Chemoimmunotherapy for esophageal squamous cell carcinoma—Summary and discussion of recent clinical trials
Abstract
As a kind of carcinoma with increasing morbidity, poor prognosis, and high mortality, esophageal squamous cell carcinoma (ESCC) is challenging for clinical management. Chemotherapy has been the standard treatment for ESCC over decades, while its clinical outcomes remain unsatisfying. And the regimen that combine standard chemotherapy with targeted therapy also demonstrates little effect. However, the advent of immune checkpoint inhibitors (ICI) proved to be a game changer in cancer treatment. Recent clinical trials had sprung up to evaluate the combined effect of ICI and chemotherapy regarding first-line ESCC treatment. What's more, researchers attempt to explore the possibility to implement ICI monotherapy regarding second-line ESCC treatment. In conclusion, most of the first-line trails present inspiring achievement, while ICI monotherapy indicates little improvement for ESCC treatment. To point out the heterogenicity that could be the potential reasons biasing the pooled results, the differences of PD-L1 immunohistochemistry (IHC) assays, geographic regions, chemotherapy regimens, and sex disparity among these trails are discussed respectively. In addition, the adverse events occurred during the trails are summarized, which confirm the safety of immunotherapy and chemoimmunotherapy. The article comprehensively reviews the representative explorations of using chemoimmunotherapy strategies in ESCC, as well as the deficiencies among them. Moreover, we highlight some feasible approaches. It will be beneficial for conducting more precise clinical trials on chemoimmunotherapy for ESCC in the future, including the use of more appropriate PD-L1 IHC assays, careful consideration of the heterogeneity of the enrolled population and the optimal combination of chemotherapy and ICI.
1 INTRODUCTION
Esophageal cancer is the sixth leading cause of cancer mortality worldwide and responsible for one in every 18 cancer deaths globally in 2020.1 Esophageal squamous cell carcinoma (ESCC) and esophageal adenocarcinoma (EAC) are the two most common histological types. An interesting geographic pattern was observed regarding the prevalence of EAC and ESCC. Eastern Asia, especially China, exhibits the highest regional incidence rates with ESCC being the predominant histological type, and thus many trials evaluating the combination of chemotherapy and immunotherapy (chemoimmunotherapy) in ESCC were mainly conducted in China.2-6 On the other hand, EAC contributes to approximately two-thirds of esophageal cancer population in western countries, such as the United States, the United Kingdom, Australia, and so on. 30%–40% of patients were diagnosed with advanced or metastatic esophageal cancer at initial diagnosis and their 5-year relative survival rate is only around 5%.7, 8
Traditional standard first-line therapy for advanced or metastatic esophageal cancer is doublet platinum-based chemotherapy with median overall survival (OS) about 1 year,9-15 while combining human epidermal growth factor receptor 2 (HER2) antibody with the standard chemotherapy is recommended for HER2 positive GAC.16 A case report demonstrated the promising treatment effect of adding anti-HER2 to chemotherapy in HER2-positive ESCC,17 but relevant randomized trials are lacking largely due to the very low HER2-positive rate in ESCC.18, 19 There is no evidence that targeted therapies are of clinical significance in phase III trials regarding advanced or metastatic ESCC.13, 20, 21
In fact, there have been few advances in survival outcomes of first-line treatment for advanced or metastatic ESCC over the past 30 years.22 However, results from recent landmark trials using chemoimmunotherapy have brought revolutionary changes to the existing treatment strategy for ESCC patients. These practice-changing trials consist of KEYNOTE-590, CheckMate-648, RATIONALE-306, ESCORT-1, JUPITOR-06, ORIENT-15, and ASTRUM-007.2-5, 23-25 Among these trials, the regimens might have some differences, but most of them investigate ICI or placebo plus investigator-chosen chemotherapy (ICC; platinum and fluoropyrimidine or platinum and paclitaxel) as treatment for ESCC. Majority of trials have uncovered the contribution of chemotherapy to antitumor immunity, which boosts the effect of immunotherapy through different mechanisms. For example, the direct cytotoxic effects of chemotherapy might induce immunogenic cell death (ICD) to facilitate the recruitment of antigen-presenting cells (APCs) and T cells, and thus potentiate immunotherapy. Corresponding to the immunosuppressive tumor microenvironment resulted from tumor cells and suppressive immune cells, such as myeloid-derived suppressor cells (MDSCs) and regulatory T cells, it is significant for chemotherapy to reduce or deplete these cells through the enhancement of antigenicity of cancer cells, the activation of apoptosis, and the support of differentiation of immune cells. Moreover, other noncytotoxic chemotherapy, such as epigenetic modulators, could augment response to immunotherapy by modulating gene expression. Because of the advantages of cooperating two kinds of treatment mechanism, chemoimmunotherapy may not only lead to more effective outcomes, but with fewer side effects (Figure 1).26, 27 In the context of poor efficacy of chemotherapy and targeted therapy in the treatment of ESCC, the encouraging results from the aforementioned clinical trials with the application of chemoimmunotherapy provoke thinking about better strategy to implement.
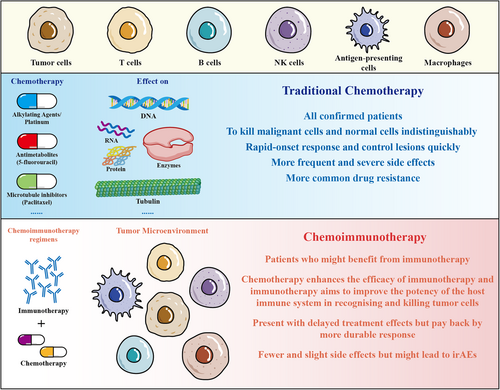
This review aims to discuss the results of previous trials, which could be divided into first-line and second-line treatment, or could be classified as global or Asian based on the patients enrolled. To address the controversial issues and conduct more efficient clinical trials in the future, we discuss the disparities among these trails, included PD-L1 immunohistochemistry (IHC) assays, geographic regions, chemotherapy regimens, and sex. Additionally, we summarize the adverse events (AEs) happened during the implementation of chemoimmunotherapy to confirm the safety. Herein, we highlight it will be of interest to investigate low-dosage chemotherapy plus immunotharapy as a better strategy in ESCC.
2 FIRST-LINE GLOBAL TRIALS
There have been three global ESCC ICI trials so far, which investigated the efficacy and safety of programmed cell death protein 1 antibody (anti-PD1) plus chemotherapy for advanced or metastatic ESCC (Table 1).
| Trial | Design | Population | OS | PFS | ORR | PD-1 Assays | Reference | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Months | Hazard ratio (HR) (95% confidence interval) | p | Months | HR (95% CI) | p | ||||||
KEYNOTE-590 NCT03189719 (Global) first-line advanced ESCC (73%) EGA (15%) EA (12%) |
Pembrolizumab + CF versus placebo + CF |
ESCC PD-L1 CPS ≥ 10 |
13.9 versus 8.8 | 0.57 (0.43–0.75) | <0.0001 | - | - | <0.0001 | - | 22C3 | [23] |
| All ESCC | 12.6 versus 9.8 | 0.72 (0.60–0.88) | 0.0006 | 6.3 versus 5.8 | 0.65 (0.54–0.78) | <0.0001 | - | ||||
PD-L1 CPS ≥ 10 |
13.5 versus 9.4 | 0.62 (0.49–0.78) | <0.0001 | 7.5 versus 5.5 | 0.51 (0.41–0.65) | <0.0001 | - | ||||
| All patients | 12.4 versus 9.8 | 0.73 (0.62–0.86) | <0.0001 | 6.3 versus 5.8 | 0.65 (0.55–0.76) | <0.0001 | 45.0% versus 29.3% | ||||
Check Mate-648 NCT03143153 (Global) first-line advanced ESCC |
Nivolumab + CF versus CF |
Tumor cell PD-L1 ≥ 1% |
15.4 versus 9.1 | 0.54 (0.37–0.80) | <0.0001 | 6.9 versus 4.4 | 0.65 (0.46–0.92) | 0.0023 | 53.0% versus 20.0% | 28-8 | [24] |
| All patients | 13.2 versus 10.7 | 0.74 (0.58–0.96) | 0.0010 | 5.8 versus 5.6 | 0.81 (0.64–1.04) | 0.0355 | 47.0% versus 27.0% | ||||
Nivolumab + Ipilimumab versus CF |
Tumor cell PD-L1 ≥ 1% |
13.7 versus 9.1 | 0.64 (0.46–0.90) | 0.0021 | 4.0 versus 4.4 | 1.02 (0.73–1.43) | 0.8958 | 35.0% versus 20.0% | |||
| All patients | 12.8 versus 10.7 | 0.78 (0.62–0.98) | 0.0110 | 2.9 versus 5.9 | 1.26 (1.04–1.52) | - | 28.0% versus 27.0% | ||||
RATIONALE-306 NCT03783442 (Global) first-line advanced ESCC |
Tislelizumab+ TP/CF versus placebo + TP/CF | PD-L1 TAP ≥ 10% |
16.8 versus 10.0 | 0.61 (0.44–0.85) | 0.0017 | - | - | - | - | SP263 | [2] |
| All patients | 17.3 versus 10.6 | 0.66 (0.54–0.80) | <0.0001 | 7.3 versus 5.6 | 0.62 (0.52–0.75) | <0.0001 | 63.5% versus 42.4% | ||||
ESCORT-1 NCT03691090 (Asian) first-line advanced ESCC |
Camrelizumab + TP versus placebo + TP |
Tumor cell PD-L1 ≥ 1% |
15.3 versus 11.5 | 0.59 (0.43–0.80) | - | 6.9 versus 5.6 | 0.51 (0.39–0.67) | - | 74.1% versus 65.6% | 6E8 | [3] |
Tumor cell PD-L1 < 1% |
15.0 versus 12.0 | 0.79 (0.57–1.11) | - | 6.9 versus 5.7 | 0.62 (0.46–0.83) | - | 69.8% versus 57.7% | ||||
| All patients | 15.3 versus 12.0 | 0.70 (0.56–0.88) | 0.0010 | 6.9 versus 5.6 | 0.56 (0.46–0.68) | <0.0010 | 72.1% versus 62.1% | ||||
JUPITER-06 NCT03829969 (Asian) first-line advanced ESCC |
Toripalimab + TP versus placebo + TP |
PD-L1 CPS ≥ 10 |
17.0 versus 10.9 | 0.64 (0.40–1.03) | 0.0618 | 5.7 versus 5.6 | 0.65 (0.45–0.92) | 0.0162 | - | JS311 | [4] |
PD-L1 CPS < 10 |
16.9 versus 11.4 | 0.61 (0.40–0.93) | 0.0209 | 5.8 versus 5.5 | 0.56 (0.41–0.78) | 0.0004 | - | ||||
| All patients | 17.0 versus 11.0 | 0.58 (0.43–0.78) | 0.0004 | 5.7 versus 5.5 | 0.58 (0.46–0.74) | <0.0001 | 69.3% versus 52.1% | ||||
ORIENT-15 NCT03748134 (Asian) first-line advanced ESCC |
Sintilimab + TP/CF versus placebo + TP/CF | PD-L1 CPS ≥ 10 |
17.2 versus 13.6 | 0.64 (0.48–0.85) | 0.0018 | 8.3 versus 6.4 | 0.58 (0.45–0.75) | <0.0001 | 78.7% versus 57.5% | 22C3 | [5] |
| All patients | 16.7 versus 12.5 | 0.628 (0.51–0.78) | <0.0001 | 7.2 versus 5.7 | 0.56 (0.46–0.68) | <0.0001 | 75.5% versus 56.9% | ||||
ASTRUM-007 NCT03958890 (Asian) first-line advanced |
Serplulimab + CF versus placebo + CF |
PD-L1 CPS ≥ 10 |
18.6 versus 13.9 | 0.59 (0.40–0.88) | 0.0082 | 7.1 versus 5.3 | 0.48 (0.34–0.68) | <0.0001 | 66.0% versus 41.8% | 22C3 | [25] |
PD-L1 1 ≤ CPS < 10 |
14.2 versus 11.4 | 0.74 (0.54–1.03) | 0.0006 | 5.8 versus 5.3 | 0.70 (0.52–0.94) | 0.0170 | 51.0% versus 42.3% | ||||
| All patients | 15.3 versus 11.8 | 0.68 (0.53–0.87) | 0.0020 | 5.8 versus 5.3 | 0.60 (0.48–0.75) | <0.0001 | 57.6% versus 42.1% | ||||
- Abbreviations: CF, cisplatin and 5-fluorouracil; EA, esophageal adenocarcinoma; ESCC, esophageal squamous cell carcinoma; ICC, investigator-chosen chemotherapy; ORR, objective response rate; OS, overall survival; PFS, progression free survival; TP, paclitaxel and cisplatin. 22C3, 28-8, SP263, 6E8, and JS311 were different immunohistochemistry (IHC) assays used to assess PD-L1 expression status.
2.1 KEYNOTE-590
The first study, KEYNOTE-590, is a randomized, double-blind, phase 3 trial evaluating pembrolizumab (anti-PD1) or placebo plus cisplatin and 5-fluorouracil (CF) as first-line treatment for advanced esophageal or gastroesophageal junction (GEJ) carcinoma. Seven hundred and forty-nine patients were enrolled across 26 countries, with almost equal population from Asian and non-Asian regions. Squamous cell carcinoma histology accounted for 73% of all eligible patients. Subgroup analysis stratified patients according to PD-L1 combined positive score (CPS), which is defined as the number of all PD-L1-positive cells within tumor microenvironment divided by the total number of viable tumor cells. Pembrolizumab significantly improved OS and progression-free survival (PFS) in both all patient analysis and ESCC subgroup analysis. For all randomized patients, median OS was extended by 2.6 months (median 12.4 vs. 9.8 months; p < 0.0001). OS benefit was most prominent in ESCC patients with PD-L1 CPS ≥ 10 (improved by 5.1 months; median 13.9 vs. 8.8 months; p < 0.0001). However, pembrolizumab failed to improve survival in ESCC patients with PD-L1 CPS < 10 (median 10.5 vs. 11.1 months). It seems responsiveness of ESCC to pembrolizumab depends on PD-L1 expression status and higher PD-L1 CPS indicates better outcomes. It is also notable that Asian subgroup demonstrated more survival benefit from pembrolizumab than non-Asian counterparts (hazard ratio [HR] of Asian vs. non-Asian: 0.64 vs. 0.83). Distinct genetic background of ESCC between Asian and non-Asian population could be a possible culprit.28 Considering higher incidence of ESCC in Asian population,1 it could be due to the different proportion of histologic types as well. Therefore, detailed histology information within each geographic subgroup was required to answer the question, which was not reported in the original study.
2.2 CheckMate-648
The second study, CheckMate-648, is an open-label, randomized, phase 3 trial, which assigned 970 treatment-naïve advanced or metastatic ESCC patients to receive nivolumab (anti-PD1) plus CF, nivolumab plus ipilimumab (anti-PD-L1), or CF with a ratio of 1:1:1. Asian and non-Asian patients takes up 70% and 30% respectively. Both PD-L1 CPS and PD-L1 tumor proportion score (TPS) were adopted in this study for subgroup analysis. Nivolumab plus CF led to a significant improvement of OS in patients with high PD-L1 expression (TPS ≥ 1%, median OS: 15.4 vs. 9.2 months, HR: 0.55 [0.42–0.72]; CPS ≥ 10, median OS: 16.1 vs. 11.6 months, HR: 0.63 [0.47–0.84]) as well as all randomized patients (median OS: 13.2 vs. 10.7 months, HR: 0.74 [0.61–0.89]). Similar results were detected for nivolumab and ipilimumab as well (TPS ≥ 1%, median OS: 15.4 vs. 9.1 months, HR: 0.63 [0.48–0.82]; CPS ≥ 10, median OS: 16.7 vs. 11.6 months, HR: 0.64 [0.47–0.86]; All patients, median OS: 12.7 vs. 10.7 months, HR: 0.78 [0.65–0.94]). However, ESCC with low PD-L1 expression level (TPS < 1%/CPS < 10) did not benefit from neither nivolumab plus chemotherapy nor nivolumab plus ipilimumab. It is consistent with the results of KEYNOTE-590, indicating the predictive value of PD-L1 expression level in anti-PD1 plus chemotherapy for advanced ESCC patients. When it comes to the geographic subgroup analysis, Asian and non-Asian patients shared similar benefit from the addition of nivolumab, but Asian patients demonstrated generally longer survival in both experimental and control arms.
2.3 RATIONALE-306
The third global study, RATIONALE-306, is a double-blind, randomized, phase 3 trial investigating tislelizumab (anti-PD1) or placebo plus ICC as first-line treatment for advanced ESCC. Six hundred and forty-nine patients across 16 countries were enrolled, with 74.9% from Asian and 25.1% from non-Asian regions. Tumor area positivity (TAP) score is introduced in this study to assess PD-L1 expression status, which is defined as the total percentage of the tumor area covered by tumor cells with any membrane staining above background and tumor-associated immune cells with any staining above background.29 Tislelizumab plus ICC significantly prolonged survival in all patients regardless of PD-L1 expression (all patients, median OS: 17.3 vs. 10.6 months, HR: 0.66 [0.54–0.80]; PD-L1 TAP ≥ 10%, median OS: 16.8 vs. 10.0 months, HR: 0.61 [0.44–0.85]; PD-L1 TAP < 10%, median OS: 16.7 vs. 10.4 months, HR: 0.72 [0.55–0.94]).
3 FIRST-LINE ASIAN TRIALS
Almost all Asian trials enrolled exclusively Chinese patients, including ESCROT-1, JUPITER-06, ORIENT-15, and ASTRUM-007 (Table 1). Notably, ORIENT-15 was initially designed as an international study, but the enrollment process outside of China was halted by the Covid pandemic and 97% of eligible patients were from China at the end of the enrollment.
3.1 ESCORT-1
ESCORT-1 is a double-blind, randomized, and phase 3 trial assessing camrelizumab (anti-PD1) or placebo plus paclitaxel and cisplatin (TP) as first-line treatment for advanced ESCC. Camrelizumab plus TP significantly improved OS in all patients and patients with PD-L1 TPS ≥ 1% (all patients, median OS: 15.3 vs. 12.0 months, HR: 0.70 [0.56–0.88]; PD-L1 TPS ≥ 1%, median OS: 15.3 vs. 11.5 months, HR: 0.59 [0.43–0.80]). Numerical survival benefit was also observed in patients with PD-L1 TPS < 1% (median OS: 15.0 vs. 12.0 months, HR: 0.79 [0.57–1.11]). No significant interaction was found between PD-L1 expression and efficacy in this study.
3.2 JUPITER-06
JUPITER-06 is a double-blind, randomized, and phase 3 trial investigating toripalimab (anti-PD1) or placebo plus TP as first-line treatment for advanced ESCC. Toripalimab plus TP extends OS irrespective of PD-L1 expression (all patients, median OS: 17.3 vs. 11.0 months, HR: 0.58 [0.43–0.78]; PD-L1 CPS ≥ 10, median OS: 17.0 vs. 10.9 months, HR: 0.64 [0.40–1.03]; PD-L1 CPS < 10, median OS: 16.9 vs. 11.4 months, HR: 0.61 [0.40–0.93]).
3.3 ORIENT-15
ORIENT-15 is a double-blind, randomized, and phase 3 trial comparing sintilimab (anti-PD1) plus ICC and placebo plus ICC as first-line treatment for advanced ESCC. Although ICC were adopted as standard chemotherapy, 93% patients received TP instead of CF. Remarkably, both TPS and CPS were applied in subgroup analysis. Patients receiving sintilimab plus ICC achieved better clinical outcomes regardless of PD-L1 expression. OS was improved by 5.2 months in all patients, 3.6 months in CPS ≥ 10, 4.1 months in CPS < 10, 5.3 months in TPS ≥ 10%, and 3.8 months in TPS < 10%.
3.4 ASTRUM-007
ASTRUM-007 is a double-blind, randomized, phase 3 trial, which assigned 551 patients with previously untreated, locally advanced or metastatic ESCC and PD-L1 CPS ≥ 1 to receive serplulimab (anti-PD1) or placebo plus CF (cisplatin and continuous infusion of 5-fluoroiracil, once every 2 weeks) with a ratio of 2:1. Serplulimab plus CF significantly prolonged PFS and OS compared with placebo plus CF in all patients (median PFS: 5.8 vs. 5.3 months, HR: 0.60 [0.48-0.75]; median OS: 15.3 vs. 11.8 months, HR: 0.68 [0.53-0.87]). Consistent survival benefits were observed across prespecified subgroups. It is remarkable that patients with PD-L1 CPS ≥ 10 shared more survival benefit from serplulimab plus chemotherapy than that in all randomized patients (median PFS: 7.1 vs. 5.3 months, HR: 0.48 [0.34-0.68]; median OS: 18.6 vs. 13.9 months, HR: 0.59 [0.40-0.88]).
4 SECOND-LINE TRIALS
ICI monotherapy has been investigated in the second-line setting (Table 2).29-32
| Trial | Design | Population | OS | PFS | ORR | PD-1 assays | Reference | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Months | Hazard ratio (HR) (95% confidence interval) | p | months | HR (95% CI) | p | ||||||
KEYNOTE-181 NCT02564263 (Global) ESCC (64%) EA (36%) |
Pembrolizumab versus ICC |
PD-L1 CPS ≥ 10 |
9.3 versus 6.7 | 0.69 (0.52–0.93) | 0.0074 | 2.6 versus 3.0 | 0.73 (0.54–0.97) | - | 21.5% versus 6.1% | 22C3 | [30] |
| All ESCC | 8.2 versus 7.1 | 0.89 (0.63–0.96) | 0.0095 | 2.2 versus 3.1 | 0.92 (0.75–1.13) | - | 16.7% versus 7.4% | ||||
| All patients | 7.1 versus 7.1 | 0.89 (0.75–1.05) | 0.0560 | 2.1 versus 3.4 | 1.11 (0.94–1.31) | - | 13.1% versus 6.7% | ||||
ATTRACTION-3 NCT02569242 (Global ESCC |
Nivolumab versus ICC |
Tumor cell PD-L1 ≥ 1% |
10.9 versus 8.1 | 0.69 (0.51–0.94) | - | - | - | - | - | 28-8 | [31] |
Tumor cell PD-L1 < 1% |
10.9 versus 9.3 | 0.84 (0.62–1.14) | - | - | - | - | - | ||||
| All patients | 10.9 versus 8.4 | 0.77 (0.62–0.96) | 0.0190 | 1.7 versus 3.4 | 1.08 (0.87–1.34) | - | 19.0% versus 22.0% | ||||
ESCORT NCT03099382 (Asian) ESCC |
Camrelizumab + TP versus placebo + TP |
Tumor cell PD-L1 ≥ 1% |
9.2 versus 6.3 | 0.58 (0.42–0.81) | 0.0014 | - | 0.60 (0.43–0.84) | - | - | 6E8 | [32] |
Tumor cell PD-L1 < 1% |
- | 0.82 (0.62–1.09) | - | - | 0.79 (0.59–1.05) | - | - | ||||
| All patients | 8.3 versus 6.2 | 0.71 (0.57-0.87) | 0.0010 | 1.9 versus 1.9 | 0.69 (0.56–0.86) | 0.0006 | 20.2% versus 6.4% | ||||
RATIONALE-302 NCT03430843 (Global) ESCC |
Tislelizumab versus ICC |
PD-L1 TAP ≥ 10% |
10.3 versus 6.8 | 0.54 (0.36–0.79) | 0.0006 | 2.7 versus 2.6 | 0.88 (0.59–1.32) | - | 28.1% versus 11.8% | SP263 | [29] |
PD-L1 TAP < 10% |
6.9 versus 5.8 | 0.82 (0.62–1.09) | - | - | - | - | - | ||||
PD-L1 TAPunknown |
9.8 versus 7.0 | 0.67 (0.41–1.12) | - | - | - | - | - | ||||
| All patients | 8.6 versus 6.3 | 0.70 (0.57–0.85) | 0.0001 | 1.6 versus 2.1 | 0.83 (0.67–1.01) | - | 20.3% versus 9.8% | ||||
- Abbreviations: EA, esophageal adenocarcinoma; ESCC, esophageal squamous cell carcinoma; ICC, investigator-chosen chemotherapy; ORR, objective response rate; OS, overall survival; PFS, progression free survival; TP, paclitaxel and cisplatin. 22C3, 28-8, SP263, 6E8, and JS311 were different immunohistochemistry (IHC) assays used to assess PD-L1 expression status.
4.1 KEYNOTE-181
In KEYNOTE-181 trial, 628 patients with advanced or metastatic esophageal carcinoma, who were refractory to first-line therapy, were randomly assigned (1:1) to pembrolizumab or ICC (i.e., paclitaxel, docetaxel, or irinotecan). Both squamous cell carcinoma and adenocarcinoma were included and accounted for approximately 64% and 36% of total trial population respectively. PD-L1 expression played an essential role in pembrolizumab monotherapy. Favorable antitumor response was observed with pembrolizumab versus chemotherapy in patients with CPS PD-L1 ≥ 10 regardless of histology (object response rate [ORR]: 23% vs. 7% [overall]; 22% vs. 7% [squamous cell carcinoma]; 18% vs. 3% [adenocarcinoma]). Despite the promising result of response, pembrolizumab failed to delay disease progression in subgroup analysis. Pembrolizumab significantly extended OS only in patients with squamous cell carcinoma (median OS: 8.2 vs. 7.1 months, HR: 0.78 [0.63–0.96]), but not in those with adenocarcinoma. The greatest OS benefits were observed in patients with ESCC and PD-L1 CPS ≥ 10 (HR: 0.64 [0.46–0.90]). On the other hand, pembrolizumab showed no survival advantages in patients with CPS PD-L1 < 10.
4.2 ATTRACTION-3
In ATTRACTION-3 trial, 410 ESCC patients who progressed after first-line therapy were randomly assigned (1:1) to nivolumab or ICC. Although patients were enrolled from 90 hospitals across multiple countries, a large majority of patients were Asian (96%). Nivolumab failed to show any advantages in terms of ORR or PFS when compared to chemotherapy. Median time to response was reported and it took average of 2.6 months for nivolumab to take effect, which is 1 month longer than conventional chemotherapy. However, such a delayed effect was paid back by more durable response (duration of response: nivolumab vs. chemotherapy: 6.9 months vs. 3.9 months). OS was prolonged by 2.5 months by nivolumab (median OS: 10.9 vs. 8.4 months, HR: 0.77 [0.62–0.96]). In contrast to KEYNOTE-181, survival benefits of nivolumab were independent of PD-L1 expression levels.
4.3 ESCORT
In ESCORT trial, 410 ESCC patients who were resistant or intolerant to first-line therapy were enrolled from China and randomly assigned (1:1) to camrelizumab or ICC. Different from pembrolizumab and nivolumab, camrelizumab significantly reduce the risk of progression compared with chemotherapy (HR: 0.69 [0.56–0.86]). Although median PFS was the same, percentages of patients who did not progress within 6 months were 22% and 4% with camrelizumab and chemotherapy respectively. Similar with ATTRACTION-3, there was no significant association between PD-L1 expression and efficacy of camrelizumab. In all patients, camrelizumab improved OS by 2.1 months (median OS: 8.2 vs. 6.2 months; HR: 0.71 [0.57–0.87]).
4.4 RATIONALE-302
In RATIONALE-302 trails, 512 ESCC patients with advanced or metastatic ESCC, who progressed after first-line therapy, were randomly assigned (1:1) to tislelizumab or ICC (i.e., paclitaxel, docetaxel, or irinotecan). Similar to ATTRACTION-3, patients were enrolled from 11 countries/regions, but most of them were Asian (78.9%). Tislelizumab led to a significant improvement of OS in all petients and patients with TAP ≥ 10% (all patients, median OS: 8.6 vs 6.3 months, HR: 0.70 [0.57–0.85]; TAP ≥ 10%, median OS: 10.3 vs. 6.8 months, HR: 0.54 [0.36–0.79]). Although tislelizumab failed to exhibit improvement of PFS, ORR was twice as high with tislelizumab versus chemotherapy (20.3% vs. 9.8%). In addition, a more durable antitumor response was also observed in tislelizumab arm (median, 7.1 vs. 4.0 months).
Results from KEYNOTE-181, ATTRACTION-3, ESCORT, and RATIOANLE-302 indicate that ICI monotherapy can effectively improve survival of ESCC patients in the second line setting. Crossing of PFS or OS curves was observed in all ICI monotherapy ESCC trials, which means a small proportion of ICI-treated patients progressed or died at the initial treatment stage. This finding is consistent with reports suggesting delayed treatment effects of immunotherapy.33 There are alternative statistical methods to avoid potential loss of power in identifying effective treatments during clinical trial design with the crossing of survival curve.34, 35 It is essential to avoid the unfavorable clinical outcomes of such a small group of patients at the beginning of treatment, which remains unsolved. Fortunately, this phenomenon does not occur in patients with chemoimmunotherapy. Chemotherapy from the combined regimen might be able to compensate for the delayed treatment effect of immunotherapy.
5 PD-L1 EXPRESSION AND THE EFFECTIVENESS OF CHEMOIMMUNOTHERAPY IN ESCC
The aforementioned trials have provided strong and consistent evidence that anti-PD1 plus chemotherapy is superior to chemotherapy alone as the first-line regimen for advanced ESCC, especially for those with high PD-L1 expression. As for patients with relatively low PD-L1 expression, the effectiveness of chemoimmunotherapy remained uncertain. KEYNOTE-590 and CheckMate-648 demonstrated no clinical benefit of chemoimmunotherapy for ESCC with PD-L1 TPS < 1% or PD-L1 CPS < 10, while the other studies presented with opposite conclusions. Therefore, heterogenic approvals of chemoimmunotherapy as first-line treatment for advanced ESCC can be found from different authorities. US Food and Drug Administration (FDA) approves pembrolizumab plus chemotherapy, nivolumab plus chemotherapy, or nivolumab plus ipilimumab as standard first-line regimen for patients with advanced ESCC regardless of PD-L1 status. European Medicines Agency (EMA), by contrast, authorizes the use of pembrolizumab plus chemotherapy only in patients with PD-L1 CPS ≥ 10 and nivolumab plus chemotherapy or ipilimumab only in patients with PD-L1 TPS ≥ 1%.
Does chemoimmunotherapy confer a survival benefit for ESCC patients with low PD-L1 expression in the first-line setting? A few meta-analyses were performed to tackle this question.36-40 In the study reported by Yap et al.,38 results of CheckMate-648, ESCROT-1, KENOTE-590, and ORIENT-15 were collected as first-line trials and pooled analyses were conducted separately for different methods (i.e., TPS and CPS) assessing PD-L1 expression status. Their findings revealed a lack of survival benefit of first-line chemoimmunotherapy for advanced ESCC with PD-L1 TPS < 1% (HR: 0.91 [0.74–1.12], p = 0.38), while modest advantages could be found for those with PD-L1 CPS < 10 (HR: 0.77 [0.62–0.94], p = 0.01). Based on this result, Yap et al. suggest first-line chemoimmunotherapy confer limited survival benefit to patients with low PD-L1 expression. On the other hand, Wu et al.'s study shares a different opinion. Chemoimmunotherapy was found as an effective first-line regimen for both patients with PD-L1 TPS < 1% (HR: 0.74 [0.56–0.97], p = 0.03) or PD-L1 CPS < 10 (HR: 0.77 [0.66–0.89], p < 0.01).39
Interestingly, opposite conclusions were derived from two separate meta-analysis study using similar trials. Wu et al.'s study added one more trial, JUPITER-06, to their meta-analysis. In fact, four trials (CheckMate-648, ESCORT-1, JUPITER-06, and ORIENT-15) were pooled in TPS subgroup meta-analysis in Wu et al.'s study, while only two trials (CheckMate-648 and ESCORT-1) in Yap et al.'s study. RATIONALE-302 and RATIONALE-306 employed TAP score to measure PD-L1 expression instead of CPS or TPS, and thus was nor included by any of these meta-analyses. Theoretically, larger sample size provides more reliable evidence. Still, cautions should be taken when interpreting the results from these meta-analyses.
6 HETEROGENICITY OF TRIALS
Apart from different PD-L1 expression status, heterogenicity of trials, in terms of PD-L1 IHC assays, geographic regions, chemotherapy regimens, and sex disparit could be the potential reasons biasing the pooled results. Herein, we point them out respectively to guide clinical trials of chemoimmunotherapy for ESCC in the future.
6.1 Concordance of PD-L1 IHC assays
Among six published ESCC ICI trials, five different assays were employed to assess PD-L1 expression status, including 22C3 (KEYNOTE-590 and ORIENT-15), 28-8 (CheckMate-648), SP263 (RATIONALE-306), 6E8 (ESCORT-1), and JS311 (JUPITER-06). Both 22C3 and 28-8 recognize extracellular domain of PD-L1 and interchangeability of these two assays has been proved by multiple comparison studies.41-44 SP263 binds to cytoplasmic domain of PD-L1. Controversial results regarding the concordance between SP263 and 22C3 has been widely reported, mostly focusing on nonsmall cell lung carcinoma (NSCLC).45-53 High agreement between SP263 and 22C3 was observed in melanoma54 and gastric cancer,55 while moderate agreement in head and neck squamous cell carcinoma (HNSC).56 Some believe that the large number of concordant negative cases could contribute to the high overall percent agreement (OPA) of SP263 and 22C3, which, in fact, show great discrepancies in distinguishing positive cases.49, 52 Similarly, the only study comparing the performance of SP263 and 22C3 in ESCC presented with high negative predictive value (NPV, 56.7%) and low positive predictive value (PPV, 56.7%) when using CPS 1 as cutoff.57 JS311 also binds to cytoplasmic domain of PD-L1,4 which demonstrates similar staining results with 22C3 and SP263 in various cancer types, including NSCLC, melanoma, and urothelial carcinoma (UC).58, 59 When it comes to 6E8, data comparing its performance with other assays is lacking. Lawson et al.'s study have mapped the binding sites of PD-L1 from different assays and showed similar staining pattern regarding different epitopes.60 Their findings suggest that discrepancies of different assays are likely to be attributable to trial design, tumor heterogenicity, or platform variables, rather than antibody epitope.60 Generally, different PD-L1 assays were comparable to some degree, but not completely interchangeable. Pooling results from different assays with the same cutoff might not be rigorous.
6.2 Asian versus non-Asian ESCC
Combing the results of published ESCC ICI trials, Asian patients with advanced ESCC receiving chemoimmunotherapy demonstrated apparently longer survival than non-Asian patients, especially Caucasians. Squamous cell carcinoma is the dominant histologic type of esophageal cancer in Asia, while adenocarcinoma is more common in non-Asia regions.1 Etiologies differ substantially between Asian and non-Asian areas, even if for the same histologic type of esophageal cancer.61 Cigarette62-64 and alcohol65-68 are the major risk factors of ESCC in western countries, while betel quid,69, 70 pickled vegetables,71-73 and hot drinks74 are responsible for ESCC in Asia. Apart from the geographic differences, genomic variation of ESCC seems to be another potential factor contributing to the discrepancies between Asian and non-Asian patients.75, 76 A comparative study screened for differentially mutated genes between Asian and Caucasian ESCC patients, using combined data from a Chinses cohort and TCGA cohort. The data set consists of 39 Caucasians, 41 Vietnamese, and 78 Chinese.76 Vietnamese and Chinese patients share genetic similarity in hierarchical clustering analysis and thus they were combined as Asian group for further analysis. TP53, NFE2L2, and EP300 were found with higher mutation rate in Asian patients. Another study, which compared genomic features between Asian and Caucasian ESCC, demonstrated similar results, with NFE2L2 and EP300 more likely mutated in Asian patients.75 Moreover, a study reported on the American Society of Clinical Oncology conference further explored the difference of immunogenomic features between Asian and non-Asian ESCC. The authors compared immune subtypes, composition of immune cell infiltrates, and T-cell-related signatures, but none of them is significantly different between Asian and non-Asian patients. Although a few differences were observed between Asian and Caucasian patients, the genomic patterns of these two groups were generally similar in the published studies.75, 76 One common limitation of these studies is the small sample size of non-Asian patients retrieved from TCGA cohort. There is no evidence backing up the association between genomic variation and chemoimmunotherapy efficacy in Asian and non-Asian patients with advanced ESCC. To better dissect the roles of genomic variation in chemoimmunotherapy of ESCC, future research with larger sample size is in need.
6.3 Chemotherapy regimens
Doublet platinum-based chemotherapy is the standard first-line treatment for advanced ESCC with CF or TP as two common regimens recommended by National Comprehensive Cancer Network (NCCN). ESCC ICI trials chose different chemotherapy regimens: CF was adopted by KEYNOTE-590 and CheckMate-648, TP by ESCORT-1 and JUPITER-06, ICC by ORIENT-15, RATIONALE-302 and RATIONALE-306. Both CF and TP have been validated by randomized trials as effective first-line therapy for metastatic or advanced ESCC.9, 14, 15, 21, 77-79 A retrospective study compared CF and TP as first-line treatment in Chinese patients with ESCC.12 A total of 398 patients were included and divided into two groups: CF (n = 195) and TP (n = 203). Significant benefit was observed in TP group for PFS (TP vs. CF: 7.85 vs. 6.53 months, p = 0.02), but not for OS (TP vs. CF: 13.46 vs. 12.67 months, p = 0.20). Considering the favorable PFS, TP is recommended especially in Chinses patients, and thus the Asian ESCC ICI trials chose TP in their study design.3, 4 Head-to-head studies comparing CF and TP in the first-line setting of ESCC are lacking. However, comparable efficacy and safety profiles of CF and TP were found in many many other settings, such as in definitive concurrent chemoradiotherapy (dCRT) of locally advanced ESCC,80, 81 in concurrent chemoradiotherapy of locally advanced cervical carcinoma,82 in first-line treatment of advanced or metastatic HNSC.83
When it comes to chemoimmunotherapy, it seems that anti-PD1 plus TP could provide greater survival benefit than anti-PD1 plus CF.2-5, 23-25 Immunogenicity of paclitaxel and fluorouracil might play an essential role. Accumulating evidence indicates that chemotherapy can induce ICD involving modification of cellular surface as well as release of intracellular molecules, which may enhance the immunogenicity of tumor microenvironment and thus drive anticancer immunity.84-87 Immunogenicity varies greatly among different chemotherapies. It has been widely reported that paclitaxel can effectively elicit ICD and promotes antitumor immune response in various cancer types, including triple negative breast cancer (TNBC),88 ovarian cancer,89 melanoma,90 and colon cancer.90 On the other hand, immunogenicity of fluorouracil has limited evidence. Moreover, a well-established ICD prediction model proposed by Galluzzi et al. demonstrated higher ICD prediction scores for paclitaxel than fluorouracil.91 Therefore, anti-PD1 plus TP might have stronger synergetic effects due to the better immunogenic capability of paclitaxel.
Current ESCC ICI trials mostly focus on CF and TP, but alternative regimens, such as docetaxel, irinotecan, carboplatin, and oxaliplatin are recommended by NCCN as well. Interestingly, oxaliplatin has been reported with superior immunogenicity than cisplatin. Further evidence is needed to determine which chemotherapy regimens can better synergize with immunotherapy. Given the heterogenic immunogenicity of chemotherapies, it is of great challenge to understand the mechanism behind chemoimmunotherapy. However, growing studies on the interaction between anticancer drugs and immunotherapy can help us better understand the combined treatment effects under the tumor immunity context.
6.4 Chemotherapy-free regimens
The favorable outcomes of nivolumab plus ipilimumab in CheckMate-648 indicates a potential chemotherapy-free option as first-line treatment for ESCC. Compared with chemoimmunotherapy, dual immunotherapy provided longer duration of response (11.1 months vs. 8.2 months). Controversially, an increased incidence of early death was also observed in the dual immunotherapy group. The Kaplan-Meier curves of OS crossed at around 6 months, suggesting that a proportion of patients do not benefit from the immune-doublet therapy at the initial treatment stage compared with chemotherapy alone. Immunotherapy functions indirectly by activating self-immunity against tumors and thus commonly presents with delayed treatment effects.33, 92-94 Esophageal patients often suffered from poor nutrition and considerable weight loss due to dysphasia and sometimes the therapeutic goal is to achieve a rapid clinical response to alleviate patient's symptoms.95-97 In such cases, chemoimmunotherapy is a better choice given higher objective response rate (47% vs. 28%). Functional assessment of cancer therapy-esophageal (FACT-E) scores showed a better improvement of quality of life in patients receiving chemoimmunotherapy within 20 weeks of initial treatment. As for treatment-related toxicities, fewer patients reported being bothered by treatment side effects in dual immunotherapy, but the incidence rate of severe AE was higher in this group. Chemotherapy-free regimens does not necessarily mean toxicity free. Nivolumab plus ipilimumab is an appropriate choice for patients who are willing to risk higher chance of progression at initial treatment and aiming at possible long-term control of disease. Predictive biomarkers can select patients who will response to dual immunotherapy to avoid early progression or death.98 PD-L1 expression alone is not enough in stratification of patients and more effective biomarkers are in need. Meanwhile, exploration of combination strategies (i.e., timing, dosing, and sequential treatments) could also be a possible solution to enhance treatment response and there are ongoing trials trying to improve these strategies.99-101
There are many ongoing trials investigating possible chemo-free regimens involving ICIs and other therapeutics in patients with advanced or metastatic ESCC (Table 3). Besides the combination of PD-1 and PD-L1 antibodies, alternative immune checkpoints outside the PD-1/PD-L1 axis, including LAG-3, TIM-3, and TIGIT, are under evaluation in ESCC. Associations between LAG-3/TIM-3/TIGIT blockades and antitumor immunity were well established in published preclinical data.102-104 Favorable clinical outcomes of dual immunotherapy with anti-PD1/anti-PD-L1 and these ICIs were also observed in other solid tumors except ESCC.105-107 Dual ICI blockades could be a promising treatment regimen for ESCC.
| Trials | Drugs | Regimens | Lines | Phases |
|---|---|---|---|---|
| NCT05038813 | TQB2450 + Anlotiniba | PD-L1 antibody + broad-spectrum TKI | First | Phase II |
| NCT04984018 | Camrelizumab + Chidamide | PD-1 antibody + HDAC inhibitor | Second | Phase II |
| NCT04880811 | Toripalimab + Afatinib | PD-1 antibody + TKI (ErbB family) | Second | Phase II |
| NCT04785820 | RO7121661 or RO7247669 | PD1-TIM-3 antibody or PD1-LAG3 antibody | Second | Phase II |
| NCT05007613 | atezolizumab + Cabozantinibb | PD-L1 antibody + broad-spectrum TKI | Second | Phase II |
| NCT05461794 | Tislelizumab + Sitravatinibc | PD-1 antibody + broad-spectrum TKI | Second | Phase II |
| NCT03766178 | Camrelizumab + Nimotuzumab | PD-1 antibody + EGFR antibody | Second | Phase II |
| NCT04732494 | Tislelizumab + Ociperlimab | PD-1 antibody + TIGIT antibody | Second | Phase II |
| NCT04866381 | Camrelizumab + SHR-6390 | PD-1 antibody + CDK4/6 inhibitor | Second | Phase II |
| NCT05049681 | Camrelizumab + Apatinib | PD-1 antibody + TKI (VEGFR-2) | Second | Phase III |
| NCT05163483 | Toripalimab + Bevacizumab + Chidamide | PD-1 antibody + VEGF antibody + HDAC inhibitor | - | Phase II |
| NCT01351103 | PDR001 + LGK | PD-1 antibody + WNT inhibitor | - | Phase I |
| NCT04721223 | Toripalimab + JAB-3068 | PD-1 antibody + SHP2 inhibitor | - | Phase Ib, Phase II |
- Abbreviations: HDAC, histone deacetylase; TKI, tyrosine kinase inhibitor.
- a Anlotinib: broad-spectrum TKI that targets VEGFR-1/2/3, FGFR, PDGFR, and KIT.
- b Cabozantinib: broad-spectrum TKI that targets c-Met, VEGFR2, AXL, and RET.
- c Sitravatinib: broad-spectrum TKI that targets HGFR, c-MET, AXL, KIT, MER, DDR2, VEGFR-1/2/3, PDGFR family, RET, and Ephrin family.
A considerable number of patients developed resistance to immunotherapy, largely due to the suppressive tumor immune microenvironment (TIME). Treatments that can positively reshape TIME and reverse the immune desert might have synergic effects with immunotherapy and thus overcome immune resistance. Therefore, numerous targeted therapeutics are investigated as well, including histone deacetylases (HADC) inhibitor, SHP2 inhibitor, EGFR inhibitor, CDK4/6 inhibitor, WNT inhibitor, and various tyrosine kinase inhibitors (TKIs). For example, HADC inhibitors were found able to modulate suppressive immune cells (i.e., TAMs and MDSCs).108 Meanwhile, HADC inhibitors could also upregulate PD-L1 expression that suppresses antitumor immunity. It is of limited therapeutic value when used alone, but it could function effectively as an immune booster when combined with PD-1/PD-L1 blockade. However, simply assessing the efficacy of various combined regimens in different clinical trials is not enough. It is of great importance to determine specific drivers of immune resistance and classify patients based on them. Grouping patients in this way can efficiently guide the combination of immunotherapy with specific therapeutics that aims at the exact driving factor to achieve better clinical outcomes.
6.5 Sex disparities in ESCC patients with immunotherapy
Influences of sex on both innate and adaptive immunity are well recognized,109 but the association between sex and immunotherapy of cancer patients remains controversial. Different results were observed in studies trying to dissect the exact relationship between sex and immunotherapy efficacy by integrating results from multiple randomized ICI trials.110-114 Some studies reported that male patients treated with ICIs achieved longer survival than female counterparts,110, 111, 113 while others showed no significant sex differences.114 Another study performed separate meta-analysis on how sex affect clinical outcomes in NSCLC patients with ICI monotherapy or ICI plus chemotherapy.112 In this study, male patients derived better clinical benefits than female with ICI monotherapy, while female outweighed male when receiving chemoimmunotherapy. It is believed that the addition of chemotherapy to ICI in female patients might positively reshape the tumor microenvironment and increase the antigenicity, which could eventually turn into a better treatment response.115 Sex plays different roles given different conditions, which partially explains the controversial results from previous studies, since the one reported no significant sex disparities pooled results from patients with both ICI monotherapy and chemoimmunotherapy.
However, we observed a different trend regarding the role of sex in ESCC patients when compared with the published results in NSCLC patients (Figure 2). We combined results from published ESCC ICI trials with available sex subgroup OS data and performed meta-analysis. KEYNOTE-590 and KEYNOTE-181 were excluded because they only reported data that combined both squamous cell carcinoma and adenocarcinoma. According to the results, we found that ICI monotherapy-treated female patients with advanced or metastatic ESCC presented with better survival benefits than male patients (HR of OS [ICI monotherapy vs. chemotherapy]: 0.59 [0.36–0.96] for female, 0.77 [0.66–0.90] for male), but chemotherapy-treated male patients are superior to female patients (HR of OS [chemoimmunotherapy vs. chemotherapy]: 0.91 [0.66–1.25] for female, 0.65 [0.58–0.73] for male).
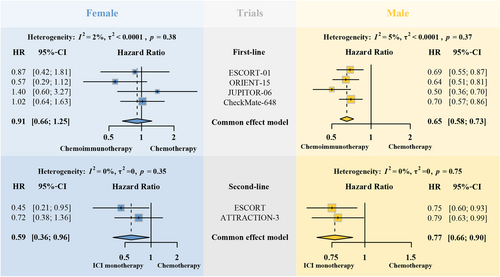
Patterns of sex disparities in anticancer immunity varied widely among different cancer types.116 When it comes to NSCLC patients, tumors in men had an immunosuppressive but high antigenic microenvironment where ICI alone can exert effective antitumor immunity, while tumors in women had an inflamed but low antigenic microenvironment where chemotherapy-induced ICD could increase antigenicity and thus boost the efficacy of ICI. However, this hypothesis does not apply to patients with ESCC. Mechanisms behind sex disparities in immunotherapy-treated ESCC patients remain uncovered and the combination of immunotherapy and chemotherapy certainly contribute to the complexity of relevant research. Ratio of female and male patients ranged from 1:4 to 1:7 in published ICI ESCC trials. Female patients are underrepresented in current clinical trials.117 Considering the remarkable roles of sex in immunotherapy, greater emphasis should be placed on the sex disparities in the future ESCC ICI trials.
7 ADVERSE EVENTS
Although the heterogenicity mentioned above might bias the results, it is of great importance to create a conducive environment for successful trial outcomes. Ensuring the safety and well-being of participants is of paramount importance in clinical trails. AEs, including any unexpected or undesirable occurrences, can pose risks to the individuals involved, as well as hinder the progress of the research. These events are carefully monitored, recorded, and analyzed to assess their severity, frequency, and potential relationship to the regimens. Therefore, it is crucial to strive for fewer and milder AEs.
Most common treatment-related adverse events (TRAEs) shared by both ICI plus chemotherapy and placebo plus chemotherapy groups were neutropenia, anemia, nausea, fatigue, and decreased appetite, which are largely due to the cytotoxic effects of chemotherapy. As expected, the addition of ICI also caused immune-related AEs (irAEs), commonly presenting with thyroid dysfunction, pneumonitis, rash, and pruritus. According to common terminology criteria for AEs (CTCAE), the grade of AEs refers to the severity of the AE, and it displays Grade 1 through 5 with unique clinical description of severity for each AE based on certain general guideline. Grade 3 is describes as “severe or medically significant but not immediately life-threatening; hospitalization or prolongation of hospitalization indicated; disabling; limiting self-care ADL, which refer to bathing, dressing and undressing, feeding self, using the toilet, taking medications, and not bedridden.” And serious AEs include life-threatening consequences or urgent intervention indicated (Grade 4) and death (Grade 5).
Notably, reactive cutaneous capillary endothelial proliferation (RCCEP) is a characteristic manifestation of camrelizumab.108 The potential mechanism behind RCCEP is that camrelizumab might activate CD4+ T cells, increase the production of cytokine interleukin (IL)-4 by T helper two cells, and then promote differentiation of macrophages toward M2 that release VEGF-A to stimulate vascular proliferation. Almost 80% patients developed RCCEP after camrelizumab treatment in ESCORT-1 cohort, but large majority of patients presented with mild symptoms (Grade 1 to 2 AEs). Interestingly, RCCEP was found significantly associated with the efficacy of camrelizumab monotherapy. In a multicenter phase 2 trial evaluating camrelizumab in pretreated advanced HCC patients, patients with RCCEP showed better response and prolonged survival.118
There are also several studies systemically reviewing the potential predictive value of irAEs in the ICI monotherapy setting.119-121 ICI-treated patients with irAEs demonstrated consistently longer OS than those without irAEs across multiple studies that cover different solid tumors, including melanoma, NSCLC, urothelial cell carcinoma (UCC), renal cell carcinoma (RCC), gastrointestinal carcinoma (GIC), and HNSC.114 Some studies suggest that common antigens shared by tumor and preinflamed organ could contribute to the association between irAEs and ICI efficacy.122, 123 ICI unleashed cytotoxic T cells that might target both tumor and normal tissues and thus resulted in both toxicity and response.
Does this association exist in the chemoimmunotherapy combination setting? A study pooled results from three randomized trials evaluating the efficacy and safety of chemoimmunotherapy involving atezolizumab in 2503 NSCLC patients.124 Longer OS was detected in chemoimmunotherapy-treated patients with Grades 1–2 irAEs, but not in those with Grades 3–5 irAEs.124 However, subgroup analyses regarding irAEs were not reported in the published first-line randomized ESCC trials and whether ESCC patients with irAEs could benefit more from chemoimmunotherapy remains uncovered.
When it comes to overall TRAEs, chemoimmunotherapy seems much more toxic than ICI monotherapy when compared to standard chemotherapy (Table 4). In second-line randomized ESCC trails, the percentage of ≥3 grades AEs were 20% to 46% lower in ICI monotherapy arm than in chemotherapy arm (ICI vs. Chemo: 18% vs. 64% [ATTRACTION-3],31 5% vs. 45% [KEYNOTE-181],30 19% vs. 39% [ESCORT],32 19% vs. 56% [RATIONALE-302]29). In first-line ESCC randomized trials except ESCORT-1, the percentage of ≥3 grades AEs were 3%–11% higher in chemoimmunotherapy arm than in chemotherapy arm (chemoimmunotherapy vs. chemotherapy: 72% vs. 68% [KEYNOTE-590],23 47% vs. 36% [CheckMate-648],24 67% vs. 65% [RATIONALE-306],2 73% vs. 70% [JUPITOR-06],4 60% vs. 55% [ORIENT-15],5 63% vs. 68% [ESCORT-1]3). Furthermore, TRAEs caused by chemoimmunotherapy led to treatment discontinuation in 3%–15% of ESCC patients. Despite the encouraging results of ICI plus chemotherapy in ESCC patients regarding first-line settings, such a considerable percentage of treatment discontinuation due to AEs certainly compromised its actual efficacy. Toxicity of chemoimmunotherapy should not be overlooked, especially for elderly who are underrepresented in the current clinical trials and less likely to tolerate the combined regimen.122 Appropriate management of AEs is essential to improve the clinical benefits of chemoimmunotherapy. Early detection, precise diagnosis, and timely treatment of AEs could be very helpful and there are many published guidelines regarding management of TRAEs for clinicians to follow.125-128 Proper supportive care is effective in improving patients' quality of life and thus increases patients' compliance.129 Prediction of AEs risk to guide early interventions during chemoimmunotherapy could be another potential solution. However, existing biomarkers mostly focus on ICI monotherapy, including germline genetic status, mRNA expression levels, cytokines, autoantibodies, and specific blood cells from peripheral blood samples, gut microbiome from stool samples, and multi-omics from tumor samples.130 Future studies are needed to explore biomarkers that can predict AEs in ESCC patients with chemoimmunotherapy.
| Cohort | ≥3 grades AE | Serious AE | Treatment discontinuation | Deaths | ||||
|---|---|---|---|---|---|---|---|---|
| Second-line | ICI | Chemo | ICI | Chemo | ICI | Chemo | ICI | Chemo |
| KEYNOTE-181 | 0.18 | 0.86 | - | - | 0.06 | 0.06 | 0.02 | 0.02 |
| Attraction-3 | 0.18 | 0.64 | 0.16 | 0.23 | 0.09 | 0.09 | 0.01 | 0.02 |
| ESCORT | 0.19 | 0.39 | 0.16 | 0.15 | 0.07 | 0.05 | 0.01 | 0.01 |
| Rationale-302 | 0.19 | 0.56 | 0.14 | 0.20 | 0.07 | 0.14 | 0.03 | 0.03 |
| First-line | ICI + Chemo | Chemo | ICI + Chemo | Chemo | ICI + Chemo | Chemo | ICI + Chemo | Chemo |
| KEYNOTE-590 | 0.72 | 0.68 | - | - | 0.24 | 0.2 | 0.02 | 0.01 |
| CheckMate-648 | 0.47 | 0.36 | 0.24 | 0.16 | 0.34 | 0.19 | 0.02 | 0.02 |
| Rationale-306 | 0.67 | 0.65 | 0.29 | 0.19 | 0.32 | 0.22 | 0.02 | 0.01 |
| ESCORT-1 | 0.63 | 0.68 | - | - | 0.12 | 0.09 | 0.03 | 0.04 |
| JUPITER-06 | 0.73 | 0.70 | 0.36 | 0.29 | 0.12 | 0.06 | 0.004 | 0.01 |
| ORIENT-15 | 0.60 | 0.55 | 0.28 | 0.20 | 0.21 | 0.12 | 0.03 | 0.02 |
| ASTRUM-007 | 0.53 | 0.48 | 0.36 | 0.32 | 0.34 | 0.23 | 0.03 | 0.02 |
- Abbreviations: AE, adverse event; chemo, chemotherapy; first-line, first-line clinical trials; ICI, immune checkpoint inhibitor; second-line, second-line clinical trials; -, lack of data.
8 LOW-DOSE CHEMOTHERAPY PLUS ICI
Despite the encouraging results of chemoimmunotherapy in treatment naïve ESCC patients, evidence is lacking regarding the appropriate dosage of chemotherapy in the combined regimen. Maximum tolerated dose (MTD) of chemotherapy was adopted in current randomized ESCC trials: paclitaxel (175 mg/m2) plus cisplatin (75 mg/m2) on Day 1 of each 3-week cycle,3-5 5-fluorouracil (800 mg/m²) on Days 1–5 plus cisplatin (80 mg/m²) on Day 1 of each 3-week cycle.23, 24 MTD chemotherapy plus immunotherapy might not be the best combination. Cytotoxic effects of MTD chemotherapy can impair normal immune cells, especially hematopoietic bone marrow precursor cells, and cause neutropenia and lymphopenia, hence lessen antitumor immune response.85, 91, 131, 132 It has been documented that, when delivered at low dose, chemotherapy can not only reduce its unfavorable immunosuppressive effects, but also stimulate antitumor immunity by interacting directly with immune cells.91 Low-dose chemotherapy could selectively deplete immunosuppressive cells, including regulatory T (Treg) cells, myeloid-derived suppressor cells (MDSCs), and M2-like tumor-associated macrophages (TAMs).91 For instance, low-dose 5-fluouracil demonstrated capability of eliminating MSDCs without affecting other immune cells via activation of caspase-3 and caspase-7 both in vivo and in vitro.133 Similar findings observed in low-dose paclitaxel that led to significant decreases in the number of both Treg and MDSCs.134 Furthermore, low-dose chemotherapy could also activate immune effector cells, including M1-like macrophages, dendritic cells (DCs), cytotoxic T lymphocytes (CTLs). There are a few studies providing evidence for the synergic effects between low-dose chemotherapy and immunotherapy.131, 134-136 In addition, low-dose chemotherapy is likely to cause less AEs when compared to MTD chemotherapy. Given the considerable percentages of treatment discontinuation caused by TRAEs in ESCC patients that received MTD chemoimmunotherapy, low-dose chemotherapy plus immunotherapy could be a potential solution to obtain better clinical benefits. Therefore, low-dose chemotherapy plus immunotherapy is worth further exploration in the future trail design that aims to discover better therapeutic strategies for ESCC patients.
9 CONCLUSIONS AND PERSPECTIVES
Promising results from the published randomized ICI trials have radically changed the therapeutic landscape for patients with advanced ESCC, and chemoimmunotherapy as novel standard first-line treatment will certainly be the game changer. Discrepancies among subgroup analyses across different trials reflect the complicated mechanisms behind chemoimmunotherapy. Various PD-L1 testing assays, different genetic backgrounds, sex, and diverse chemotherapy backbones all contribute to the heterogenicity of these trials. Chemo-free regimens, such as dual immunotherapy or ICI plus targeted therapeutics, are alternative treatment to chemoimmunotherapy. As one of causes of treatment discontinuation and poor prognosis, AEs should not be overlooked, particularly the irAEs.
Due to the heterogenicity of these trials is impossible to ignore, pooling results from different trials seems unscientifically. Therefore, future research with large sample size is in need. In addition, sex is associated with clinical outcomes in ESCC patients with immunotherapy and the mechanism behind such a relationship is worth further investigating. To understand the underlying logic of chemoimmunotherapy and to find novel combination regimens with stronger synergic effects, growing studies with a focus on the immunogenicity of chemotherapy are critical. Although some undesirable results from previous dual immunotherapy regimens, while some favorable results exist simultaneously, many ongoing trials are investigating possible chemo-free regimens. It is significant to discover and utilize biomarkers that could select responsive patients and avoid early progression. In the context of toxicity of immunotherapy, it is also of importance to explore biomarkers that could predict AEs in ESCC patients with immunotherapy and chemoimmunotherapy. Approval of chemoimmunotherapy as practical treatment is not the end, but a temporary victory that inspires us to continue the battle against esophageal squamous cell carcinoma.
AUTHOR CONTRIBUTIONS
Zhen Zhang: Data curation (lead); formal analysis (equal); writing—original draft (equal); writing—review and editing (equal). Jiaqian Huang: Data curation (supporting); formal analysis (equal); writing—original draft (equal); writing—review and editing (equal). Yuhong Xu: Writing—original draft (supporting); writing—review and editing (equal). Huiyan Luo: Conceptualization (lead); funding acquisition (lead); supervision (lead). All authors have read and approved the final manuscript.
ACKNOWLEDGMENTS
Thanks to all investigators who contributed to these clinical trails. This research was supported by the National Key Research and Development Program of China (2021YFF1201300), the National Natural Science Foundation of China (81930065, 82073112), the Natural Science Foundation of Guangdong Province (2014A030312015), the Science and Technology Program of Guangdong Institute of Esophageal Cancer (M202109), the Science and Technology Program of Guangzhou (20220608008, 202002030208). All elements of Figure 1 were created by Adobe Illustrator.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
The ethics approvals are openly available from the reports of each clinical trial.
Open Research
DATA AVAILABILITY STATEMENT
The data are openly available from the reports of each clinical trial.




