Journal list menu
Export Citations
Download PDFs
ISSUE INFORMATION
ORIGINAL ARTICLE
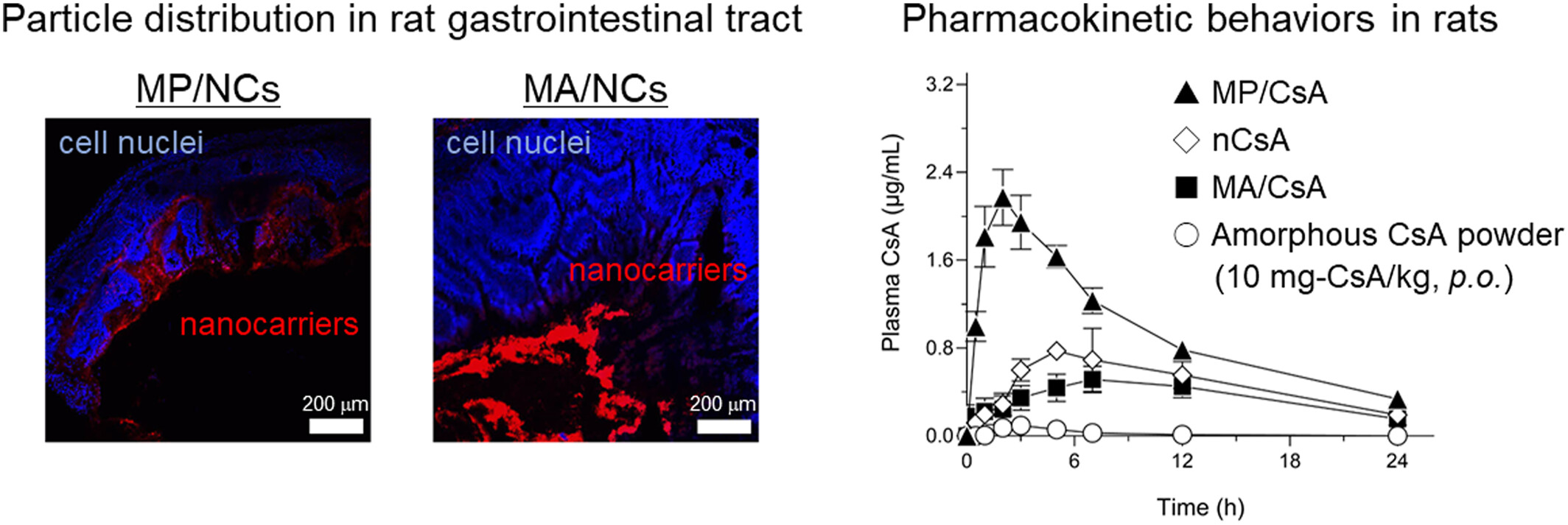
Pharmacokinetic control of orally dosed cyclosporine A with mucosal drug delivery system
- Pages: 117-126
- First Published: 22 April 2024
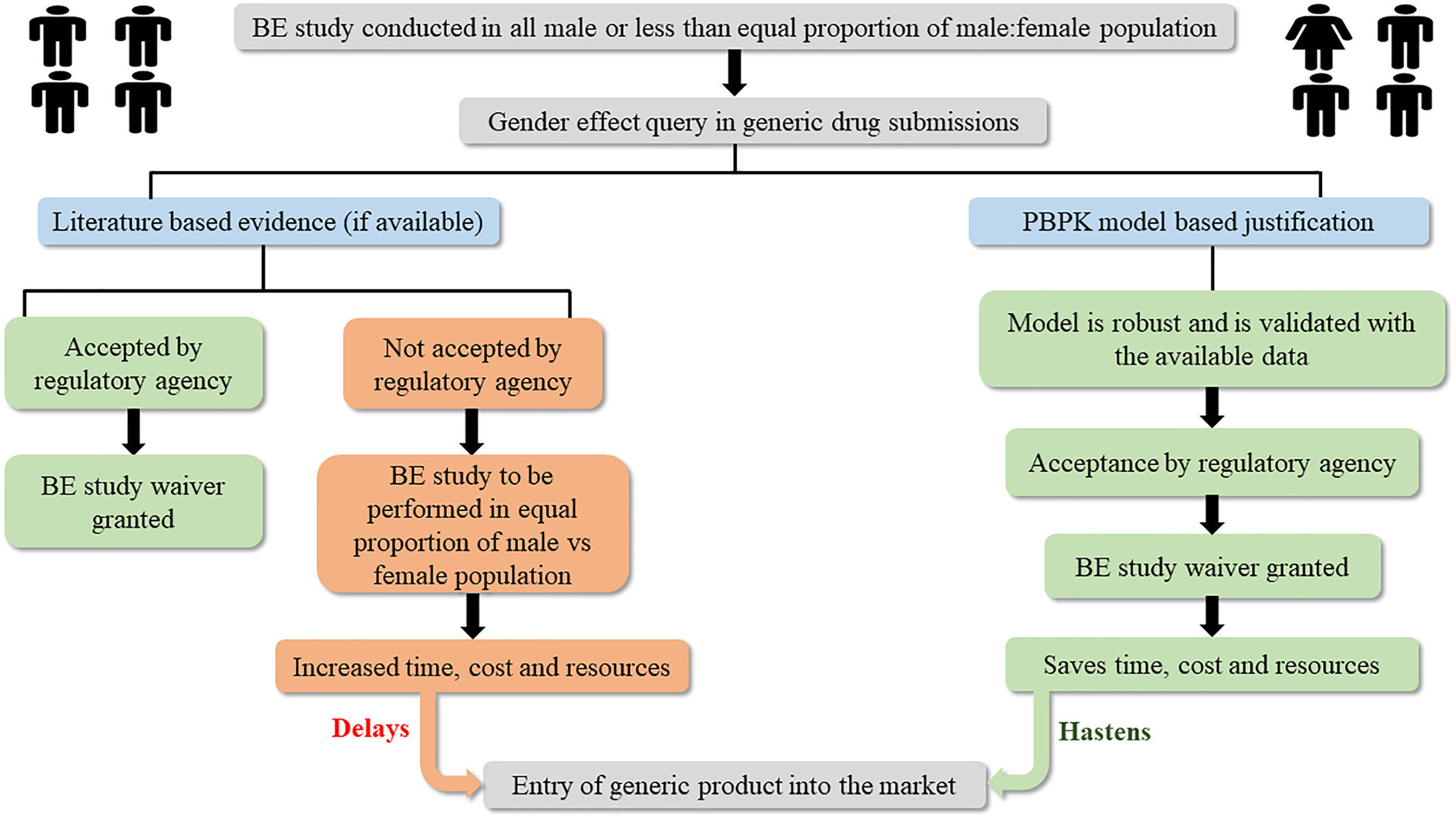
Evaluating gender effect in the generic bioequivalence studies by physiologically based pharmacokinetic modeling – A case study of dextromethorphan modified release tablets
- Pages: 127-137
- First Published: 22 May 2024
Heterogeneous brain distribution of bumetanide following systemic administration in rats
- Pages: 138-148
- First Published: 01 June 2024
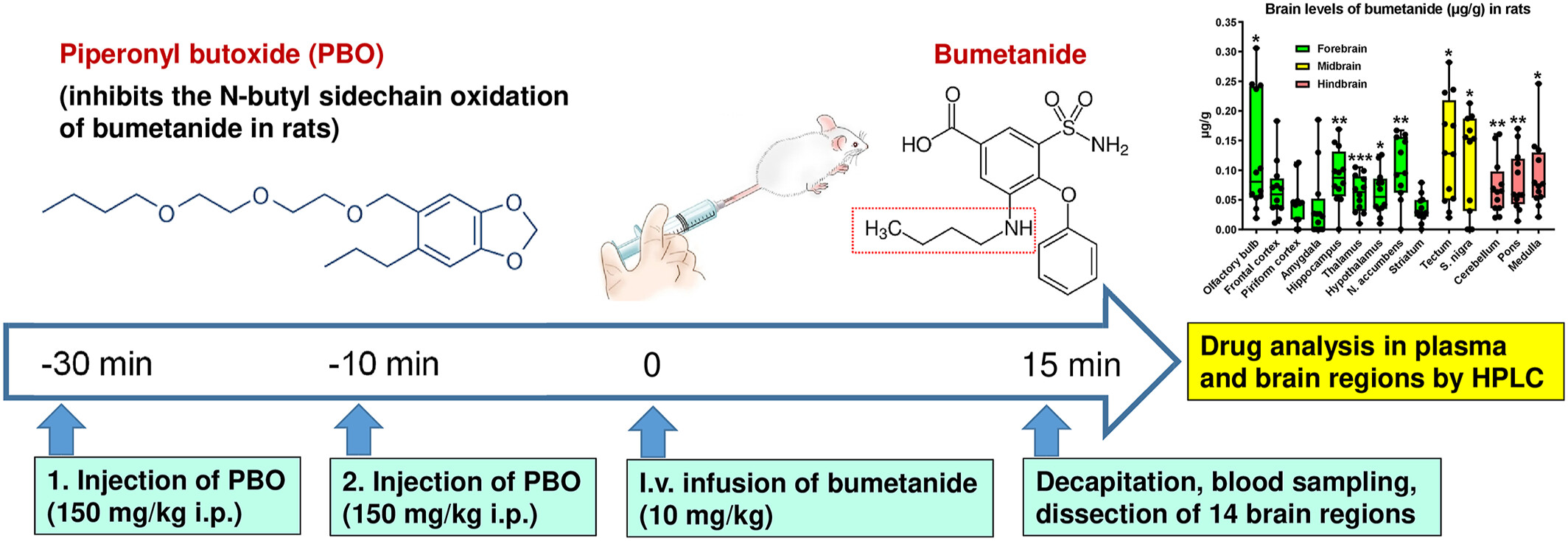
Bumetanide was determined in 14 brain regions following i.v. administration of 10 mg/kg in rats after inhibition of its metabolism by piperonyl butoxide. Significant, up to 5-fold differences in regional bumetanide levels were determined with the highest levels in the midbrain and olfactory bulb and the lowest levels in the striatum and amygdala.
Icaritin exhibits potential drug–drug interactions through the inhibition of human UDP-glucuronosyltransferase in vitro
- Pages: 149-158
- First Published: 17 June 2024

This study provides supporting information for understanding the drug–drug interaction (DDI) potential of the flavonoid icaritin and other UGT-metabolized drugs in clinical. In addition, the findings provide safety evidence on DDI when patients with liver cancer receive a combination therapy including icaritin.