Journal list menu
Export Citations
Download PDFs
Communications
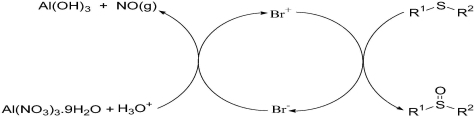
Aluminium Nitrate/Silica Sulfuric Acid/Bromide Ion: As an Effective and Catalytic Oxidizing Media for the Selective Oxidation of Sulfides to Sulfoxides
- Pages: 1191-1194
- First Published: 25 September 2013
Multifunctional Catalysis of Heteropoly Acid: One-Pot Synthesis of Quinolines from Nitroarene and Various Aldehydes in the Presence of Hydrazine
- Pages: 1195-1198
- First Published: 25 September 2013
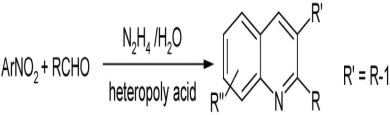
12-Molybdophosphoric acid catalyzed transfer hydrogenation of nitroarene by hydrazine in a homogeneous phase. This catalytic system was applicable to one-pot quinolines synthesis in the presence of various aldehydes in water. This method provides a new and efficient protocol in terms of mild reaction conditions, clean reaction profiles, small quantity of catalyst, and simple work-up procedure.
The One-Pot Synthesis of 2,4,5-Triaryl-Imidazoles Using Heteropolyacids as Heterogeneous and Recyclable Catalysts
- Pages: 1199-1203
- First Published: 25 September 2013
Hypervalent Iodine(III) Sulfonate Mediated Synthesis of α-Thiocynanatoketones in a Task-Specific Ionic Liquid [bmim]SCN
- Pages: 1204-1207
- First Published: 25 September 2013
![Hypervalent Iodine(III) Sulfonate Mediated Synthesis of α-Thiocynanatoketones in a Task-Specific Ionic Liquid [bmim]SCN](/cms/asset/50664987-a775-4d83-a794-e29c31dee3d1/mcontent.jpg)
The task-specific ionic liquid (TSIL) and 1-n-butyl-3-methylimidazolium thiocynanate, ([bmim]SCN) were used as the medium as well as the reactant for the synthesis of a-thiocynanatoketones by the reaction with a-sulfonyloxy aryl ketones. Significant rate enhancements and improved yields have been observed.
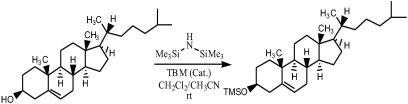
Trimethylsilylation of Hydroxyl Group with 1,1,1,3,3,3-Hexamethyldisilazane (HMDS) Catalyzed by Tribromomelamine (TBM)
- Pages: 1208-1213
- First Published: 25 September 2013
Articles
TH-11, a Streptomyces sp. Strain that Degrades Poly(3-Hydroxybutyrate) and Poly(Ethylene Succinate)
- Pages: 1214-1220
- First Published: 25 September 2013

A mesophilic aliphatic ester degrading strain, TH-11, was isolated from environment and further classified as a Streptomyces. When detected using p-nitrophenyl butyrate as substrate, the esterase activity was not affected when the bacteria were removed. Our results suggested that the depolymerase activity were performed by enzymes released into the culture medium.
A Mixed Solvothermal Route to Synthesis of Dice-Like PbS
- Pages: 1221-1224
- First Published: 25 September 2013

The authors developed a simple solvothermal route to synthesize PbS nanocrystals in the mild binary mixed solvent made of diethylenetriamine and water. Two kinds of PbS nanostructures (dice-like and cubic) have been successfully prepared at 150°C. The results show that the different forming rate of H2S strongly affect growth rate of crystals, and finally decide the morphologies of PbS.
Plasmonic Properties and Size Programming: A Quantitative Study for Photosynthesized Gold Nanoparticles
- Pages: 1225-1234
- First Published: 25 September 2013
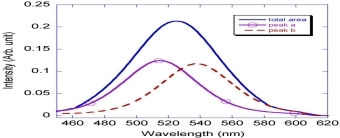
A novel two-component peak quantitative spectra deconvolution model is employed to elucidate the relationship between the plasmonic properties and the concentration-dependent Au nanocrystals nucleation. The variation of peak area (b/a) shows that the second crystal growth progress, induced the blue-shift phenomenon of surface plasmon absorption, is determined by the initial concentration of starting reagent NaAuCl4.
Enhanced Removal of Cu(II) and Pb(II) from Aqueous Solutions by Pretreated Biomass of Fusarium Solani
- Pages: 1235-1242
- First Published: 25 September 2013
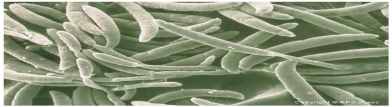
Fusarium solani biomass was used as biosorbent for Cu(II) and Pb(II) removal. An enhanced Cu(II) removal (96.53%) was observed for aluminum hydroxide pretreated biomass. Maximum Pb(II) removal (95.48%) was observed with native biomass. The kinetic studies showed that the biosorption process followed pseudo second-order model for Cu(II) and Pb(II). The equilibrium data fitted well to the Langmuir isotherm model.
Facile Synthesis of 3(2H)-Pyridazinones and 2(3H)-Furanones of Anticipated Biological Activities
- Pages: 1243-1250
- First Published: 25 September 2013
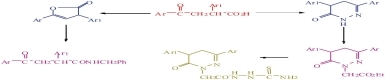
3-Aroyl-2-arylpropionic acids 2a-e were utilized to synthesize 3(2H)-pyridazinones 3a-e and 2(3H)-furanones 6 through reaction with hydrazine hydrate and freshly distilled acetic anhydride, respectively, in the hope of obtaining new 3(2H)-pyridazinones with no ulcerogenic side effect or with negligible general side effects as those currently used NSAIDS as well as biologically active 2(3H)-furanones.
FT-IR Studies of Plasticization of Propylene Carbonate and Ethylene Carbonate in PEO-NaSCN Polymer Electrolytes
- Pages: 1251-1257
- First Published: 25 September 2013
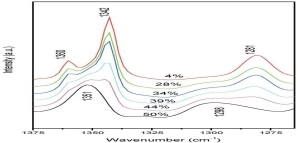
Using the FT-IR spectroscopy, the interactions are revealed for the gel polymer electrolytes (GPEs) of poly(ethylene oxide) (PEO)-NaSCN-propylene carbonate (PC) and PEO-NaSCN-ethylene carbonate (EC). This work quantitatively measures the fraction of PEO amorphous phase, and compares the plasticization of PC and EC in PEO-NaSCN polymer electrolytes with different NaSCN content.
Synthesis and Structural Characterisation of Derivatives of Tricyclo[5.2.1.02,6]Dec-8-Ene-3,5-Dione with an Expected Antimicrobial Activity
- Pages: 1258-1265
- First Published: 25 September 2013
![Synthesis and Structural Characterisation of Derivatives of Tricyclo[5.2.1.02,6]Dec-8-Ene-3,5-Dione with an Expected Antimicrobial Activity](/cms/asset/0b011d27-40a0-4b4f-83bf-220aa0a46fec/mcontent.jpg)
Anhydrides, imides, N-ethylimides, N-hydroxyimides and N-aminoimides of 1,4,5,6-tetramethyl-bicyclo[5.2.1.02,6]hept-5-ene-2,3-dicarboxylic acid, 1,4,5,6,7-pentamethyl-bicyclo [5‥2.1.02,6]hept-5-ene-2,3-dicarboxylic acid and 7-ethyl-1,4,5,6-tetramethyl-bicyclo[5.2.1.02,6]hept-5-ene-2,3-dicarboxylic acid were obtained. Antimicrobial activity of the newly obtained derivatives was tested against selected Gram-positive and Gram-negative bacteria and fungi of the Candida species. The structures of obtained compounds and their antimicrobial activity were compared. Structure of 1b, 2b and 1e were determined by an X-ray analysis.
Synthesis of Nano-Sized Zinc Oxide Photocatalyst by Combustion Method
- Pages: 1266-1271
- First Published: 25 September 2013
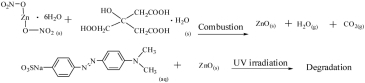
The objective of this research was to use combustion synthesis to create a nano-sized ZnO photocatalyst using citric acid as the fuel and zinc nitrate as the oxidant. Experimental observations reveal that both UV light and ZnO powders are needed for the photocatalytic reaction and that, in addition, the scattering problem of UV light also needs to be considered. In this work, the optimized photocatalytic degradation ratio reaches 92.7%.
Theoretical Study of Heterocyclic Nitro Compounds for Use as Novel Explosives and Propellants
- Pages: 1272-1276
- First Published: 25 September 2013
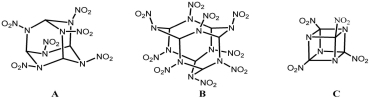
The heats of formation are predicted to be 567.90, 874.29 and 975.83 kJ/mol at the B3LYP/6-31G* level for hexanitrohexazaadamantane (A), nonanitrononaza-tetracyclo[7.3.1.13,7.15,11] pentadecane (B) and tetranitrotetrazacubane (C) respectively. The detonation velocities of the title compounds are larger than, and detonation pressures are much larger than that of the widely used 1,3,5,7-tetranitro-1,3,5,7-tetraazacyclooctane. The dissociation energies for the weakest C-N bonds in the cage skeleton of the title compounds are 137-144 kJ/mol.
A New Steroid Glycoside Derivative from Acorus Calamus L.
- Pages: 1277-1279
- First Published: 25 September 2013
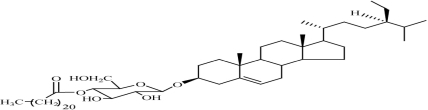
A new steroid glycoside derivative, 4′-O-docosanoyl-3-O-β-D-glucosyl-sitosterol, along with six known steroids has been isolated from the ethanol extract of the rhizoma of Acorus calamus L‥ The structures of the new and known compounds were established on the basis of extensive 1D and 2D NMR spectral data.
Fe3+-Montmorillonite: An Efficient Solid Catalyst for One-Pot Synthesis of Decahydroacridine Derivatives
- Pages: 1280-1285
- First Published: 25 September 2013
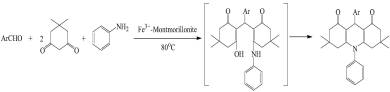
A series of 1,8-dioxo-decahydroacridine derivatives were synthesized through condensation of aromatic aldehydes, dimedone and aniline catalyzed by Fe3+-montmorillonite. Benzaldehyde and other aromatic aldehydes were employed and reacted well to give the corresponding decahydroacridine in the yields ranging from 80% to 92% over Fe3+-montmorillonite catalyst. Variations of the electronic nature of substituents on the aromatic ring did not show obvious effects in terms of yields.
Sesquiterpenoids-Related Metabolites from the Soft Coral Sinularia sp.
- Pages: 1286-1289
- First Published: 25 September 2013
Synthesis of Morphlinotetrahydrothieno[2,3-c]Isoquinolines
- Pages: 1290-1299
- First Published: 25 September 2013
![Synthesis of Morphlinotetrahydrothieno[2,3-c]Isoquinolines](/cms/asset/37829f78-fa69-4ef7-8baa-6a93b97a7f7a/mcontent.jpg)
1-Morpholin-4-yl-5,6,7,8-tetrahydroisoquinoline-4-carbonitrile was synthesized from 3-amino-1-thioxo-5,6,7,8-tetrahydro-1H-isothiochromene-4-carbonitrile and used as starting material to synthesize many thienotetrahydroisoquinolines, which inturn were used in the synthesis of many pyrimido-thienotetrahydroisoquinolines.
Synthesis and Characterization of Novel Monoazo Naphthalimide Disperse Dyes Containing Carboxylic Acid Group with High Heat Fastness Properties
- Pages: 1300-1307
- First Published: 25 September 2013
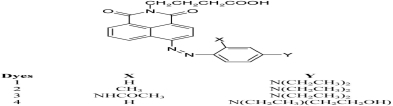
N-ester group of naphthalimide disperse dyes caused to increase the dyeing capability while these dyes do not have good heat fastness at high temperatures (> 180 °C). So, we decided to substitute N-ester group with carboxylic group. Thus, in the present study, the synthesis, characterization and dyeing properties of novel monoazo disperse dyes having carboxylic functional group were reported.
Substitute Effect on the Structure, Stability of Valence Isomers and Aromaticity of 1-H-Boratabenzene
- Pages: 1308-1312
- First Published: 25 September 2013

Density functional theory (B3LYP) calculations were performed on the Me and F substituted valence isomeric forms of 1-H-boratabenzne. The calculations reveal that planar benzene analog is the lowest energy isomer. The aromaticity of it is analyzed in the light of the nucleus-independent chemical shift (NICS) and shows that aromaticity increase in F substituted, but decrease in Me substituted.
Synthesis and Fluorescent Properties of 2-Arylamino-5-(3-Aryl-1-Phenyl-Pyrazol-4-Yl)-1,3,4-Thiadiazoles
- Pages: 1313-1316
- First Published: 25 September 2013
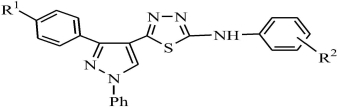
A series of novel pyrazolyl-substituted 1,3,4-thiadiazole derivatives were prepared by cyclization of the intermediate 3-aryl-l-phenyl-pyrazol-4-ylformaldehyde 4′-phenylthiosemicarbazones with 0.5 M ferric chloride solution. The structures of the new compounds were confirmed by IR, 1H NMR and elemental analysis. Simultaneously, the compounds were detected by fluorescence spectrophotometer and had preferable fluorescence activity.
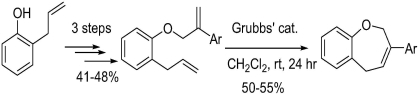
Synthesis of 3-Aryl-2,5-Dihydro-1-Benzoxepines from Phenol via Ring-Closing Metathesis
- Pages: 1317-1321
- First Published: 25 September 2013
The Regiospecific Synthesis of Some New 3,5-Disubstituted Isoxazoles
- Pages: 1322-1325
- First Published: 25 September 2013
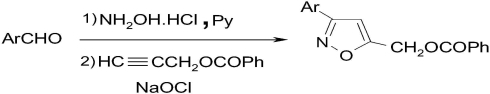
Propargyl alcohol (1) reacted with benzoyl chloride to give propargylphenylcarboxylate (2). Then, the aldehydes (3a-3g) were converted to the related oximes (4a-4g) and nitrileoxides in situ by NaOCl, consequently. Reaction of compound 2 with nitrileoxides in a [3+2] cycloaddition reaction gave regiospecifically isoxazoles (5a-5g). 1H NMR, 13C NMR, FT-IR, and elemental analyses confirmed the structure of the synthesized compounds.
Synthesis and In Vivo Anticonvulsant Screening of Coumarin Incorporated Schiff Bases of 1,3,4-Oxadiazoles
- Pages: 1326-1331
- First Published: 25 September 2013
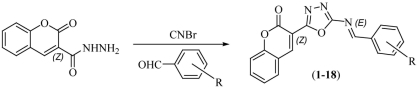
3-(5-{[(1E)-(substituted phenyl) methylene] amino}-1,3,4-oxadiazol-2-yl)-2H-chromen-2-ones (1-18) were synthesized starting from 2-Oxo-2H-chromene-3-carboxylic acid hydrazide in a simple two step process which involves the cyclization of carbohydrazide using cyanogen bromide to oxadiazole. The Schiff bases were then prepared by reacting the later with appropriate substituted benzaldehydes. All the compounds displayed appreciable anticonvulsant activity with less neurotoxicity when tested against the MES induced seizures.
Study on Determination of Triterpenoids in Chaenomeles by High Performance Liquid Chromatography and Sample Preparation with Matrix Solid Phase Dispersion
- Pages: 1332-1337
- First Published: 25 September 2013
Speciation Analysis of Dissolved Copper in Wastewater with Azocarmine B by Light-Absorption Ratio Variation Combined with Continuous Flow Analysis
- Pages: 1338-1344
- First Published: 25 September 2013
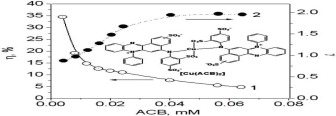
The complexation between Cu(II) and of azocarmine B was characterized by the spectral correction method and used for determination of Cu(II) by the light-absorption ratio variation approach combined with continuous flow analysis. The limit of detection of Cu(II) is 0.04 mg/L, the recovery is between 97 and 100% and about 30 samples could be analyzed per hour by CFA.
A Novel Ion-Selective Electrode for Determination of the Mercury(II) Ion Based on Schiff Base as a Carrier
- Pages: 1345-1350
- First Published: 25 September 2013

The ionophore bis-salicyladehyde-diaminjodipropylamine (BSDDA) was synthesized by the reaction of salicyladehyde with diaminjodipropylamine in a 2:1 molar ratio in ethanol. The O and N atoms in ionophore are in favor of the coordination between the ionophore and metal ions. The experimental results demonstrated that the ionophore exhibited excellent potentiometric response characteristics for Hg2+ ion, in addition, the electrode can be used to the detection of Hg2+ ion in the sample.
Assay of Phenformin Hydrochloride in Pharmaceutics by Coupling with Sodium Nitroprusside
- Pages: 1351-1356
- First Published: 25 September 2013
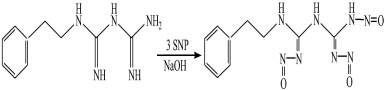
One phenformin hydrochloride molecule and three sodium nitroprusside(SNP) molecules can take place nucleophilic substitution reaction in basic solution. The formed product has maximal absorption wavelength at 520nm. Phenformin hydrochloride and SNP almost have no absorption in the range of 500nm − 900nm. According to the absorbance of product, the amount of phenformin hydrochloride can be obtained.
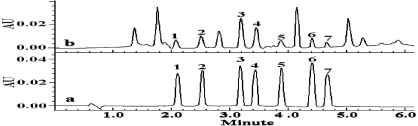
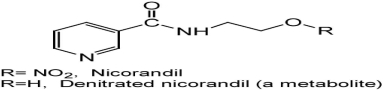
Quantitative Analysis of Nicorandil in Commercial Tablets by Spectrophotometry
- Pages: 1357-1366
- First Published: 25 September 2013
Separation and Retention Behavior of Aromatic Carboxylic Acid Isomers by High-Performance-Liquid-Chromatography Using β-Cyclodextrin Bonded Phase with Diamine-s-Triazine Moiety
- Pages: 1367-1372
- First Published: 25 September 2013
A b-cyclodextrin (b-CD) bonded phase with diamine-s-triazine moiety was prepared. The separation and retention behavior of the isomers of five aromatic carboxylic acids, including toluic acid, aminobenzoic acid, nitrobenzoic acid, hydroxybenzoic acid, and naphthoic acid were investigated by a high-performance liquid chromatography (HPLC) using the b-CD bonded phase prepared. The influence of mobile phase pH in the range of 2.7-3.6 on the retention of these analytes was examined. The isomers of the aromatic carboxylic acids, with the exception of nitrobenzoic acid, were optimally and effectively separated at pH 2.7, while the three isomers of nitrobenzoic acid could be well separated at pH 3.3. Compared with the chromatographic results obtained previously on the amine-s-triazine-b-CD bonded phase, the retention factors of the isomers of aromatic carboxylic acid on the diamine-s-triazine-b-CD bonded phase increase to a relatively much greater extent. Thus, the functionality of the spacer arm of the bonded phase playing an important role in the retention of aromatic carboxylic acid isomers is demonstrated. The results also imply that the hydrogen-bonding interaction and the mechanism of anion exchange sorption as well may contribute significantly to the retention mechanisms.
Notes
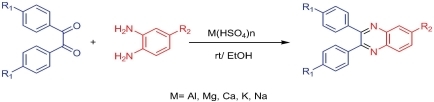
Metal Hydrogen Sulfates M(HSO4)n: As Efficient Catalysts for the Synthesis of Quinoxalines in EtOH at Room Temperature
- Pages: 1373-1378
- First Published: 25 September 2013