Journal list menu
Export Citations
Download PDFs
Cover Picture
Macromol. Chem. Phys. 21/2015
- Page: 2065
- First Published: 02 November 2015

Front Cover: The size-dependent pairing between an-isotropic multimaterial nanoparticles is demonstrated. The association is provided by the spontaneous assembly of Janus nanoparticles upon addition of a silica precursor. A face-selective silica coating on the surface of the Janus particles induces a morphology change, which then mediates the irreversible size-dependent pairing of the particles. Further details can be found in the article by D. Estupiñán, M. B. Bannwarth,* K. Landfester, and D. Crespy* on page 2070.
Masthead
Contents
Contents: Macromol. Chem. Phys. 21/2015
- Pages: 2067-2069
- First Published: 02 November 2015
Full Papers
Size-Dependent Self-Assembly of Anisotropic Silica-Coated Hybrid Nanoparticles
- Pages: 2070-2079
- First Published: 11 September 2015
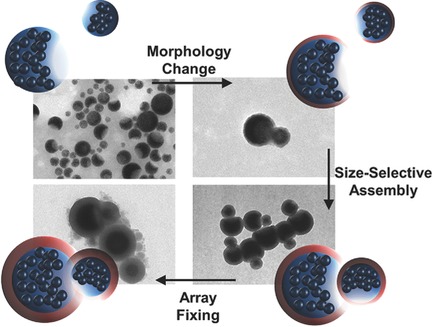
The size-dependent interaction between anisotropic hybrid colloids is investigated. The particles spontaneously assemble in pairs by a three-step process: (i) site-selective morphology change of Janus particles, (ii) size-dependent assembly, and (iii) fixing of the particle association. This is achieved simultaneously by adding a silica precursor that gradually creates a cavity for large particles, in which smaller ones can fit.
Thermally Induced Crosslinking of Poly(N-Propargyl Glycine)
- Pages: 2080-2085
- First Published: 02 September 2015
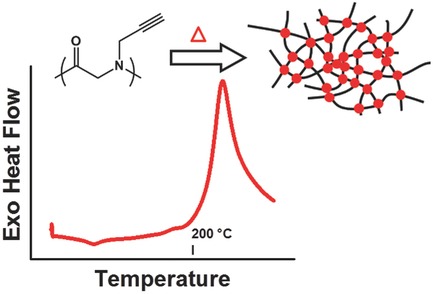
Poly(N-propargyl glycine) undergoes crosslinking simply upon heating in the absence of any additives or metal catalysts. The crosslinking process is analyzed by thermal analysis (differential scanning calorimetry and thermogravimetric analysis), Fourier-transform infrared, electron paramagnetic resonance, and solid-state NMR spectroscopy.
Surface Chemistry of Amphiphilic Polysiloxane/Triethyleneglycol-Modified Poly(pentafluorostyrene) Block Copolymer Films Before and After Water Immersion
- Pages: 2086-2094
- First Published: 24 September 2015
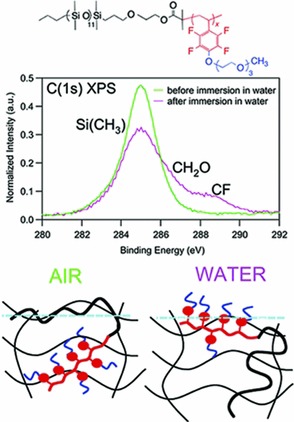
Films of triethylene glycol-modified pentafluorostyrene block copolymers undergo significant surface reconstruction upon immersion in water, resulting in a preferential exposure of the oxyethylenic chains to contact water. Such modification is even more marked when the block copolymer is dispersed in a cross-linked silicone matrix, as the surface concentration of the fluorinated block increases after immersion in water.
Multicyclic Polyesters of Trimesic Acid and Alkanediols and the Theory of Network Formation
- Pages: 2095-2106
- First Published: 12 October 2015
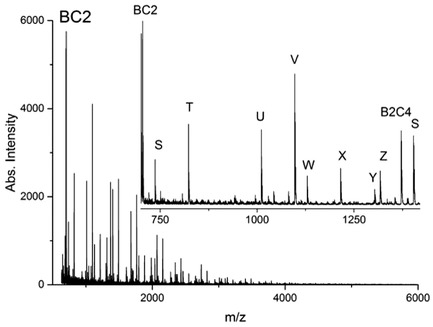
Depending on the reaction conditions, polycondensations of trifunctional monomers yield soluble nanogels or insoluble networks. At moderate monomer concentrations the initially formed functional cyclic and multicyclic oligomers (the “eggs”) serve as building blocks for the larger nanogels or networks. Polycondensations in bulk yield at first hyperbranched oligomers and polymers (the “hens”) which form small cyclic units in a later stage of the polycondensation.
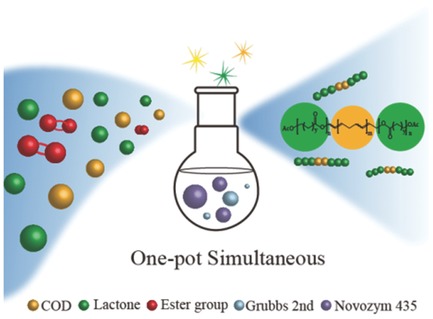
One-Pot Combination of eROP and ROMP for the Synthesis of Block Copolymers
- Pages: 2107-2114
- First Published: 09 October 2015
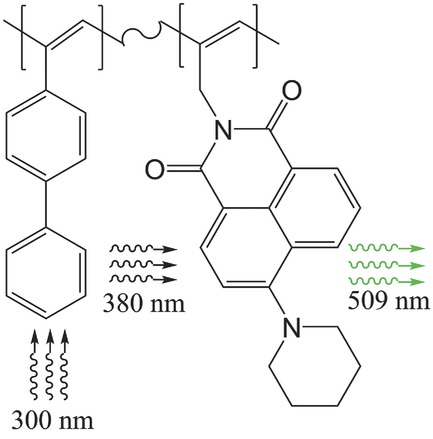
Copolymerization of N-(prop-1-yne-3-yl)-4-(piperidine-1-yl)-1,8-naphthalimide with Arylacetylenes into Fluorescent Polyacetylene-Type Conjugated Polymers
- Pages: 2115-2128
- First Published: 12 October 2015
Partial Molar Volumes and Thermal Expansion Coefficients as an Explanation for Co-Solvent Effect of Penetrants in Multicomponent Polymer Mixtures
- Pages: 2129-2140
- First Published: 13 October 2015
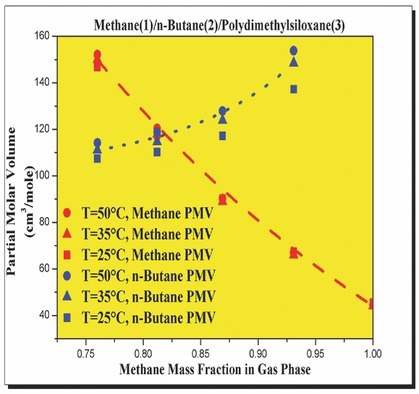
For multicomponent gases/polymer mixtures, partial molar volume and volumetric thermal expansion coefficient of each gas dissolved in the polymer phase are estimated by using the experimental solubility or swelling data in conjunction with the Sanchez–Lacombe equation of state. The results suggest that the co-solvent effect is provoked by the gas with higher solubility and higher volumetric thermal expansion coefficient.
Synthesis and Ring-Opening Polymerization of Cyclic Butylene 2,5-Furandicarboxylate
- Pages: 2141-2146
- First Published: 13 October 2015
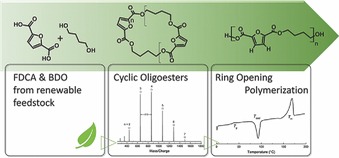
Furanic polyesters, derived from renewable 2,5-furandicarboxylic acid, are highly attractive polymers and sustainable substitutes of some performance-related fossil-based commodities like polyethylene terephthalate. In this work a new synthetic pathway for the production of furan-based polyesters is reported. Cyclic oligoesters of 2,5-furandicarboxylic acid and butane diol are chemically synthesized by semi-batch esterification. The cycles are then successfully polymerized via ring-opening polymerization.