatment targets.
2 Tolerance to Microbes is the Result of Bilateral Shaping of Host and Non-Self
Infection has been the major cause of human mortality throughout history.2 As highlighted by Jared Diamond in “Guns, germs, and steel,” infectious disease, more so than military force, enabled European conquests over the Incas of Peru and the Aztecs of Mexico.3 In Australia, accidental or deliberate release by British colonists of Variola (smallpox) virus kept in ship surgeon's stores decimated the native Aboriginal population of the Sydney region.4 For these introduced infections, case fatality rates were much higher than in populations where disease was endemic. This suggests the evolution in hosts of either heightened immune response to control pathogens, or reduced capability to tolerate pathogens.
Evolved host differences were evident following the deliberate release of Myxoma virus, a related poxvirus, to control the European rabbit plague in Australia.5 The natural host of Myxoma virus is a South American rabbit species in whom infection is rarely fatal. In this host, immune reactions do not clear the virus, but form chronic fibromas that shed virus for mechanical spread by mosquitos. In wild European rabbits in Australia during initial release of Myxoma virus, the fatality rate was exceptionally high at >99.5%. Over serial Myxoma virus epidemics, a virus population with decreased virulence arose and, once case fatality rates were <90%, a rabbit population with heightened viral resistance emerged, demonstrating the ability of host, and pathogen to shape one another. The reduction in fatality rates may have been due to genetically heightened immunity to Myxoma virus in the evolved survivors, or greater tolerance of the virus and less fatal immunopathology. Heightened immunity is the presumed mechanism,6 but in support of greater tolerance is the observation of marked lymphadenopathy in humans infected by the zoonotic orthopoxvirus, monkeypox, but little to none in the same (non-immune) population infected with the closely related human disease, smallpox.5
2.1 Immune Tolerance Provides an Equal and Balancing Form of Immune Defense Against Infection
Another example of immune tolerance to microbes is infection with Epstein Barr Virus (EBV). Most humans are infected with EBV, presenting little or no clinical signs at the time of infection, but harboring the virus life-long in the B cell reservoir. Some humans, however, develop infectious mononucleosis (IM), a debilitating chronic immune reaction that persists for many months. The development of IM may be due to reduced ability to mount an immune response that limits EBV replication, although this is not supported by the known expanded protein-specific CD4+ and CD8+ T lymphocytes during the primary IM response.7 Alternatively, humans susceptible to IM may lack tolerance mechanisms that curtail host reaction, and so generate a response sufficient to limit viraemia but insufficient to trigger chronic lymphadenopathy, fever, and malaise.
From an evolutionary perspective, tolerance, rather than resistance, is a more stable defense against microbes. Resistance, based on greater immune reaction to a microbe, is less beneficial once it is carried by a majority of a population because herd immunity reduces its utility. Consequently, resistance traits will tend to remain genetically polymorphic in a host species, hence preventing eradication of infectious disease by this means alone. Resistance traits also select for evasion by mutated microbes, because they disadvantage susceptible strains. By contrast, a trait that allows a host to tolerate infection, so that it imposes little fitness cost on host or microbe, does not select for microbial evasion. As the proportion of resistant hosts rises, each shedding the tolerated microbes, any residual hosts without the resistance trait are strongly selected against, so that tolerance traits tend to become genetically fixed in a species.8 Tolerance traits are thus abundant in biological systems, and so it is reasonable to assume that they may readily hijacked in cancer.
2.2 Learning From the Microbiome: Shared Tolerance Mechanisms Between Gut-Associated Lymphoid Tissue (GALT) and the Tumor Microenvironment (TME)
Humans are colonized by an enormous community of foreign microbes on skin and mucosal surfaces that do not harm an immunocompetent host. Many are opportunistic pathogens, exemplified by Staphylococcus aureus, Streptococcus pneumoniae, and Candida albicans. Others are symbionts that provide nutrients and aid resistance to the opportunistic pathogens. The microbiome is an essential repository of foreign antigen, and serves, in part, to induce tolerance to innocuous allergens, both local and systemic. A diverse microbiome is increasingly recognized as a strong determinant of health, while dysbiosis, an impaired or unbalanced microbiome, contributes to disease, and compromises its treatment.9
The microbiome is a functional organ; not only do we tolerate this repository of over 100 trillion foreign cells, we are shaped by its presence. Within the gut, the gut-associated lymphoid tissue (GALT) maintains a balance of stimulation and induced tolerance, which is impacted both by the composition of, and interaction with, the bacteria present.10 Commensals such as Bacteroides fragilis, segmented filamentous bacteria, and bacterial metabolites including short chain fatty acids, contribute to local and systemic tolerance by inducing immuno-modulatory CD4+ subsets such as Treg and Th17.9 An excess of pathogenic organisms, such as listeria monocytogenes, can stimulate autoimmunity in predisposed hosts by polarizing CD4+ T cell differentiation toward the Th1 subset.11 Modification of the microbial environment, for example via proton pump inhibitors, can reduce microbial diversity and increase dyspepsia and enteric infections, while probiotics and fecal microbial transplantation (FMT) have therapeutic benefit in diseases such as ulcerative colitis, gastroenteritis, and Clostridium difficile infection.12
Akin to the placental interface and immunomodulatory tumor microenvironment (TME), interaction whilst maintaining immunological tolerance in a setting of close proximity requires anatomical separation. A barrier of mucus, epithelial cells and antimicrobial peptides achieves this separation between microbes and host in the gut. This barrier prevents microbial translocation into the intestinal wall and facilitates regulation of “exposed” cell types. Focus on modulating interaction at this interface may play a significant role in success of microbiome-based treatment approaches − if an intact barrier is required to maintain tolerance, its disruption may help to break it. Approaches targeting this principle may include co-administration of immunostimulatory treatments such as checkpoint inhibitors with membrane-disruptive chemotherapy such as platinum salts or cyclophosphamide.13
The role of the microbiome in cancer predisposition, progression, and response to treatment is increasingly recognized.14 Specific organisms such as Helicobacter pylori can be oncogenic, while commensals appear to have tumor-suppressive properties.14 The gut microbiome has been shown to modulate toxicity and efficacy of chemotherapy, while microbial composition directly correlates with response to immunotherapy with the current focus on defining features of a “favorable” treatment environment 15. Depletion of microbial diversity with antibiotics reduces clinical response to checkpoint inhibitors in patients with advanced cancer, while reconstitution of germ-free mice with FMT from responding patients improves checkpoint inhibitor efficacy.16 Bacteria such as Akkermansia and Ruminococcus have been shown to drive this response through IL12-dependent induction of Th1-response. Presence of such favorable bacteria may be useful as both a biomarker for chemotherapy and immunotherapy response, and to identify potential FMT donors.17 Whether microbial diversity or, rather, the presence of specific bacteria in the gut is more important remains unclear. It is likely that both factors are contributory; a diverse microbiome provides the critical antigen repository for immune priming, whilst specific bacteria may drive immune responses in favor of cancer resistance.
Cytokines enriched within the GALT such as type I interferons, which drive continuous expression of Foxp3+ CD4+ regulatory T cells, are essential for maintaining tolerance.18 A significant proportion of microbiome-induced T reg cells also produce high levels of IL10 and highly express CTLA4 − shared modulators of tumor immunity.19 The significance of CTLA4 in intestinal homeostasis is highlighted by the high rate of enterocolitis in patients receiving the CTLA-blocking antibody, ipilimumab. Immune-mediated colitis, often pathologically indistinguishable from inflammatory bowel disease, occurred in 8–22% of patients receiving single agent ipilimumab and 23% of those receiving combination therapies with anti-PD1.20
3 Placental Reproduction Relies on Maternal Tolerance of the Fetus and Provides a Fitness Benefit for the Host
In vertebrates, placental reproduction serves as a fundamental example of a repeatedly selected function of tolerance of “altered self.” The placenta represents an interface for physiological exchange between maternal and fetal tissues, and has evolved through diverse means in diverse species. Many genes underlying placental development are conserved in other organs of the same species, due to the usual pattern of development of novel organs from pre-existing structures.21 Conversely, truly placenta-specific genes such as placental-specific protein 1 (PLAC1) are few in number and not conserved across placental species.22 In fact, there is little uniformity across placental evolution between species. Evidence for this is found in the order of scaled reptiles called squamates, in which the placenta has evolved over 100 times with a diverse range of placental structure, degree of nourishment (placenta versus yolk) and age of origin.23 In the seahorse, placentation occurs in males, where it cannot repurpose female hormonal circuitry. Fetal tolerance persists despite eliciting an immune response that is greater than the immune response elicited by cells from distant species.24 The mechanisms of tolerance remain partly unknown.
3.1 Time and Proximity: Shared Tolerance Mechanisms Between the Placental Interface and the TME
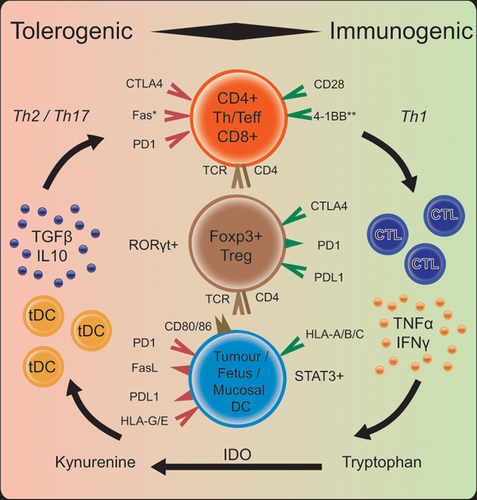
Neutralization of allo-reactive cells at the maternal-fetal interface is facilitated by T regs, which can be peripherally induced in the presence of the immunomodulating cytokine, TGFβ, and maintained in a functional ratio with effector T cells (specifically Th17), in part, by the programmed death 1-PD1-ligand (PD1-PDL1) pathway.25 PDL1 has an important role in feto-maternal tolerance because of its usual role as a negative feedback molecule to limit immune stimulation. PDL1 is constitutively expressed in (fetal) trophoblasts and incrementally increases from first- to third-trimester human placental tissue.26 Blockade of PDL1 in murine models leads to T-cell mediated rejection of allogeneic (non-self-derived) but not syngeneic (self-derived) embryos, highlighting its specific role in tolerance to non-self.26 Additionally, decreased expression of PD1 and PDL1, and relative predominance of Th17 immune cells was observed in placental tissue from women with pre-eclampsia compared with tissue from women without pre-eclampsia.27 In cancer, checkpoint inhibition with monoclonal antibodies to PD1 or PDL1 have an established treatment role in many types of solid cancers. The interaction of the PD1 pathway with IL17-producing Th17 cells is not well understood. In one reported case, an IL17-blocking antibody used to reverse severe immune-related skin and gut toxicity of checkpoint inhibition provided dramatic relief from toxicity but was associated with tumor growth.28
Maternal interaction with the fetus is further modulated by a unique trophoblastic MHC expression pattern, which is subject to selective pressure and replicated in human pathology. In humans, HLA-class I and II molecules encoded by this locus play a crucial role in presenting antigen to T cells to enable distinction between self and non-self. Altering HLA-expression patterns through transcriptional regulation is a strategy for both pathogen and cancer cell immune-evasion.29 Fetal trophoblasts do not express class II (CD4+ binding) HLA antigens and have restricted classical class I expression; HLA-A and HLA-B are absent, but HLA-C is retained and non-classical (limited polymorphism) HLA-E and HLA-G are expressed.22 HLA-G has well-established immunosuppressive properties and limited non-pathologic expression outside the trophoblast. HLA-G transcription and expression however is switched on in the majority of solid tumors, and its expression has been shown to negatively impact prognosis.30
Maternal decidua and fetal trophoblasts upregulate expression of the tryptophan-catabolising enzyme indoleamine 2,3-dioxygenase (IDO); IDO then suppresses T cell activity through depletion of the essential amino acid.31 Tolerogenic IDO+ dendritic cells (DC) are induced in the tumor microenvironment (TME) primarily by IFN-γ; a key instigator of Th1 anti-tumor activity, thus providing simultaneous negative feedback to the activated system.32 In many cancers, tumor cells constitutively express IDO, and IDO-high expression tumors have reduced cytotoxic T cell infiltrate and poorer prognosis.33 Pre-clinical models of IDO inhibitors have shown increased anti-tumor activity, however the first phase 3 trial of combined IDO/PD-1 inhibition to announce results was negative, and several pivotal late-phase trials have been halted based on emerging data.34 Research into combination with non-PD-1/PD-L1-targeted drugs is ongoing.35
Immunogenic co-stimulatory molecule 4-1BB (CD137) is co-induced post-coitus with IDO in murine models. Stimulating the signal in vivo resulted in fetal rejection, while endogenous signaling did not have this effect but protected the mother from infection, suggesting a synergistic effect.36 Agonists of 4-1BB in the cancer setting are currently in clinical trials combined with PD1 blockade, based on the increased cytotoxicity and anti-tumor effects observed in murine models.37 Combination of 4-1BB agonists either systemically or intra-tumorally with IDO-inhibitors may have therapeutic potential.
Fetal trophoblasts also express the inhibitory molecule CD95L (Fas ligand), a protein ordinarily expressed in activated T cells but constitutively expressed in immune-privileged sites, which induces apoptosis in CD95 (Fas)-expressing cytotoxic lymphocytes.22 Cancer cells express both Fas and Fas ligand, and are generally resistant to this path of apoptotic death.38 This is achieved either through altered expression of Fas or co-expression of a protein that prevents correct assemblage of the death-inducing signaling complex (DISC), and is an important mechanism of treatment-resistance.39
4 Tolerance of Cancer Represents a Pathological Hijacking of Physiological Functions
Cancer cells are derived from self, but in this case, a “successful” clone has mutated to circumvent physiological cell cycle checkpoints, amongst other malignant hallmarks. This transformation may occur in a step-wise fashion in a conducive environment, or may result from a single acquired mutation, in a gene such as EGFR within the receptor tyrosine kinase pathway. In both situations, the burden of “passenger” mutations, defined as having no impact on reproduction of the cell, serves as a molecular clock to the event and may contribute to the immunogenicity of a tumor.40 Some tumors evoke an immune flare, termed an elimination phase, which may develop into spontaneous remission. The magnitude of the elimination response is variable, and, at one extreme, absent (termed a “cold” or poorly immunogenic tumor). When present, this initial flare most often subsides to equilibrium − a state of balanced tolerance and resistance, and ultimately transforms into dominant immune tolerance of cancer, termed malignant escape.41
This evolution of cancer and host to arrive at an immune homeostasis skewed in favor of cancer tolerance occurs with time and proximity. Malignant clones have acquired distinguishing “non-self” features, but − analogous to a fetus in-utero or resident gut microbes − interact with the host immune system and are shaped by it. In cancer, the process of selection against immunogenic clones is termed immunoediting; an update of the originally proposed concept of immunosurveillance to recognize the active role of the immune system. The outcome of persistent host-cancer interaction is malignant escape, which is ultimately harmful. Therapeutic agents, such as checkpoint inhibitors targeting CTLA-4 and PD-1/PD-L1, aim to halt or reverse this progression to tolerance of cancer and achieve an immunogenic, rather than tolerogenic, homeostasis.
Cancer cells utilize many of the same pathways of physiological tolerance as microbes or fetal cells. A metabolically active TME supports tumor growth and regulates immune interaction by recruiting cell types that create an anatomical (stroma augmenting) and biological (immune suppressive) barrier.42 Malignant cells can evade detection by host immune cells by loss of MHC class I expression or functional impairment of other components of antigen processing.43 Physiological death can be evaded via both contact dependent (receptor/ligand interface) and contact-independent (cytokine mediated) means. Contact-dependent means include upregulation of Fas ligand, CTLA4, and PDL1 and down-regulation type 1 IFN receptors, while contact-independent means include secretion of immune suppressive products such as TGF-β, often driven by constitutive STAT3 activation.44
The goal of immunotherapy is to restore immune response to cancer and prevent progression to tolerance or “immune escape.” This can be achieved by perpetuating an initial elimination phase to the point of eradication, or by sustaining long-term equilibrium. Checkpoint inhibition aims to activate immune cells to achieve these goals. In some cancer types, combination immunotherapies are more effective, and deliver higher response rates at the cost of more frequent and severe toxicity. Exploring shared tolerance mechanisms in physiology and pathology will likely provide pathways for rational combination therapy that is effective and deliverable.
5 Conclusions and Outlook
Mechanisms of peripheral tolerance to cancer exploit everyday physiological processes, which are readily available for analysis. Furthermore, many of these mechanisms are targetable, and may be adaptable to the therapeutic setting. Tolerance of microbes and the fetal allograft are two physiological states of immunological tolerance that provide benefit to the host. Understanding the mechanisms leading to a selective advantage for tolerance in these settings may provide insight into the mechanisms that facilitate survival of cancer cells.
Manipulation of peripheral tolerance to cancer represents the augmentation of a complex and tightly evolved system − one that is successfully utilized in everyday physiological function. This should not encourage therapeutic nihilism, but drive better understanding of tolerance in all its incarnations, and the subtle shifts that allow its maladaptation. Further characterization of physiological tolerance may provide pathways to new therapeutic targets and, significantly, assist in inducing, identifying and maintaining states of favorable immune homeostasis.
Abbreviations
EBV, Epstein Barr Virus; FMT, fecal microbial transplantation; GALT, gut-associated lymphoid tissue; IM, infectious mononucleosis; TME, tumor microenvironment.
Conflict of Interest
M.B.B. reports non-financial support from Bristol Myers Squibb. P.B. has no disclosures. W.C. is a scientific advisory board member for Bristol Myers Squibb, MSD, Astra Zeneca. M.J.B. reports personal fees from Roche, Bristol Myers Squibb, MSD. S.K. reports non-financial support from BMS, Boeringer, personal fees and non-financial support from Roche, Astra Zeneca, personal fees from Pfizer. C.C.G. has no disclosures.




