Targeting the tumor microenvironment and T cell metabolism for effective cancer immunotherapy
Abstract
The successful implementation of immunotherapies has provided new impetus in the fight against cancer. Antibody-mediated blockade of immune checkpoint molecules PD-1/PD-L1 and CTLA-4 has had a dramatic impact upon the treatment of previously intractable cancers such as malignant melanoma, while adoptive cell therapies using chimeric antigen receptor-bearing T cells have proven highly efficacious in B cell leukemia. Furthermore, significant progress has been made in understanding the mechanisms by which tumors evade or become resistant to these immunotherapies. In this regard, approaches to broaden the applicability and enhance the efficacy of immunotherapies increasingly include modulation of tumor and immune cell metabolism. In this mini-review, we highlight the most recent studies describing novel approaches and targets for the manipulation of the tumor microenvironment and T cell metabolism and describe how these approaches are being combined with current immunotherapies in preclinical studies.
Introduction
During the past decade, the development and increased use of cancer immunotherapies has resulted in impressive clinical benefits. Function blocking antibodies targeted to the immune checkpoint molecules PD-1/PD-L1 and CTLA-4 have been approved by the US Food and Drug Administration for the treatment of advanced melanoma, non-small cell lung cancer, renal cell carcinoma and Hodgkin's disease, among others 1. Furthermore, adoptive cell transfer (ACT) of ex vivo-expanded autologous tumor-infiltrating lymphocytes (TILs) or genetically engineered chimeric antigen receptor T (CAR-T) cells has been the other pillar of cancer immunotherapy 2, 3. Despite these successes, responses to cancer immunotherapy are variable and only a subset of patients exhibit durable responses 4, 5.
It is likely that variation in tumor immunogenicity and the presence of immunosuppressive signals within the tumor microenvironment determine the efficiency of patient antitumor immune responses as well as immunotherapies 1, 4, 6. Weakly immunogenic tumors are typically characterized by a lack of immune infiltration, and in particular a lack of CD8+ CTLs 6. Poor immune reactivity and infiltration to tumors is prognostic for resistance to checkpoint inhibitors while immunotherapeutic responses are enhanced in so-called immune “hot” tumors 4. Moreover, there is increasing appreciation of the interplay between tumor cell and immune cell metabolism in the outcome of antitumor immune responses. In this regard, cancer cells and antitumor lymphocytes, such as effector CD8+ T cells, have similar metabolic requirements and compete for nutrient resources, including glucose and amino acids, within the tumor microenvironment (TME) 7. Furthermore, in addition to depriving antitumor immune cells of key nutrients, tumor cells create a challenging environment characterized by hypoxia, acidic pH, and high levels of immunosuppressive metabolites. Thus, new approaches that combine existing immunotherapies with strategies that overcome the metabolically challenging TME and enhance infiltration, effector responses and longevity of antitumor T cells are being investigated 6. In this mini-review, we focus on the most recent studies describing mechanisms for manipulating the immunosuppressive TME and enhancing antitumor T cell responses through modulating metabolic pathways, and how these approaches can be used to improve the efficacy of immune checkpoint blockade and T cell ACT for the treatment of cancer.
Impact of the TME and checkpoint inhibitors on T cell metabolism
T cells undergo a metabolic switch upon engagement of the T cell receptor (TCR). Whereas naïve T cells uptake low levels of glucose and use mitochondrial oxidative phosphorylation (OXPHOS) to fuel their energy requirements, effector T cells increase nutrient uptake and rely on aerobic glycolysis, glutaminolysis, and lipid synthesis to support metabolic and functional demands. The metabolic regulation of T cell activation has been reviewed extensively in recent times 7-9. Briefly, aerobic glycolysis is relatively inefficient in the production of ATP but enables T cells, and other proliferating and inflammatory immune cell types, to use glucose to fuel the pentose phosphate pathway (PPP). The PPP generates NADPH that in turn is rate-limiting for the production of amino acids, nucleic acids, and fatty acids, and the production of biomass. Furthermore, a number of recent studies have shown important roles for glycolytic enzymes in non-glycolytic processes that regulate T cell activation and effector function (reviewed in ref. 8). Tumor cells frequently share a similar glycolytic metabolic profile with activated T cells. Consequently, cancer cell nutrient uptake creates a TME characterized by the limited availability of glucose, lipids, and amino acids and, therefore, lymphocytes are suppressed due to the inability of the cells to sustain energetic needs 10-12. Furthermore, T cells and other antitumor immune cells are inhibited by tumor-derived waste products, such as lactate or kynurenine, and low oxygen levels 10.
The mechanisms by which immune checkpoint receptors block T cell responses also involve manipulation of T cell metabolism. Thus, PD-1 engagement results in a progressive state of metabolic exhaustion in T cells by inhibiting glucose and amino acid uptake and catabolism 13, 14. Instead, PD-1 signals favor the engagement of fatty acid oxidation (FAO), which is required for the long-term survival and fitness of chronically activated T cells 13, 15. In effector T cells, immune checkpoint blockade shifts T cell metabolism back toward glycolysis. Thus, T cell metabolic impairment plays a pivotal role in the suppression of antitumor responses.
Approaches to manipulate T cell and tumor glycolytic metabolism
Glycolytic metabolism is required for efficient T cell effector function and is dampened within the TME, in part, as a result of low levels of glucose availability 11, 12. However, a recent study demonstrated that glucose deprivation is not the only limiting factor for engagement of the glycolytic pathway by CD8+ TILs. Gemta and colleagues demonstrated that TILs have reduced enolase-1 activity, a glycolytic enzyme that catalyzes the conversion of 2-phosphoglycerate to phosphoenolpyruvate (PEP) 16. Importantly, this deficit could be reversed as provision of the downstream metabolite pyruvate improved the metabolic fitness and functional capacity of TILs 16. Furthermore, previous studies showed that PEP is a key metabolite in the regulation of antitumor responses as, in addition to its function in glycolysis, PEP regulates the cytoplasmic Ca2+ concentration and NFAT1 activation in T cells 12. Under conditions of reduced glucose availability and glycolytic flux, restoration of intracellular PEP levels by overexpression of the gluconeogenesis enzyme PEP carboxykinase 1 in either CD4+ or CD8+ T cells results in improved antitumor responses upon ACT 12, 17.
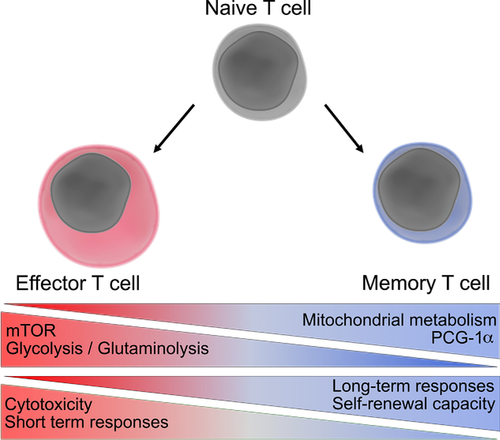
Constitutive activation of glycolysis is linked to terminal differentiation of effector T cells. While glycolysis permits enhanced cytokine secretion and production of cytolytic proteins, it is also associated with decreased T cell persistence and nondurable tumor responses (Fig. 1). Furthermore, it is well established that ACT of memory phenotype T cells results in superior and sustained antitumor responses as compared to effector T cells 18. Thus, several studies have focused on attenuating glycolysis to promote a memory-like phenotype. Treatment with the glycolysis inhibitor 2-deoxyglucose enabled the differentiation of long-lived memory T cells with enhanced antitumor function upon ACT 19. Furthermore, recent work has demonstrated that T cells lacking the serine/threonine Pim kinases or control T cells treated with a Pim kinase inhibitor had substantially reduced glycolytic metabolism and adopted a memory-like phenotype 20. Consequently, ACT using melanoma-specific pmel T cells pretreated with the PIM kinase inhibitor AZD1208 improved tumor clearance and survival when compared to controls. Furthermore, combination of AZD1208, PD-1 blockade, and T cell ACT had synergistic effects against B16 melanoma in mice 20. Another potential approach to improve antitumor responses was revealed by the finding that tumor-associated Tregs are also dependent upon the glycolytic pathway for their suppressive function. A new study showed that TLR8 ligation inhibits glucose uptake and glycolytic metabolism specifically in Tregs 21. Furthermore, TLR8 agonists promoted antitumor responses following T cell ACT in a xenograft model of human melanoma 21.
Increased tumor glycolysis correlates with poor therapeutic responses to ACT 22. Nonetheless, dampening glycolytic metabolism in vivo is complex due to the difficulties of specifically targeting tumor cells. A role for tumor-associated macrophages (TAM) in promoting tumor cell glycolytic metabolism was recently described. TAM production of inflammatory cytokine TNF enhanced tumor cell glycolysis, while depletion of TAMs using chlodronate rendered otherwise resistant Lewis lung cancers sensitive to anti-PD-L1 therapy 23. A potential cheap and nontoxic approach to manipulating tumor cell metabolism was reported by Gonzalez and colleagues who demonstrated that in vivo administration of the monosaccharide mannose interferes with tumor cell glycolysis 24. Mechanistically, mannose treatment regulates apoptotic proteins resulting in an enhanced sensitivity of cancer cells to chemotherapeutic drugs. Sensitivity to mannose varied between tumor cell lines and was associated with expression levels of phosphomannose isomerase 24. A further consideration is the role of mannose in enhancing Treg differentiation and suppressing inflammatory T cell responses 25. Therefore, the outcome of mannose treatment in cancer will likely depend on the balance between anti-tumour effects and immunosuppressive effects.
Approaches to manipulate T cell and tumor mitochondrial metabolism
Mitochondrial metabolism has a key role in the development and maintenance of memory T cells with long-lived antitumoral activity (Fig 1). Pearce and colleagues demonstrated that CD28 co-stimulation during T cell priming regulates memory cell formation by eliciting enhanced FAO and spare respiratory capacity (SRC), as well as morphological mitochondrial remodeling 26, 27. In the absence of CD28 co-stimulation, tumor-specific T cells suppressed tumor growth within the first 15 days after ACT but failed to maintain durable responses, resulting in tumor relapse. Treatment of anti-CD3/CD28-primed T cells with etoxomir, an irreversible inhibitor of carnitine parmitoyltransferase (Cpt) 1, dampened durable antitumor responses, highlighting the importance of long-chain FAO for the efficacy of memory phenotype T cell ACT 27.
Within the TME, exhausted TILs frequently have diminished or absent CD28 expression. However, recent work determined that expression of the costimulatory TNF receptor family member 4-1BB was elevated on CD8+PD-1+Tim3+LAG3+ TILs from B16 melanomas 28. 4-1BB co-stimulation enhanced mitochondrial function in CD8+ T cells via peroxisome proliferator activated-receptor (PPAR)-γ coactivator 1α (PGC-1α), a transcription factor involved in mitochondrial biogenesis. Furthermore, 4-1BB agonists enhanced the efficacy of anti-PD-1 therapy and T cell ACT in B16 melanoma models in vivo 28. Several additional lines of evidence have also determined the utility of targeting PPAR signaling pathways to improve T cell immunotherapy. In glucose- and oxygen-deprived mouse melanoma models, TILs upregulate PPAR-alpha signaling and use FAs as an energy source 29. Promotion of FA catabolic pathways in vitro improved the subsequent antitumor function of adoptively transferred CD8+ T cells, and synergized with PD-1 blockade resulting in superior control of tumor growth 29. Similarly, Chamoto and colleagues show that Luperox, an ROS generator, synergizes with PD-L1 mAb therapy against MC38 colon adenocarcinoma cells in mice. ROS accumulation did not directly affect tumor cell killing but elevated T cell responses through upregulation of PGC-1α 30. Small molecule activators of PGC-1α (oltipraz and bezafibrate) potentiated PD-1 blockade therapy. More recently, the same group reported that bezafibrate in combination with anti-PD-L1 induces expression of genes involved in mitochondrial biogenesis and FAO, such as PGC-1α or Cpt1b, and anti-apoptotic genes including Bcl2, Birc3, and API5. Overall, PGC-1α activation results in an increased number of tumor-reactive T lymphocytes infiltrating the TME thereby boosting PD-1 blockade therapy 31. By contrast to the effects of Luperox in vivo, Pilipow and colleagues demonstrated that ROS limitation, using the antioxidant N-acetylcysteine (NAC), favors the generation of long-lived stem cell memory T (Tscm) cells 32. ACT using NAC-generated Tscm CD19 CAR-T lymphocytes demonstrated enhanced clearance of lymphoblastic leukemia cells in immunodeficient mice 32. These studies demonstrate that targeting ROS in antitumor therapy has complex context-dependent effects.
A study from the Delgoffe laboratory indicated that targeting tumor cell mitochondrial metabolism might also represent a viable approach to enhancing immunotherapies. Their results demonstrated that different human melanoma cell lines varied substantially in their reliance upon mitochondrial or glycolytic metabolic pathways 33. Cell lines with dysregulated oxidative metabolism induced higher levels of intratumoral hypoxia in immunodeficient mice while knockdown of mitochondrial complex 1 subunit Ndufs4 in melanoma cells reversed this effect 33. Importantly, anti-PD-1 induced CD8+ T cell infiltration and function was enhanced in Ndufs4, but not GLUT1, knockdown tumors, as compared to control. These data suggest that, in this model, tumor cell-intrinsic oxidative but not glycolytic metabolism dampens anti-PD-1 efficacy.
Targeting the tumor microenvironment to improve immunotherapies
The uncontrolled growth of tumor cells leads to the formation of hypoxic non-vascularized areas, while high degrees of tumor hypoxia are associated with poor clinical outcomes. Hypoxic areas within tumors typically lack infiltrating T cells 34 and several recent studies have addressed the question of whether reversing tumor hypoxia can enhance T cell infiltration and responses to cancer immunotherapy. One such study investigated the impact of evofosfamide (TH-302), an alkylating chemotherapeutic pro-drug that is activated under conditions of hypoxia. Jayaprakash and colleagues reported that treatment of mice with TH-302 restored normoxia and enhanced T cell infiltration into TRAMP-C2 prostate tumors 34. Importantly, TH-302 treatment markedly enhanced the therapeutic efficiency of PD-1/CTLA-4 checkpoint blockade 34. Similarly, respiratory hyperoxia (breathing 60% O2) enhanced infiltration of antitumor CD8+ T cells and reduced tumor burden within the lung when combined with dual CTLA-4/PD-1 blockade in a MCA205 fibrosarcoma pulmonary mouse model 35. In this study, supplemental oxygenation repressed Treg numbers and levels of the inhibitory cytokine TGF-β 35. Furthermore, metformin treatment also reduces tumor hypoxia and potentiates the effects of PD-1 blockade against B16 melanoma and MC38 colorectal carcinomas in mice 36.
Alternative approaches to counteracting the effects of tumor hypoxia have included targeting the immunoinhibitory effects of adenosine signaling. Within hypoxic tumors, stabilization of hypoxia-inducible factor (HIF) transcription factors increases expression of CD73, a cell surface nucleotidase that promotes synthesis of extracellular adenosine. Binding of the A2A adenosine receptor (A2AR) suppresses inflammatory immune responses by antitumor cells, including T lymphocytes, NK cells, DCs, and macrophages. Antagonizing A2AR or inhibiting CD73 has synergistic effects against tumors when combined with anti-PD-1 37. Furthermore, a recent study showed that resistance to PD-L1 blockade in mouse models of non-small cell lung cancer was associated with enhanced expression of CD38 by tumor cells and A2AR-induced immune suppression 38. Consistent with an important role for CD38 expression in mediating tumor escape, CD38, or A2AR antagonists enhanced the therapeutic effects of anti-PD-L1.
Glycolytic solid tumors excrete high levels of lactate while tumor expression of high levels of lactate dehydrogenase A (LDHA) is associated with poor prognosis in human melanoma 39. LDHA function is particularly important for tumor cell growth under hypoxic conditions 40 while the acidic TME generated by lactate secretion blocks T cell cytokine production and cytolytic capacity 39 and induces NK cell apoptosis 41. In addition, a recent study demonstrated that an acidic microenvironment within melanoma induces expression of the transcriptional repressor protein ICER in macrophages and promotes their polarization to an anti-inflammatory, pro-tumorigenic phenotype 42. Preclinical studies to determine the impact of neutralization of tumor acidity on antitumor immunity have yielded promising results. Thus, sodium bicarbonate therapy slowed the growth of Yumm1.1 melanomas in mice 43. Bicarbonate therapy alone did not impede the growth of aggressive B16 melanomas in mice but enhanced the therapeutic efficacy of anti-PD-1 or anti-CTLA-4 43. Furthermore, long-term survival rate after ACT of melanoma-specific pmel TCR transgenic T cells were improved when combined with bicarbonate (40% vs. 10% with ACT alone) 43.
Indoleamine 2,3-dioxygenase (IDO), produced within the TME by malignant cells, Tregs, stromal cells, and tolerogenic DCs, converts the essential amino acid tryptophan (Trp) to kynurenine (Kyn) 44. The lack of Trp results in decreased mTORC1 signaling, activation of the stress kinase GCN2, and triggers cell cycle arrest and apoptosis in effector T cells 44. Recent studies have shown that Kyn further contributes to immunosuppression in the TME by promoting PD-1 expression by TILs through upregulation and activation of the aryl hydrocarbon receptor (AHR) transcription factor 45. The discovery of a novel role for the enzyme cofactor tetrahydrobiopterin (BH4) in T cells has revealed potential new approaches to counteract the inhibitory effects of Kyn in the TME. Penninger and colleagues demonstrated that the synthesis of BH4 was important for TCR-induced T cell proliferation and influenced mitochondrial metabolism 46. Furthermore, in vitro BH4 supplementation could reverse the inhibitory effects of Kyn on T cells while delivery of BH4 in vivo enhanced T cell infiltration and reduced growth of E0771 and TC1 orthotopic tumors 46. High levels of IDO expression in the TME is correlated with poor prognosis and promotes resistance to CTLA-4 blockade 47 and CD19-CAR-T cells 48. A recent study evidenced the beneficial role of IDO1 inhibitors in long-term survival in combination with radiotherapy and PD-1 blockade in a mouse model of glioblastoma 49. Similarly, studies performed in the murine B16.SIY melanoma model showed improved tumor control when combining anti-PD-L1 or anti-CTLA-4 with the IDO inhibitor INCB23843 50.
Concluding remarks
The TME has long been recognized as hostile to effective immune responses. The development of checkpoint inhibitors demonstrated the capacity of endogenous immune responses to be reinvigorated and combat tumor growth, while advances in ACT approaches have also resulted in dramatic improvements in patient outcomes. Complimentary approaches to reduce the impact of the nutrient-deprived and immunosuppressive TME on antitumor responses may help to optimize immunotherapies. Furthermore, identification of the pathways and targets that determine T cell metabolic fitness are likely to result in new approaches to enhance the durability and efficacy of antitumor responses following T cell ACT. The discovery of such novel approaches to target tumor cell and immune metabolism will likely broaden the applicability and enhance the efficacy of immune-based anticancer therapies in the future.
Acknowledgements
Work in the Salmond laboratory is supported by a grant from Cancer Research UK (23269). H.C.H. is supported by a University of Leeds PhD scholarship. The authors are grateful to Prof. G. Cook and Dr. R. Brownlie for constructive comments on manuscript drafts.
Conflict of interest
The authors declare no financial or commercial conflict of interest.
References
Abbreviations
-
- A2AR
-
- adenosine A2A receptor
-
- ACT
-
- adoptive cell transfer
-
- BH4
-
- tetrahydrobiopterin
-
- CAR
-
- chimeric antigen receptor
-
- FAO
-
- fatty acid oxidation
-
- Kyn
-
- kynurenine
-
- OXPHOS
-
- oxidative phosphorylation
-
- PEP
-
- phosphoenolpyruvate
-
- PPAR
-
- peroxisome proliferator activated-receptor
-
- TIL
-
- tumor-infiltrating lymphocytes
-
- TME
-
- tumor microenvironment