Missed Druggable Cancer Hallmark: Cancer–Stroma Symbiotic Crosstalk as Paradigm and Hypothesis for Cancer Therapy
Abstract
During tumor evolution, cancer cells use the tumor-stroma crosstalk to reorganize the microenvironment for maximum robustness of the tumor. The success of immune checkpoint therapy foretells a new cancer therapy paradigm: an effective cancer treatment should not aim to influence the individual components of super complex intracellular interactomes (molecular targeting), but try to disrupt the intercellular interactions between cancer and stromal cells, thus breaking the tumor as a whole. Arguments are provided in favor of a hypothesis that such interactions include formation of synapse-like structures (interfaces) where the interacting cells are located at a distance of ∼10–15 nm. Within these interfaces, molecules initiating and strengthening the interaction are organized, and allow optimum cross-signaling; a very confined intercellular space facilitates the concentration of secreted cytokines, enhancing the paracrine cross-communication. These features of tumors form a druggable cancer hallmark the tumor-stroma symbiotic crosstalk—which represents a new target for efficient cancer drug discovery.
1 Introduction
1.1 Cancer Hallmarks—Excellent in Theory, Hopeless in Practice
The cancer microenvironment contributes to the evolution of cancer cells, thus increasing the complexity of cancer, a phenomenon that has been emphasized in recent reviews.1, 2 Dealing with this complexity requires finding certain universal principles that may reduce the diversity of tumors to a relatively small number of traits common to all of them. Such reduction was proposed by Hanahan and Weinberg in their well-known review “The Hallmarks of Cancer”: “We foresee cancer research developing into a logical science, where the complexities of the disease… will become understandable in terms of a small number of underlying principles.”3 Their analysis resulted in the concept of six common hallmarks of cancer: stimulation of growth, resistance to growth suppressors and apoptosis, replicative immortality, induction of angiogenesis, and activation of invasion and metastasizing.3 Later, anomalous metabolic pathways and evasion of the immune system were added to this list. In addition, the enabling features were also included, such as genome instability and inflammatory process prior to and during the emergence of tumor.4, 5
These generalizations were enthusiastically accepted, and the citation rate of these papers was very high. However, this list of common traits, each of which is a product of interactions of many molecular mechanisms, does not contribute much to the practical tasks of finding therapeutic approaches. Many of these mechanisms overlap, and are therefore unsuitable for the identification of proper therapeutic targets.6, 7 The review by Fouad formulates yet another version of the hallmarks of cancer: “selective growth and proliferative advantage, altered stress response favoring overall survival, vascularization, invasion and metastasis, metabolic rewiring, an abetting microenvironment, and immune modulation” that support tumor progression.7
In 2000, Hanahan et al. commented on the medical implications of the concept of common hallmarks of cancer as follows: “We envision [development of] anticancer drugs targeted to each of the hallmark capabilities of cancer; some, used in appropriate combinations … will be able to prevent incipient cancers from developing, while others will cure preexisting cancers, elusive goals at present.”3 The hallmarks were principally deduced for cancer cells,4, 5 although almost all of them—except replicative immortality, which is questionable—implicated the participation of the tumor microenvironment cells.4 Therefore, the therapeutic concepts resulting from this approach implied acting against cancer cells. As early as 2006, Orimo and Weinberg noted the importance of the stroma for tumor progression.8 From approximately 2010, the number of publications describing the stroma's contribution to cancer development quickly increased,4, 9, 10 though the “abetting microenvironment” was included in the list of the main hallmarks only in 2017.7 This inclusion makes sense. The current view of the tumor stroma is not just a physical support of mutated epithelial cells. All tumors engage a broad repertoire of normal cells in their evolution, and adopt them for their needs. The recruited normal cells facilitate the acquisition of characteristic traits, and form what is called the tumor microenvironment (TME), its ecological niche, which plays the most important role in both the development of a primary tumor and its metastasis.4, 11-16 Neither cancer cells nor stromal cells alone, but rather their interactions, lead to the evolution of a tumor as an organ-like entity. These interactions include: (i) direct binary contacts between ligands and receptors exposed on the surfaces of cancer and stroma cells, and (ii) paracrine communications between cancer (usually epithelial) cells and various cells of TME17, 18 (Figure 1). Some authors use the term “symbiotic”19, 20 for the tumor–stroma interaction: “The relationship between tumor and stroma is symbiotic. Stromal cells are corrupted by malignant epithelium, creating a permissive microenvironment, which drives cancer progression”21 (also see 19, 20). Usually symbiosis here means a complementary exchange of paracrine factors. This exchange modifies its characteristics during the conversion of usual fibroblasts into CAFs. A broadly accepted viewpoint claims that the changes of this kind can lead to recapitulation of cancer atavistic characteristics which commonly occur in unicellular organisms. However, this process is different from the one that occurs in multicellular organisms in that the latter strictly controls the growth and death of particular cells. These two viewpoints are hotly debated. However, I believe that at present, there is no data that proves either one of them. Therefore, I refer the reader to the two most recent publications on this subject.22, 23
It is now clear that to defeat cancer we should extract ourselves from the indecipherable complexity of intracellular interactomes, and try to disrupt the system as a whole by destroying the interactions between its parts. This simple “home-grown intuition”24 determines a new paradigm of cancer therapy: the finding and destruction of the intercellular crosstalk that lies at the root of the success of the malignant tumor's murderous mission. Adopting this concept as a basis, we should exclude paracrine interactions from the list of targets we aim to destroy, despite their undoubted importance. The reach of paracrine signals can extend to tens of cell diameters,17 thus forming a gradient profile of signaling that can, depending on the concentration, generate different outcomes instead of a simple “yes or no” binary response. That is why here I will not consider the so-called cytokine networks that specifically mediate the crosstalk between immune cells.25 Only direct interactions produce relatively simple binary contacts necessary for a predictable therapeutic effect. However, as shown later, a paracrine contact is efficient when it is concentrated in a confined space, in particular in a cleft of an immune synapse—a narrow gap between directly interacting cells of the immune system. A telling example of the success of such a concept is tumor immune checkpoint therapy (see later).
Based on the assumption that we make here, not only the direct contacts between immune cells, but also direct contacts of other stromal components with cancer or immune cells might be important therapeutic targets. These considerations imply the expedience to formulate a new hallmark of cancer that would form a firm basis for cancer therapy. A desirable new hallmark must be “druggable,” meaning that it can be probed in the context of a relatively simple search for a therapeutic intervention. Such a novel hallmark might be defined as “cancer-stroma symbiotic crosstalk.”
2 The Stromal Component of Tumors—An Indispensable Associate of Cancer Evolution
The American National Cancer Institute defines TME as “normal cells, molecules, and blood vessels that surround and feed a tumor. A tumor can change its microenvironment, and the microenvironment can affect how a tumor grows and spreads.” In solid tumors, the cancer microenvironment consists of two main components, cellular and non-cellular, whose proportion and composition vary depending on the location and stage of the tumor. The non-cellular components mainly include the extracellular matrix (ECM), composed of proteins, glycoproteins, and proteoglycans, serving as a scaffold that supports tissue architecture.4, 11, 26 The cellular components include fibroblasts, such as cancer associated fibroblasts (CAFs), mesenchymal stem cells, adipocytes, pericytes, endothelial cells, networks of lymphatic vessels, and tumor-infiltrating cells of the immune system.21, 27-30 From the therapeutic point of view, immune cell interactions with cancer cells might be the most successful targets for cancer treatment, and they could serve as a paradigm for a more general approach.
3 Brief Summary of Immune Checkpoints
To elucidate the issue, I provide some basic information on how T cells of the immune system are activated and deactivated (Figure 1). At the same time, I do not touch upon the aspects of immune editing and immune surveillance, which, although very important, are not directly related to the problem. For further information, the reader is referred to the reviews that are cited later.
3.1 Three Signals of T Cells Activation/Inhibition Within Immune Synapses
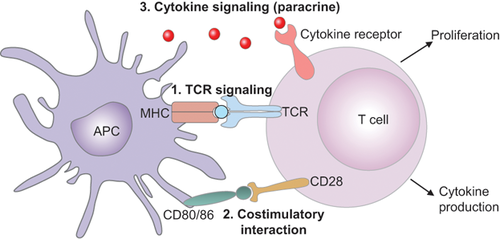
Three signals are needed to activate T cells. The first one is triggered by the interaction of T cell receptors (TCRs) with their antigen exposed on the surface of the antigen presenting cells (APCs) in a complex with the proteins of the major histocompatibility complex (MHC). The second signal is antigen-independent and caused by the interaction between the co-stimulating receptor CD28 on T cells and CD80 (B7.1) or CD86 (B7.2) proteins on APCs.32 The B7 family includes important membrane-bound ligands that bind both the co-stimulating and the co-inhibiting receptors. The third signal is stimulating cytokines (in a synapse, see later). T cells are fully activated only in the presence of all three signals, after which they begin to proliferate and destroy the carriers of the antigen presented by APCs. Apart from the co-stimulating signals, there are also co-inhibiting signals for T cells. As soon as T cells begin to proliferate, they start producing inhibiting signals using a variety of co-inhibiting interactions.33-35 The inhibiting interactions prevent an overly active response to immune stimuli, and help avoiding autoimmune reactions.
I will examine the process of co-inhibition and its role in the vital activity of the cell, using an example of the first discovered co-inhibiting signal—cytotoxic T lymphocyte-associated antigen 4 (CTLA-4).36, 37 Naïve and memory T cells express high levels of CD28 on their surface, but no CTLA-4. The latter is produced by the T cells during the primary response to the antigen. The number of CTLA-4 molecules on the surface of T cells is directly correlated to costimulatory/checkpoint receptors. The literature on this subject is vast, so here I will only refer to the recent concise and systematic reviews,38-41 limiting them to the most general concepts. The initial contact of TCRs with the antigen-loaded MHC on APCs (signal 1) induces the activation of multiple effectors, including membrane-bound molecules such as integrins, lymphocyte function-associated antigen-1 (LFA-1) and its ligand ICAM-1, signaling adaptors, cytoskeletal elements, etc. These processes strengthen the T cell–APC interactions. This contact also includes the co-stimulatory and checkpoint receptors (signal 2). Finally, the IS provides a very confined intercellular space that concentrates the cytokines secreted into it, strongly enhancing the cytokine cross-communication (signal 3).42 Immune synapses (ISs) are also critical for natural killer (NK) cell cytotoxicity. Mechanical forces generated at ISs as a result of intercellular adhesion are also important. ISs involving NK cells do not express TCRs, but they express activating and inhibitory receptors that can regulate signaling and dynamic changes in the integrin–actin network.41 Generally, the current therapies aimed at checkpoint blockade directly affect the immunological synapse.39 However, there are reports of direct interactions of T cells with checkpoint molecules expressed on the tumor surface, leading to immune inhibition,43, 44 and T cells can also function without synapse formation.42 Figure 2A shows that, similar to antigen presenting cells, the tumor exposes specific tumor antigens in a complex with proteins of MHC, and these antigens are recognized by TCRs. Some tumors also express B7 (CD80/86) components of the co-stimulating system.45 Similar to CTLA-4, co-inhibitor PD-1 is absent from the resting naïve and memory T cells and is only expressed upon TCR engagement.46 Its interaction with PD-L1 and PD-L2 located on APCs and tumors sends a negative signal to T cells, which can lead to T-cell exhaustion.43, 44
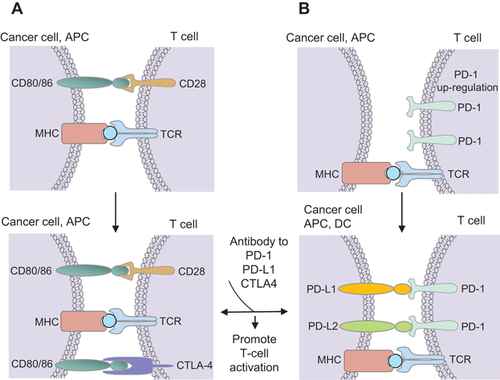
When a T cell interacts with the tumor antigen, it tries to proliferate, and begins to expose CTLA-4 on its surface. CTLA-4 interacts with the tumor B7 component of signal 2 and inhibits T lymphocytes, which blocks the anti-tumor signal. Thus, the tumor manages to avoid destruction by the immune system. Apart from CTLA-4, the PD-1, and PD-L1/PD-L2 co-inhibitory systems are widely used as targets in therapeutic practice49 (Figure 2B). PD-L1, also known as B7 homolog 1 (B7-H1), is activated in certain tumor cells, and its interaction with PD-1 induces apoptosis of T cells and leads to immunosuppression. Expression of PD-L1 is observed in a number of solid tumor types, including melanoma, bladder cancer, non-small cell lung, head, and neck cancer, and metastatic osteosarcoma. Similarly to PD-1, PD-L1 is a therapeutic target,50 although the mechanism of PD-1—mediated inhibition of the T cell function remains poorly understood.51
3.2 Immune Checkpoint Therapy—A New Paradigm of Tumor Therapy
The simple principle of how the T cells can avoid immunosuppression and resume tumor annihilation is illustrated in Figure 2. Monoclonal antibodies against CTLA-4 or PD-1, or their ligands, disrupt the interaction of these molecules with T cells, allowing them to destroy tumors. This concept was proven by a revolutionary therapeutic success of targeting the binary interactions between the stromal immune cells and APCs, stromal immune cells and tumor cells, stromal immune cells (CD8+ cytotoxic lymphocytes) and CAFs. This kind of therapy was termed immune checkpoint therapy.
The most impressive effect of the CTLA-4 blockade is its ability to induce a long-term tumor regression that lasted up to 13 years in clinical trials with some melanoma patients. However, the success rate in the case of melanoma was only about 8% (see the latest data in Ref. 52). Moreover, drug-activated T cells affect healthy tissues. Additionally, clinical trials revealed severe side effects in about 15% of patients, including several fatal outcomes. The reader can find the toxicity data as of December 2016 in53 (http://www.uptodate.com/contents/toxicities-associated-with-checkpoint-inhibitor-immunotherapy). Still, the inhibition of CTLA-4 checkpoint caused a revolutionary shift in the perception of cancer as an incurable disease. Clinical trials showed that the PD-1/PD-L1 blockade resulted in 30–50% positive outcomes for a wide range of tumors, and it was approved by the FDA for melanoma, non-small cell lung cancer, kidney cancer, Hodgkin's disease, head and neck cancer, and bladder cancer. The success of immunotherapy stimulated the search for other inhibiting checkpoints for cancer treatment.36, 37
CTLA-4 and PD-1 regulate different inhibiting pathways and have non-overlapping action mechanisms, suggesting that a combined therapy might be more efficient. Indeed, this was experimentally demonstrated in preclinical trials in mouse models. The preliminary clinical trials with anti-CTLA-4 combined with anti-PD-1 or anti-PD-L1 antibodies in other types of tumors produced promising results that hail the new combination immunotherapy as an efficient strategy for cancer patients,36, 54 albeit with a somewhat higher toxicity compared with the individual components. In some patients with metastasizing types of cancer, the use of T cell checkpoint inhibitors resulted in a complete and long-term disappearance of metastatic foci, an observation that led to a change in the paradigm of cancer therapy. Until now, cancer therapy was aimed mainly at “true” cancer cells, although much effort, with some success, was put into using the immune system-based agents such as antibodies, cytokines, and cancer vaccines for cancer treatment.55 In contrast, the new paradigm considers the interactions of cancer cells with their microenvironment as the most promising target. However, although these methods have greatly increased the lifespan of many patients with malignant neoplasms, many patients with common cancer types do not respond to this treatment. Besides, inhibition of immune checkpoints causes multiple side effects, mostly autoimmune inflammatory reactions termed immune-related adverse events.47, 53, 56, 57
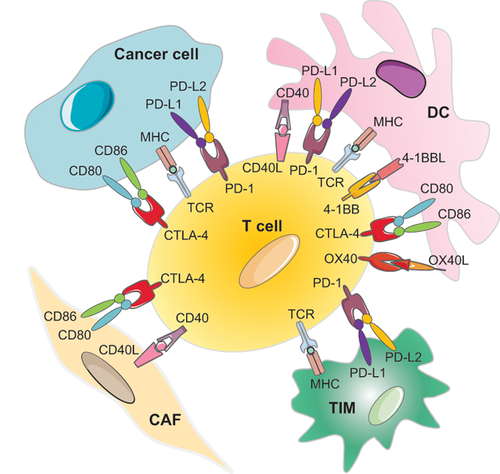
Fortunately, there are many pathways of co-inhibition and co-stimulation, some of which are shown in Figure 3. The tumor and the corresponding immune responses continuously evolve, making the identification of a single immunological blocker that could provide a universal therapeutic success nearly impossible. Therefore, there is a well-founded opinion that future immunotherapy will use combinations of immune and more traditional agents, such as chemo- or radiotherapy.36, 58, 59 Additionally, direct intercellular contacts that do not involve immune cells may be discovered, and these might serve as efficient therapeutic targets.
3.3 Intercellular (Possibly, Synapse-Like) Contacts Versus Intracellular Interactomes
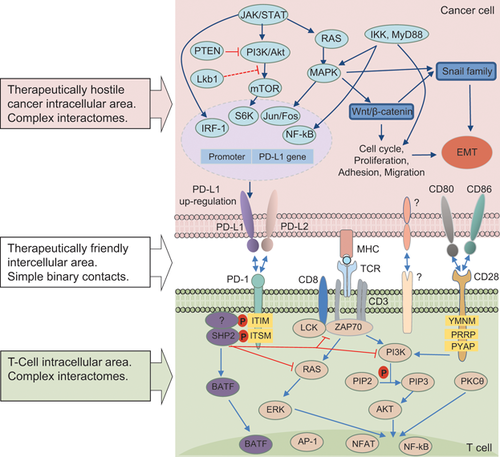
Checkpoint immunotherapy shows that the most efficient way to destroy a cancer system is by acting directly on binary contacts between ligands and their receptors exposed on the surface of APCs, cancer, and stromal immune cells. Cell-surface proteins represent attractive targets for therapy, due to their accessibility and involvement in essential signaling pathways, which are often dysregulated in cancer.61 A receptor-ligand interaction is in itself a single key event—the binding of a signaling molecule (ligand), to its receiving molecule (receptor). This binding (interaction) is relatively simple, and can be described by simple kinetic equations. Receptors and ligands have many forms, but all have one thing in common: they come in closely matched pairs in which a receptor recognizes just one or a few specific ligands, and a ligand binds to just one or a few target receptors. In other words, they are involved in relatively simple binary interactions. This is the basis of well recognized druggable properties of receptors and their cognate ligands, which make them especially useful clinical targets.62 In this case, receptors interact with ligands that themselves exhibit properties of receptors. Thus, one interaction provides two targets, which makes it possible to kill two birds with one stone. Besides, interacting cells in intercellular contacts are brought together to a distance comparable to the length of the receptor-ligand complexes, typically 15–40 nm.63 Therefore, inhibition of the two targets might also result in the inhibition of paracrine crosstalk.
These considerations lead to the concept of a therapeutically promising space consisting of direct intercellular interactions. This is the antithesis of molecular-targeted therapy, the targets of which are the components of complex intracellular interactomes (Figure 4). As in any complex system,1, 64-66 phenotypic effects of molecular targeting in this case are unpredictable. Immune checkpoint therapy is a striking example of the success of the afore-mentioned concept,67 but its complexity is manifested here by its rather high toxicity, and the enormous variability of patients’ responses—ranging from none to complete remission which presents an important problem.47, 53, 68, 69
Biological systems are extremely heterogeneous, which a priori implies heterogeneity of their response to external influences.59 Therefore, efforts are being made to classify tumors by the absence/presence of some characteristic immune traits.67, 70-72 However, the problem of reliable prognosis for therapeutic efficacy, using a limited number of traits, remains unsolved.73 Worse still, the available long-term follow-up data on melanoma show that a substantial part of patients that were earlier responding to the therapy with inhibitors of immune checkpoints, become resistant.69, 74 We do not understand why T cell checkpoints are ineffective in the majority of cancer patients. This could be because their immune system does not recognize antigens of cancer cells, or as a result of different mechanisms of immune inhibition.75 A multitude of new agents targeting other immune and non-immune processes and tumor components is under investigation.74 These include inhibitors of immune checkpoints, co-stimulating agonists, oncolytic viruses, vaccines, and adoptive cell therapy, as well as combinations with traditional methods of treatment.76
4 From the Solid Base of a Paradigm to a Flimsy but Falsifiable Hypotheses
Keeping in mind a successful approach of destroying the direct interactions between immune and cancer cells, I hypothesize that a similar strategy might be fruitful if such pro-tumor binary contacts existed between the cancer cells and other components of stroma. It is widely accepted that paracrine crosstalk between tumor stroma cells causes a transformation of stromal fibroblasts to CAFs. To make this crosstalk efficient, the interacting cells should probably form synapse-like contacts, enabling the effective exchange of paracrine signals.
It should be noted that the idea of a certain analogy between immune cells and CAFs occurred to others earlier.77 These authors pointed out that although CAFs were usually considered pro-tumorigenic, more recent studies demonstrated their tumor-inhibitory effects.78 The cells of the immune system can change their role from tumor inhibitory to tumor neutral, suggesting that both T-cells and tumor-residing fibroblasts exhibit a similar degree of plasticity. In 2014, the authors wrote in their review: “This review proposes to expand the term of cellular “polarization,” previously introduced to describe different activation states of various immune cells, onto CAFs to reflect their phenotypic diversity.” However, they did not expand this analogy to interactions between CAFs and cancer cells. Assuming that synapse-like binary contacts between cancer cells and other components of stroma might be a target for therapeutic action, I will give a very concise outline of the potentially promising exploratory approaches where tumor-stroma and stroma-stroma interactions can be detected. To this end, I will consider an example of CAFs, which are better studied than the other stromal constituents.
4.1 Brief Overview of Cancer-Associated Fibroblasts, Barely Explored Architects of Cancer Pathogenesis
CAFs are some of the most prevalent stromal cells in a number of carcinomas, including breast, prostate, pancreas, esophagus, and intestine cancer.28 In other carcinomas, including ovarian carcinoma, melanomas, and kidney tumors, CAFs are less frequent but still occur.11 CAFs attracted great attention as enhancers of cancer therapy efficacy. Some authors even call them “The Architects of Stroma Remodeling”79 or “Architects of Cancer Pathogenesis”.80 CAFs have been reported to affect the tumor progression in different ways, involving ECM degradation, release of numerous soluble factors, regulation of tumor metabolism, and promotion of cancer cell proliferation, migration, and metastasis. The most recent findings are found in the relevant reviews.28-30, 78, 79, 81
The normal fibroblasts can have a variety of suppressive functions against the initiation of cancer and metastatic cells through direct contacts with cells and paracrine signaling with soluble factors. The tumor-induced transformation of the normal fibroblasts into CAFs causes a number of pro-tumorigenic signals, followed by a distortion of the normal tissue structure, thus supporting the growth of cancer cells.82 CAFs are a heterogenous “family” or “group” of cells that exhibit mesenchymal-like features. Conversion of the normal fibroblasts to CAFs is considered a three-step process. First, distant normal cells are recruited by malignant or pre-malignant cells through paracrine and endocrine signals. Second, the recruited cells are transformed into CAFs. The final step is the maintenance, expansion, and evolution of CAF populations in the cancer microenvironment, enabled by the persistent signals produced by malignant cells.83, 84 In return, CAF populations emanate paracrine signals that affect cancer progression. Bi-directional cross-talk between cancer cells and fibroblasts is presumed to be the leading cause of malignant cancer phenotype formation.77, 85
One of the most significant features of CAFs is that their phenotype, that is, promoting tumor progression, is stably maintained in vitro and ex vivo even without steady contact with neighboring cancer cells.26, 78, 86 Recent studies report that many types of cells may be recruited as predecessors of CAFs: resident tissue fibroblasts, peritumoral adipocytes, bone marrow mesenchymal stem cells, hematopoietic stem cells, and many others.78, 81 After being recruited from various sources, a subset of these precursors acquires the CAF phenotype through complex activation processes that are still poorly understood. Most researches agree that, irrespective of the precursor, CAFs express similar sets of markers, such as α-smooth muscle actin (α-SMA), fibroblast activation protein (FAP), and the α and β platelet-derived growth factor receptor (PDGFR).81 Unlike epithelial cancer cells, the genetic changes such as oncogene/tumor suppressor mutations are rare in CAFs. In contrast, epigenetic changes, such as DNA methylation, histone modifications and nucleosome structure, changes in the expression of non-coding RNAs and abnormal activation of several signaling pathways, are often observed when the CAF phenotype is acquired. These changes affect the expression of many genes encoding growth factors, cytokines, and other products—a process that intensifies proliferation, stimulates secretion of ECM proteins and various growth factors, and causes remodeling of the cytoskeleton.4, 11, 28, 78, 81, 87 Another feature of CAFs, important from the viewpoint of new therapeutic targets, is worth noting: fibroblasts are more genetically stable than “true” cancer cells.27, 88 They divide slowly and, accordingly, mutate slowly. As a result, stromal therapeutic targets might be more stable compared to cancer cells with a permanently changing genetic structure. For this reason, the stroma is currently attracting a significant amount of attention from researchers who are developing the new approaches to cancer treatment.9, 27, 86, 89
4.2 Cancer Associated Fibroblasts Can Inhibit Antitumor Immune Response Through Direct Contacts With Immune Cells
Because of their preponderance in the tumor microenvironment, CAFs were recently studied as regulators of immune cell recruitment and function. As a result, CAFs were shown to play pro-inflammatory and immunosuppressive roles through secretion of transforming growth factor (TGF) and other cytokines, thus affecting both the innate and adaptive immune response.78, 90 In this review, I consider direct contacts of CAFs with cells of the immune system, which, in my opinion, are important for strengthening and guiding the action of paracrine factors. CAFs can establish direct contacts with immune cells and affect the efficiency of checkpoint immunotherapy by means of the expression of co-inhibitory receptor ligands60, 91-93). By now, such a possibility was experimentally demonstrated for PD-L1 and/or PD-L2 expression (Figure 2A). Nazareth and colleagues93 found a constitutively high expression of functional PD-L1 and 2 in the fibroblasts cultured from human non-small cell lung cancers. It was also shown that CAFs of large intestine cancer express PD-L1 and PD-L2 and negatively regulate the proliferative response of CD4+ Th-cells. Similar observations were reported for CAFs from melanoma cells (see review Ref. 78). However, most of these findings were made in in vitro experiments using isolated CAFs, and, therefore, require further studies to confirm the physiological significance of PD-L1/L2 expression by CAFs for their immunosuppressive role in vivo.78 A recent paper,91 presents further evidence of the immuno-inhibiting function of CAFs resulting from their direct interactions with immune cells. The authors show that CAFs can function as antigen presenting cells, able to absorb, process, and present on their surface tumor specific antigens complexed with MHC-I proteins. With the help of PD-L2 and FASL, this triggers an antigen-specific negative regulation of tumor-specific CD8 + T cells, which leads to their dysfunction and apoptosis. Neutralization of PD-L2 or FASL reactivates the cytotoxic capacity of T cells in vitro and in vivo. Thus, CAFs might support T cell suppression within the tumor microenvironment by a mechanism dependent on immune checkpoint activation, hence representing another mechanism of T cell depletion and dysfunction within tumors.91
4.3 CAFs Can Directly Interact With Cancer Cells and Enhance Their Invasion and Metastasis
CAFs are often found in the vicinity of, or in direct contact with, neoplastic cells.11, 28, 29, 89 However, only a few reports provide experimental evidence for the CAF-cancer cell direct interaction and study its functional consequences. The most obvious and important consequence of such direct interactions is the involvement of CAFs in promoting cancer cell epithelial-mesenchymal transition, invasion, and metastasis.79, 94-99 This should be expected, because collective cell migration is ubiquitous in multicellular organisms, and it is recognized that the physical interaction between cells in conjunction with chemical signals plays a fundamental role in this process.100 Gaggioli et al.94 demonstrated that CAFs led the invasion of squamous cell carcinoma cells (SCCs) by generating tracks in the extracellular matrix in a co-culture system. During joint invasion, the leading cells were CAFs, and associated SCC cells followed them. Thus, SCC cell invasion needs either close proximity or direct contact to CAFs. Similar evidence is presented in the review.98 To investigate the differential contribution of direct cell–cell contacts and paracrine signaling factors to NSCLC metastasis, Choe et al.96 performed two types of co-culture: direct co-cultures of the NSCLC cell line with primary cultures of CAFs from patients with resected NSCLC, and indirect co-cultures across a separable membrane. CAFs more potently induced EMT in the case of direct co-culture, thus providing evidence that the physical contacts between NSCLC cells and CAFs might control the metastatic potential of NSCLC. This probably does not exclude the participation of paracrine crosstalk that could be strengthened by the physical cell-to-cell interaction, similar to the immune synapses. In a more recent review,79 it is asserted that CAFs adjacent to cancer regions are able to increase the invasiveness of cancer cells through both cell-cell interactions and various pro-invasive molecules, such as cytokines, chemokines, and inflammatory mediators.
It is also known79 that CAFs can co-travel in the blood with circulating murine metastatic lung carcinoma cancer cells probably supporting the cancer cell viability and growth advantage at the metastatic site. The authors hypothesized that in invasive tumors the cancer and stromal cells were in direct contact, and established a complex crosstalk that evolved during tumor development. In a very important study,99 it was demonstrated that CAFs caused a collective invasion by means of a heterophilic adhesion involving N-cadherin at the CAF membrane and E-cadherin at the cancer cell membrane. Impairment of the E-cadherin/N-cadherin adhesion abrogates the ability of CAFs to guide collective cell migration and blocks cancer cell invasion. In parallel, the organizers of intercellular junctions, nectins, and afadin, are recruited to the cancer cell/CAF interface. These findings show that a mechanically active heterophilic adhesion between CAFs and cancer cells enables cooperative tumor invasion. Contacts between cancer cells and CAFs may also be implemented through the interaction of the Eph-receptor and reciprocal ephrin ligands.101 One can assume that these direct contacts form synapse-like structures strengthening the paracrine cross-talk.
4.4 Attempts of Targeting the Interaction Between CAFs and Carcinoma Cells
The sinister role of direct interactions of CAFs with cancer cells in the process of metastasis makes it especially important to destroy these contacts for therapeutic purposes. With such a goal, Yamaguchi et al.98 tried to identify inhibitors of direct interaction between CAFs and cancer cells, and found that the Src inhibitor dasatinib effectively blocked the physical association between CAFs and scirrhous gastric carcinoma (SGC) cells with a very low cytotoxic effect. Dasatinib was also effective against peritoneal dissemination of SGC in a mouse model. Importantly, histological analysis revealed that metastasizing tumors were less associated with stromal fibroblasts in mice treated with dasatinib compared with controls. These results demonstrate that direct interaction between CAFs and SGC cells can be a target for anti-metastasis therapy.98 Nevertheless, the authors advise caution, recalling the studies that showed that the depletion of CAFs in mouse models accelerated progression of pancreatic cancer. Although these results are contradictory, they accentuate the need for thorough tests of the safety of the inhibitors of CAF-cancer interactions in anticancer therapy. On the other hand, if the therapeutic target were the CAF-cancer contacts and not CAFs themselves, the strategy might be safe, because CAFs would not be depleted.
5 Conclusion
5.1 Does Immunotherapy of Cancer Entail a Change of Paradigm?
- Cancer is no longer regarded just as a set of mutant and dysregulated cells with their “driver” mutations. Instead, cancer and TME (stroma) cells jointly form an evolving, integrated, cooperative, and dynamic organ-like system.
- Gradually it is becoming clear that in order to defeat cancer we should abandon attempts to treat it by targeting the components of complex intracellular interactomes, and instead try to disrupt the system as a whole by destroying the interactions between its parts.
- I put forward a hypothesis that in most cases such interactions include direct cell-to-cell contacts manifested by synapse-like structures. At these interfaces the interacting cells are brought together at a distance of ∼10–15 nm, which precisely organizes multiple molecules into areas of intercellular contacts. Additionally, the synapse-like structures provide a very confined intercellular space that facilitates the concentration of cytokines secreted therein, thus strongly enhancing the paracrine cross-communication. The two interdependent parts of the interactions constitute a missed cancer hallmark, which I suggest naming the “tumor-stroma symbiotic crosstalk.”
Abbreviations
APC, antigen presenting cell; CAF, cancer associated fibroblast; CD40, cluster of differentiation 40; CTLA 4, cytotoxic T lymphocyte-associated antigen 4; DC, dendritic cell; ECM, extracellular matrix; IS, immune synapse; LFA-1, lymphocyte function-associated antigen-1; MHC, major histocompatibility complex; NK cell, natural killer cell; NSCLC, non-small cell lung cancer; PD-1, programmed death-1; PD-L, programmed death ligand; TCR, T cell receptor; TIM, tumor infiltrating macrophage; TME, tumor microenvironment.
Acknowledgments
The work was supported by the Russian Science Foundation (project 14-50-00131). The author is thankful to Drs B.O. Glotov, T.V. Vinogradova, and I.V. Alekseenko for their help in preparing the manuscript.
Conflict of Interest
The authors declare no conflict of interest.




