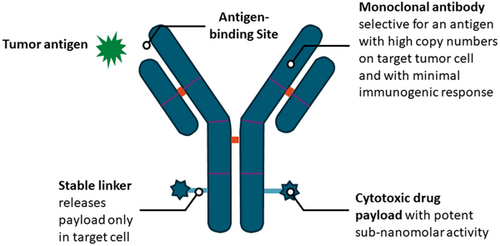
New era for emerging therapeutic targeting human epidermal growth factor receptor 3 (HER 3) in advanced nonsmall cell lung cancer and metastatic breast cancer
Abstract
Human epidermal growth factor receptor 3 (HER3) is a member of the transmembrane receptor tyrosine kinase family. Upregulation of HER3 pathway has been implicated as a mechanism of resistance in solid tumors, particularly in estrogen receptor positive, HER2 positive breast cancer and epidermal growth factor (EGFR) mutant nonsmall cell lung cancer. Several studies suggest that HER3 overexpression represents a negative prognostic biomarker associated with poor survival. Preclinical and clinical studies of anti-HER3 investigational therapies suggest that expression of the HER3 ligand, neuregulin, may predict response to treatment. Despite its emergence as a key cancer therapeutic target, HER3 cannot be targeted with traditional tyrosine kinase inhibitors therapy due to its weak kinase activity. Monoclonal and bispecific antibodies targeting HER3 have been developed and tested in early phase trials. Objective responses were limited when first-generation HER3-specific monoclonal antibodies were investigated as monotherapies in phase 1 and 2 clinical trials for nonsmall cell lung cancer (NSCLC) and metastatic breast cancer (MBC). MBC and NSCLC HER3 specific antibody-drug conjugates have shown encouraging results in resistance in cancer cells, particularly in those that overexpress HER3. These agents have shown some promise in early phase trials in both NSCLC and MBC setting in heavily pretreated patients with varying degrees of response. It is unclear which subgroup of patients will truly benefit from targeting HER3 as these therapies are under investigation.
Graphical Abstract
Human epidermal growth factor receptor 3 (HER3) overexpression represents a negative prognostic biomarker associated with poor survival. Targeting HER3 has shown some promise in early phase trials in both nonsmall cell lung cancer and metastatic breast cancer in heavily pretreated patients with varying degrees of response. Identifying a predictive biomarker will aid to better select patients that will respond to treatment.
1 INTRODUCTION
The human epidermal growth factor receptor (HER) proteins are a family of receptor tyrosine kinases (ErbBs) and consist of type I transmembrane growth factor receptors. HER regulate different cellular functions in response to extracellular ligands.1 The family includes four highly homologous members, EGFR/ERBB1/HER1, HER2/ERBB2, HER3/ERBB3, and HER4 ERBB4. These receptors consist of a glycosated ligand-binding extracellular domain, a transmembrane domain, and an intracellular kinase domain.2
It is well recognized that alterations in HER signaling, either due to genetic mutations or receptor overexpression, drive oncogenic progression in multiple solid tumor types. Various wealth of evidence supports that HER3 plays a critical role in EGFR- and HER2-driven tumors. Recent studies suggest that HER3 overexpression represents a negative prognostic biomarker associated with poor survival. Upregulation of HER3 pathway has been implicated as a mechanism of resistance in various solid tumors.3
HER3 it is not a direct target of kinase inhibitors because its kinase inactive with no or little intracellular tyrosine kinase activity. Moreover, it is not a direct target of kinase inhibitors and not presently an easily drugable target. MBC and NSCLC HER3 specific anti-HER 3 agents have shown promising results in resistance in cancer cells, particularly in those that overexpress HER3.3-5 This review summarizes the current progress and emerging clinical data related to the potential use of anti-HER3 therapies in the treatment of EGFR-mutant NSCLC and HER3-overexpressing metastatic breast cancer.
2 HER3 SIGNALING AND TARGETED THERAPY IN CANCER
2.1 The role of HER3 in oncogenesis and treatment resistance
HER3 is a protein receptor that belongs to the HER or EGFR family of receptor tyrosine kinases (RTKs), which also includes EGFR/ERBB1/HER1, HER2, and HER4. HER family members are expressed on the surfaces of a wide variety of cell types and regulate diverse cellular processes, including cell division, proliferation, and differentiation.1 In contrast to other EGFR family members and HER proteins, HER3 is not oncogenic or transforming when overexpressed alone. Extracellular interactions between HER3 and its ligand, neuregulin (NRG; also known as heregulin), trigger a conformational change in the receptor that facilitates interactions with other RTKs.2 HER3 heterodimerizes with other RTKs (preferentially HER2) to subsequently activate downstream signaling pathways, including the phosphoinositide 3 kinase/protein kinase B (PI3K/Akt) and MEK/mitogen-activated protein kinase (MAPK) signaling pathways (Figure 1). That can activate cytoplasmic oncogenic signaling; in particular via the PI3K/AKT/mTOR pathway. This signaling inhibits apoptosis and leads to cell proliferation, promotion of cancer cell survival and progression. HER3 can accord resistance to Anti-EGFR-targeting therapy through dimerization with partners other than EGFR because it dimerizes with receptors other than EGFR, such as HER2 and MET receptor.3-5
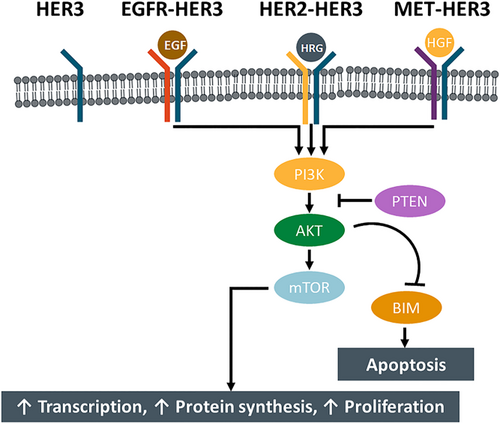
Notably, HER3 has been incriminated as a driver of cancer development in a number of different solid tumors, as overactivation of HER3-mediated signaling drives tumorigenesis and metastasis.2, 3 In patients with solid tumors, such as breast, gastric, colorectal, and ovarian cancers, HER3 expression has also been associated with a worse overall survival (OS).4
Compared with other HER family members, HER3 are almost kinase inactive and have relatively weak kinase activity.5 However, HER3 preferentially interacts with HER2, which is a constitutively active kinase.1 HER2 relies on HER3 for activation of the PI3K/Akt signaling pathway, and studies have confirmed that HER3 is required for transformation and sustained proliferation of HER2-expressing cancer cells.6, 7 Likewise, mutant HER3 relies on HER2 activity to promote oncogenic cascades, which appears to occur in a ligand-independent manner.8 As a result, overexpression of HER3 has also been implicated as a significant cause of resistance to EGFR- and HER2-targeted treatment, particularly in HER2-positive cancers.9 In HER2-positive breast cancer cells, for example, targeting of HER2 with a tyrosine kinase inhibitor (TKI) result in compensatory upregulation of HER3, which can maintain PI3K/Akt signaling.10 Because of its feedback regulation via AKT signaling, HER3 has been shown to play a significant role in mediating resistance to HER2 and PI3K (PI3K) pathway-directed therapies11-17 (Figure 2).
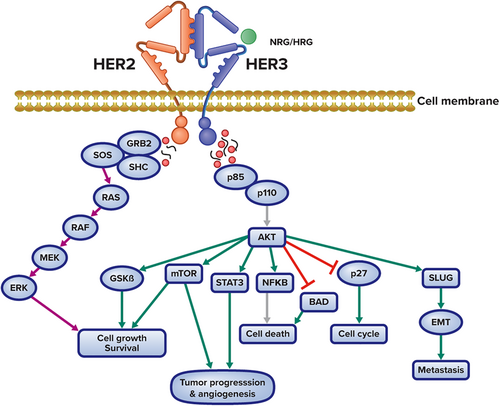
These effects have also been observed in hormone-refractory prostate cancer, platinum-resistant ovarian cancer, cetuximab-resistant colorectal cancer, and TKI-resistant nonsmall cell lung cancer (NSCLC).11-15 A subset of colorectal cancer patients develops acquired resistance to cetuximab-containing regimens due to high levels of circulating NRG, which activates HER3.15
Upregulation of HER3 is also implicated in resistance to MEK/RAF inhibitors in thyroid carcinoma and malignant melanoma.16, 17 This is due upregulation of HER3 through FOXD3 and this act as form of adaptive resistance to RAF/MEK inhibitors. Moreover, HER3 is well known as an impressive inducer of the PI3K/AKT cascade with unique negative feedback inhibition. Inhibition of the PI3K/AKT cascade, with either mTOR inhibitors, or PI3K/AKT inhibitors, as well as inhibition of the MAPK pathway with BRAF inhibitor has been demonstrated to result in transcriptional upregulation of HER3. Ultimately, this will result in the reactivation of its downstream targets.16, 17
HER3 overexpression is also thought to play a role in resistance to hormone therapy with the estrogen receptor (ER) agonist, tamoxifen. In a study of more than 400 patients with ER-positive tamoxifen-treated breast cancer, those with HER2- and HER3-positive cancers were significantly more likely to experience relapse while receiving treatment with tamoxifen than their counterparts with HER2- and HER3-negative cancers.18 Additionally, in cell culture models, the inhibition of HER3 reduces PI3K/Akt signaling, inhibits cancer cell proliferation, and increases sensitivity to tamoxifen, suggesting an integral role for HER3 in resistance to tamoxifen therapy.19 Tamoxifen-induced growth inhibition of MCF7 cells and HER2 overexpressing MCF7 cells is significantly increased by inhibiting HER3. Tamoxifen resistance in breast cancer cells is associated with increased sensitivity of MCF-7 cells to tamoxifen following HER3 downregulation.18, 19
The compensatory upregulation of HER3 along with the sustained PI3K/AKT signaling and MET amplification in NSCLC leads to anti-EGFR agent, gefitinib treatment failure because of activation of HER3 signaling.11
2.2 Prognostic value of HER3 overexpression
In addition to treatment resistance, HER3 overexpression may represent a prognostic factor for disease progression. In meta-analyses of studies examining HER3 expression in colorectal and gastric cancers, HER3 expression was found to be associated with worse response to cetuximab, as well as tumor differentiation, metastasis, and worse OS.20, 21 Similarly, in a microarray study of ovarian tumor samples, increased HER3 expression was associated with a more than twofold increased risk of poor progression-free survival (PFS).22 Additionally, in NSCLC, increased HER3 expression was found to be associated with higher tumor stage.23
The significance of HER3 expression as a breast cancer prognostic factor has been debated, with studies finding both positive and negative associations between HER3 expression and OS.3 These differences may be explained in part by differences in HER2 and ER expression, which have been found to modulate the effect of HER3 expression on OS and recurrence-free survival in breast cancer.4, 24-26 These findings are supported by the observation that HER3 is mutated in no more than 3% of primary breast tumors, but up to 14% of metastatic ER-positive breast cancers.27
3 CONSIDERATIONS AND STRATEGIES FOR TARGETING HER3
Given the increasing appreciation for the role of HER3 in both oncogenesis as well as treatment resistance, HER3 has emerged as a key therapeutic target. The variability of HER3 expression patterns both among and within different types of cancers, combined with the complexity of the downstream signaling pathways, makes targeting HER3 in cancer cells challenging. Bidirectional crosstalk between HER3 and ER has been found to contribute to anti-HER3 treatment resistance.28 Additionally, given the weak kinase activity of HER3, it is not an ideal candidate for traditional TKI therapy. As a result, current antibodies against HER3 are based on a blocking strategy.3, 29 These agents block the activity of HER3 by various processes, including locking HER3 in the tethered confirmation, trapping NRG (the HER3 ligand), blocking NRG binding sites, triggering the internalization of HER3, and preventing heterodimerization.1, 29 In some cases, these antibodies also direct immune cells to kill HER3-positive cancer cells, a process known as antibody-dependent cell-mediated cytotoxicity (ADCC).1
3.1 Anti-HER3 antibodies and antibody drug conjugates (ADC)
The first generation of HER3-specific monoclonal antibodies (mAbs) included patritumab, seribantumab (MM-121), and lumretuzumab (RG7116). These agents block the proliferation of the PI3K/Akt signaling cascade by preventing the binding of NRG to HER3, which inhibits heterodimerization and triggers internalization of the HER3 receptor.30-33 In the case of lumretuzumab, this interaction also triggers ADCC by facilitating the engagement of immune cells.32 First-generation anti-HER3 mAbs have been investigated in a number of preclinical, phase 1, and phase 2 trials in patients with diverse cancers, including head and neck squamous cell carcinoma, breast cancer, NSCLC, and ovarian cancer.1, 29, 34 Although objective responses were limited when these first-generation anti-HER3 mAbs were investigated as monotherapies, later trials suggested that anti-HER3 mAbs increase sensitivity to EGFR inhibitors and chemotherapeutics, particularly in patients with high levels of NRG expression.35-39
Currently, there are four therapeutic strategies to target HER3 (Figure 3). Monoclonal, bispecific antibodies and ADC, as well as pan-HER strategies, have been the most popular HER3 targeting strategies. Emerging HER3-targeting strategies include HER3-targeting vaccines and various methods to affect HER3 degradation at the mRNA or protein levels. Epigenetic inhibitors may be useful for inhibiting HER3 gene expression, but the mechanisms and feasibility of these strategies must be validated further. DXd is an exatecan derivative.40
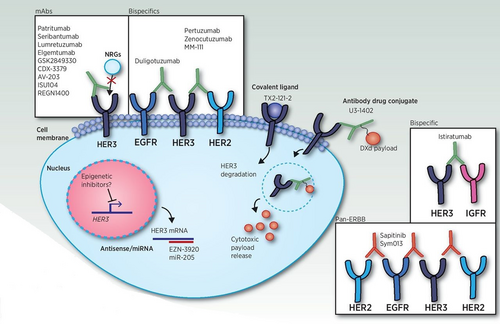
The extensive crosstalk between HER3 and other HER family members suggests that efficient targeting of multiple receptors may represent a therapeutic advantage over mAbs; therefore, bispecific antibodies that target HER3 and other HER receptors have emerged as a strategy for preventing therapeutic resistance.41 In addition, because HER3 is expressed on many different tissues, but at relatively low levels compared with other HER receptors, such as HER2, bispecific antibodies may increase the specificity of anti-HER3 agents to cancer cells overexpressing HER3 and may also facilitate increased uptake in these targets by increasing the number of targets for interaction.41, 42 Two bispecific antibodies targeting HER3 and HER2 (MM-111 and MCLA-128) have advanced to clinical trials, but only MCLA-128 remains under investigation in trials of breast cancer (ClinicalTrials.gov Identifier: NCT03321981) and NRG1 fusion-positive solid tumors (ClinicalTrials. gov Identifier: NCT02912949).
Additionally, conjugation of a HER3-specific antibody to another drug has emerged as a strategy for efficient targeting of anticancer drugs to HER3-expressing tumor cells. Several investigational antibody-drug conjugates (ADCs) have shown success in cell culture and mouse models of various cancers, including NSCLC, colorectal cancer, breast cancer, and melanoma.43-46 Although there was no effect on PI3K/Akt signaling, a HER3-DNA topoisomerase inhibitor ADC triggered apoptosis of tumor cells in a mouse model of EGFR TKI–resistant NSCLC.47 Similarly, a HER3-monomethyl auristatin F(MMAF) ADC was able to overcome trastuzumab resistance in HER2-positive MBC cells in xenografts.45 These preclinical results suggest that HER3-specific ADCs may represent a promising option for overcoming therapeutic resistance in cancer cells, particularly in those that overexpress HER3.47 Early clinical trials have reported promising results thus far in patients with either HER-3 overexpressing breast cancer or resistant EGFR-mutant NSCLC who were treated with a HER3-DNA topoisomerase inhibitor ADC (patritumab deruxtecan/HER3-DXd).48, 49
3.2 Other investigational HER3-Targeted therapies
-
In cell culture models, a HER3 peptide vaccine inhibited proliferation and promoted apoptosis and ADCC in breast and pancreatic cancer cells.50
-
An adenoviral vector encoding full-length HER3 was also found to trigger ADCC and HER3 internalization and block HER3 signaling and proliferation in mouse models of HER2-positive and triple-negative breast cancers.51
-
Inhibition of the HER3 pseudokinase activity by small molecules has been found to result in abrogation of HER3 signaling in cell culture models.52, 53
-
Strategies involving knockdown of HER3 mRNA expression using small interfering RNA have been improved by utilizing locked nucleic acid oligonucleotides (LNA-ONs), which bind mRNA with high affinity and are resistant to degradation by nucleases.54
-
In a cell culture model, a HER3-specific LNA-ON efficiently reduced HER3 protein levels by more than 90% and inhibited cellular proliferation in HER3-positive prostate and liver cancer cells.54
4 PRETREATED EGFR-MUTANT NSCLC
4.1 HER3 in the treatment of NSCLC
HER3 expression seen in the majority of EGFR-mutated NSCLC cases and found in more than 80% of the primary tumors. The role of the HER3 receptor in NSCLC has become increasingly clear in recent years, particularly in tumors harboring mutations in EGFR. HER3 is a pseudokinase that, through interactions with other HER family members such as EGFR, regulates signaling cascades that trigger cancer proliferation and metastasis.1, 55 Studies have estimated that 32%–83% of primary NSCLC tumors express HER3, and elevated HER3 activity is significantly correlated with mutations in EGFR in NSCLC.56-58 Although EGFR TKIs do not directly target HER3, their use is associated with compensatory upregulation of HER3, which can result in therapeutic resistance.9 Notably, in a mouse model of NSCLC, HER3 inhibition was sufficient to overcome resistance to EGFR inhibitors, suggesting that targeting of HER3 represents a promising option for overcoming therapeutic resistance in EGFR-mutated NSCLC.2, 59
4.2 Patient selection for anti-HER3 treatment
Identifying patients with HER3-expressing tumors will be an important step in predicting response to HER3-targeting therapies. Although HER3 expression is typically evaluated using immunohistochemistry, a standardized strategy for staining of HER3 is lacking.3, 57 When evaluating HER3 expression in patients with NSCLC, a careful and comprehensive approach should be taken, which may include assessment of phosphorylation status. In a study of HER2-positive breast cancer cells from 71 cases, phosphorylated HER3 was significantly associated with disease-free survival.60
Preclinical and clinical studies of anti-HER3 investigational treatments suggest that expression of the HER3 ligand NRG may predict response to treatment.1 In a phase 2 randomized study of the HER3-specific mAb seribantumab, patients with NSCLC and detectable NRG mRNA had improved PFS, whereas no effect was observed in patients with NRG-negative tumors.38 Additionally, in a study of NSCLC tumor cells, elevated NRG expression was associated with treatment failure and resistance to the EGFR TKI erlotinib, which was overcome following treatment with the anti-HER3 mAb patritumab.40 These results advocate that NRG expression may be a key consideration for predicting response to HER3-targeted treatment.
4.3 Investigational HER3-targeting therapies in the treatment of NSCLC
Although first-generation HER3-specific mAbs showed promise in initial studies, they exhibited limited efficacy as monotherapies, and the only phase 3 clinical trial was terminated early because of failure to meet predetermined measures of efficacy.1 Several investigational agents aimed at increasing the efficacy of anti-HER3 therapies have emerged in the NSCLC landscape. Currently, these agents are under investigation primarily for advanced tumors that have progressed when standard therapies were used.
4.3.1 U3-1402/patritumab deruxtecan
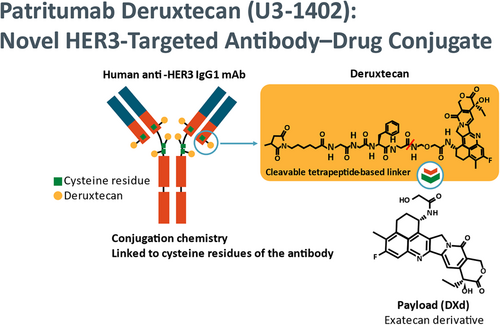
Patritumab deruxtecan,(HER3-DXd) is an ADC in which patritumab is conjugated to a topoisomerase inhibitor via a peptide linker, facilitating specific delivery of the drug to HER3-expressing tumor cells (Figure 4).47, 48 Preliminary results from an ongoing phase 1 trial of U3-1402 were released in October 2019; safety and efficacy data from 30 patients with NSCLC who had previously been treated with EGFR TKIs were reported.47 All patients had activating mutations within EGFR, and of the 25 tumors that could be evaluated, all expressed HER3. Grade 3 or 4 treatment-emergent adverse events occurred in 43% of patients, including 2 dose-limiting toxicities in one patient (grade 4 thrombocytopenia and grade 3 febrile neutropenia) and three dose-limiting toxicities in three patients (all grade 4 thrombocytopenia). At the start of the study, 15 patients had a history of central nervous system (CNS) metastases, of which nine progressed during treatment; one patient without a history developed new CNS involvement. Efficacy was evaluated in 26 patients, and six patients had a confirmed partial response, with a median best percent change in sum of diameters of −25.7%.47
In a 2021 update, the objective response rate increased to 39%, and the disease control rate was 72%.49 Grade 3 or higher treatment-emergent adverse events occurring in greater than or equal to 15% of patients included thrombocytopenia (30%), neutropenia (19%), and fatigue (14%).49 Treatment-related interstitial lung occurred in four pts (7%; 1 grade ≥3 [2%]; no grade 5).48
The trial is still recruiting patients and is open to individuals with metastatic or unresectable NSCLC, including those with acquired resistance to EGFR TKIs (ClinicalTrials.gov Identifier: NCT03260491).
4.3.2 MCLA-128/Zenocutuzumab
MCLA-128, also known as zenocutuzumab, is a bispecific antibody targeting both HER2 and HER3. This dual-targeting mechanism increases specificity for cancer cells and may also increase the uptake of treatment, as HER3 is expressed at lower levels than other HER family members.41, 42 In a phase 1/2 trial of 28 patients with solid tumors, zenocutuzumab exhibited a favorable safety profile, with only one grade 3 event (an infusion-related reaction) noted.61 In one patient with NSCLC, an ongoing partial response was observed for more than 10 months.61
An ongoing phase 2 trial is currently recruiting to test the safety and antitumor activity of zenocutuzumab in patients with solid tumors harboring NRG1 gene fusions (ClinicalTrials.gov Identifier: NCT02912949). This trial is open to patients with locally advanced or metastatic tumors that have previously been resistant to standard therapy and includes a cohort for NSCLC, which represents the cancer type most frequently found to be NRG1 fusion positive.62, 63 The study will evaluate both the ORR the duration of response, as well as PFS and OS. The updated result from the study showed ORR was 34% in all tumor types and the median duration of response was 9.1 months (95% CI, 7.4-not reached), with the 6-month rate being 76% and 27% at 12 months.63
4.3.3 Other agents in the pipeline
New agents have also progressed into early-stage clinical trials, including ISU104 (ClinicalTrials.gov Identifier: NCT03552406). ISU104 blocks HER3 activation and heterodimerization, and according to preliminary results from an ongoing phase 1/2 study in refractory solid tumors, it exhibited a disease control rate of 60%.64
Another agent in development is TAS0728, a small-molecule inhibitor of HER2 kinase activity65; it is being investigated in a phase 1/2 trial that is examining its safety and activity in patients with advanced HER2- or HER3-positive tumors (ClinicalTrials.gov Identifier: NCT03410927).
In addition, BDTX-189 is a small-molecule inhibitor that has been granted fast-track designation by the Food and Drug Administration (FDA) for patients with HER2 or EGFR mutations.66 The phase 1/2 Masterkey-01 study is currently enrolling patients with locally advanced or metastatic solid tumors who have experienced disease progression with standard therapy, including those with tumors harboring HER3 mutations (ClinicalTrials. gov Identifier: NCT04209465).
5 HER3-OVEREXPRESSING METASTATIC BREAST CANCER
5.1 HER3 in the treatment of metastatic breast cancer
Although the prognostic value of HER expression in breast cancer has been debated, studies suggest that HER expression is associated with worse prognosis and increased risk of metastasis.2, 3 Targeting of HER3 activity has therefore emerged as a key therapeutic strategy in breast cancer. This has been a focus in ER- and HER2-positive breast cancers in particular, as studies have found that HER3 overexpression increases the risk of treatment resistance.9, 10, 18, 19 Mutations in HER3 are also enriched in metastatic breast cancer, suggesting that HER3-targeted therapies may benefit patients with more aggressive disease as well.1, 27
Emerging evidence suggests that HER3 expression may be a critical biomarker for treatment response in luminal tumors, in which HER3 is essential for tumor cell survival.1, 27 In cell culture studies, downregulation of HER3 expression in luminal breast cancer cells has been found to increase sensitivity to both antiestrogen therapy and radiotherapy, suggesting that tumors of luminal origin may also be ideal targets for anti-HER3 therapy.67, 68 Results from preclinical and clinical trials of HER3-targeted therapies also suggest that expression of the HER3 ligand NRG represents a potential biomarker for predicting response to HER3 blockade treatments. For instance, a meta-analysis of 29 trials, including nine in breast cancer, found that anti-HER3 therapy confers a survival effect only in tumors with high NRG expression levels.69
5.2 Investigational HER3-targeting therapies in the treatment of metastatic breast cancer
HER3-specific mAbs exhibited modest efficacy as monotherapies, and the only phase 3 clinical trial was discontinued because of failure to meet predetermined measures of efficacy.1 Nonetheless, the landscape of investigational anti-HER3 therapies has expanded, and there are several options under investigation in metastatic breast cancer.
5.2.1 U3-1402/Patritumab deruxtecan
Preliminary safety and efficacy data from an ongoing phase 1/2 clinical study in patients with previously treated HER3-overexpressing metastatic breast cancer were reported in 2018.70 Nausea was the most common treatment-emergent adverse event (71.4%), and reversible grade 3/4 thrombocytopenia and liver enzyme elevation were observed in 19% and 9.5% of patients, respectively. In 21 patients, the objective response rate was 33%, and the disease control rate was 95%.70
In a 2022 update, the objective response rate increased to 42.9%, and the disease control rate was 90.5%.49 Grade 3 or higher treatment-related adverse events occurring in greater than or equal to 15% of patients included thrombocytopenia (30.8%), neutropenia (39.6%), leukopenia (18.1%), and anemia (18.7%).49 However, treatment-induced interstitial lung disease was reported in 6.6% of the patients, including 1 grade 5 event. The trial began in Japan but has since expanded to the United States and now the trial is completed, but has been granted a delay in reporting and results are expected at the mid of 2024 (ClinicalTrials.gov Identifier: NCT02980341).
5.2.2 MCLA-128/Zenocutuzumab
Results from an ongoing phase 2 study of zenocutuzumab in patients with endocrine therapy–refractory MBC was reported in early 2020. Among 48 patients, grade 3 or 4 adverse events were uncommon, and the most frequently reported treatment related events of any grade included fatigue (27%), diarrhea (25%), and nausea (21%). Efficacy data were available for 42 patients, for whom the disease control rate was 45%.71 Full results from the trial, including efficacy in HER2-positive and triple-negative cohorts, are expected in 2022 (ClinicalTrials.gov Identifier: NCT03321981).
5.2.3 Other agents in the pipeline
Several other investigational agents for HER3-expressing cancers have progressed into phase 1/2 clinical trials, including the HER3-specific mAb ISU104 (ClinicalTrials.gov Identifier: NCT03552406) and the small-molecule HER2 kinase inhibitor TAS0728 (ClinicalTrials.gov Identifier: NCT03410927).72 Preliminary results from an ongoing trial of ISU104 in refractory solid tumors, which is still recruiting participants, indicated that the mAb was associated with a disease control rate of 60%. Data from the TAS0728 trial are expected in early 2021, but in preclinical mouse studies, TAS0728 exhibited potent antitumor activity and restored sensitivity to HER2-targetting treatments in HER2-positive breast cancer cells.65, 73
In addition, BDTX-189 is an irreversible small-molecule inhibitor, orally available that has been granted fast-track designation by the FDA for patients with HER2 or EGFR mutations.66 The phase 1/2 Masterkey-01 trial is currently recruiting patients with metastatic or locally advanced solid tumors who have experienced disease progression with standard therapy, including those with tumors harboring HER3 mutations (ClinicalTrials.gov Identifier: NCT04209465).74
Trispecific ErbB-cMet-IGF1R antibodies which target EGFR, IGF1R, and cMet or EGFR, IGF1R, and HER3 have been reported to inhibit cellular growth and receptor activation and constitute a potential candidates 99 for future applications in various therapeutic areas.74, 75
Table 1 summarize HER3-targeted therapies under clinical development.
| Drug | Drug type | Target | Tumor tested | Highest clinical trial phase | Trial | Up-to-date clinical activity | References |
|---|---|---|---|---|---|---|---|
| U3–1402/Patritumab Deruxtecan | ADC | HER3 | HER3-expressing MBC | Phase II | NCT02980341 | ORR = 30.1%–42.9%. mDOR = 5.9–8.3 months | [49, 76] |
| CRC | Phase 1/2 | NCT04479436 | ORR 46.3%. DCR 90.2% | [77] | |||
| EGFR -mutated NSCLC | Phase 1/2 | NCT03260491 | mDOR = 7 months, DCR = 72%. ORR = 39% | [48] | |||
| (MEHD7954A) Duligotuzumab | Bispecific Abs | HER2/HER3 | HNSCC | Phase II | NCT01911598 | No results posted | [78] |
| MCLA-128/Zenocutuzumab | Bispecific Abs | HER2/HER3 | NRG1 fusion-positive advanced Solid tumors | Phase I/II | NCT02912949 | ORR = 34%. mDOR = 9.1 months | [79] |
| NSCLC harboring NRG1 fusion | Phase I/II | NCT02912949 | ORR 42% | [63] | |||
| MBC | Phase II | NCT03321981 | DCR = 45%. ORR = 27% | [71, 80] | |||
| (MM-141) Istiratumab | Bispecific Abs | HER3/IGF1R | Advanced solid tumor | Phase I | NCT01733004 | Awaiting results | [81] |
| Metastatic Pancreatic cancer | Phase II | NCT02399137 | Did not improve PFS, OS | [82] | |||
| Lumretuzumab (RO5479599, RG7116) | mAbs | HER3 | Advanced malignant HER3 + solid tumors of epithelial cell origin | Phase Ib | NCT01482377 | Awaiting results | [36, 83] |
| HER3-positive, HER2-low MBC | Phase Ib | NCT01918254 | Awaiting results | [84] | |||
| Advanced squamous NSCLC | Phase Ib/II | NCT02204345 | ORR = 25% DCR = 91.7% | [85] | |||
| U3–1287/Patritumab | mAbs | HER3 | Advanced EGFR + ve NSCLC | Phase I + II | NCT01211483 | Patritumab improved PFS in the NRG high, but not in the ITT population | [86, 87] |
| Advanced HNSCC | Phase Ib | NCT02350712, | PFS = 7.9 m OS = 13.5 ms | [88] | |||
| MM-121/Seribantumab | mAbs | HER3 | NSCLC | Phase I/II | NCT00994123 | HRG-positive. mPFS = 1.86 | [89, 90] |
| Ovarian cancer | Phase II | NCT01447706 | DCR = 92%. ORR = 33% | [35, 91] | |||
| Elgemtumab (LJM716) | mAbs | HER3 | HER2 + MBC | Phase II | NCT02167854 | Awaiting results | [92] |
| ESCC | Phase II | NCT01822613 | Awaiting results | [93] |
- Abbreviations: ADC, antibody-drug conjugate; BOR, best overall response; CRC, colorectal cancer; DCR, disease control rate; ESCC, Esophageal squamous cell carcinoma; HNSCC, head and neck squamous cell carcinoma; mAbs, monoclonal antibodies; MBC, metastatic breast cancer; mDoR, median duration of response; NSCLC, non-small cell lung cancer; ORR, objective response rate; OS, overall survival.
6 CONCLUSION AND FUTURE PERSPECTIVES
HER3 is a unique EGFR family member that is not oncogenic alone but can cooperate with other different receptors to induce HER3 mediated tumorigenesis, metastatic events and contributes toward the development of treatment resistance. HER3 is implicated in tumorigenesis through interaction with other EGFR and HER family members. Overexpression of HER3 have been associated with poor survival and a mechanism of resistance in multiple solid cancers. Targeting HER3 as a potential druggable target has been investigated in NSCLC and metastatic breast cancer. HER3 has been mainly targeted with monoclonal or bispecific antibodies due to the minimal kinase activity. Monoclonal and bispecific antibodies block HER3 ligand binding or its heterodimerasation with other family member receptors. Early clinical studies of HER3 targeting therapies have yielded conflicting results. It is unclear whether this is due to utilization of improper antibody epitopes, pharmacokinetics issues, or lack of biomarkers.94 A few studies suggest that NRG expression predicts response to anti-HER3 therapies. Identifying a predictive biomarker will aid to better select patients that will respond to treatment.95, 96
New therapeutic strategies, including HER3 directed ADCs, are being investigated in a broad range of cancers. The potential advantage of ADCs over monoclonal antibodies could be that the cancer cells need to express HER3, but do not have to be fully dependent on HER3 to induce cell death. Furthermore, HER3-directed ADC has been shown to be promising and has the advantage of facilitating the immune cells. Other investigational strategies comprise synthetic antisense oligos, proteasomal degradation, and peptide-based vaccines targeting HER3. However, these remain experimental. Indeed, ADC targeting HER3 is in clinical development for EGFR-mutant NSCLC, which has a higher frequency of HER3 expression and also where there is an unmet need for treatments after standard EGFR TKI failure.
The winning strategy to therapeutically target HER3 remains to be seen, but HER3 is as a promising drug target and the era of drugging the “undruggables” has already started. The appropriate biomarker for HER3-directed therapy is not yet known. With a move toward identifying and targeting solid tumor-specific mutations, anti-HER3 therapies represent an exciting development which may be available for patients in the near future.
AUTHOR CONTRIBUTIONS
José J. Berenguer-Pina: Project administration (supporting); writing – review and editing (supporting); visualization (equal). Maha AlSindi: Writing – review and editing(supporting); visualization (equal). Thamir Mahgoub: Writing – review and editing (supporting); visualization (equal). Yasar Ahmed: Conceptualization (lead); project administration (lead); writing – original draft (lead); writing – review and editing (lead); visualization (equal). All authors have read and approved the article.
ACKNOWLEDGMENT
Not applicable.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ETHICS STATEMENT
Not applicable.
Open Research
DATA AVAILABILITY STATEMENT
Not applicable.