Partial Molar Volumes and Thermal Expansion Coefficients as an Explanation for Co-Solvent Effect of Penetrants in Multicomponent Polymer Mixtures
Corresponding Author
Muhammad Ahsan Bashir
Laboratoire de Chimie Catalyse Polymères et Procédés (C2P2), LCPP team, Bat 308F, Université Claude Bernard Lyon 1 CPE Lyon, CNRS, UMR, 5265, 43 Bd du 11 novembre 1918, F-69616 Villeurbanne, France
Dutch Polymer Institute DPI, P.O. Box 902, 5600 AX, Eindhoven, The Netherlands
E-mail: [email protected], [email protected]Search for more papers by this authorVincent Monteil
Laboratoire de Chimie Catalyse Polymères et Procédés (C2P2), LCPP team, Bat 308F, Université Claude Bernard Lyon 1 CPE Lyon, CNRS, UMR, 5265, 43 Bd du 11 novembre 1918, F-69616 Villeurbanne, France
Search for more papers by this authorVasileios Kanellopoulos
Process Development Group – Innovation Process Technology, Borealis Polymers PO PDO, Borealis Polymers Oy, P. O. Box330, 06850 Porvoo, Finland
Search for more papers by this authorMohammad Al-Haj Ali
Process Development Group – Innovation Process Technology, Borealis Polymers PO PDO, Borealis Polymers Oy, P. O. Box330, 06850 Porvoo, Finland
Search for more papers by this authorCorresponding Author
Timothy McKenna
Laboratoire de Chimie Catalyse Polymères et Procédés (C2P2), LCPP team, Bat 308F, Université Claude Bernard Lyon 1 CPE Lyon, CNRS, UMR, 5265, 43 Bd du 11 novembre 1918, F-69616 Villeurbanne, France
E-mail: [email protected], [email protected]Search for more papers by this authorCorresponding Author
Muhammad Ahsan Bashir
Laboratoire de Chimie Catalyse Polymères et Procédés (C2P2), LCPP team, Bat 308F, Université Claude Bernard Lyon 1 CPE Lyon, CNRS, UMR, 5265, 43 Bd du 11 novembre 1918, F-69616 Villeurbanne, France
Dutch Polymer Institute DPI, P.O. Box 902, 5600 AX, Eindhoven, The Netherlands
E-mail: [email protected], [email protected]Search for more papers by this authorVincent Monteil
Laboratoire de Chimie Catalyse Polymères et Procédés (C2P2), LCPP team, Bat 308F, Université Claude Bernard Lyon 1 CPE Lyon, CNRS, UMR, 5265, 43 Bd du 11 novembre 1918, F-69616 Villeurbanne, France
Search for more papers by this authorVasileios Kanellopoulos
Process Development Group – Innovation Process Technology, Borealis Polymers PO PDO, Borealis Polymers Oy, P. O. Box330, 06850 Porvoo, Finland
Search for more papers by this authorMohammad Al-Haj Ali
Process Development Group – Innovation Process Technology, Borealis Polymers PO PDO, Borealis Polymers Oy, P. O. Box330, 06850 Porvoo, Finland
Search for more papers by this authorCorresponding Author
Timothy McKenna
Laboratoire de Chimie Catalyse Polymères et Procédés (C2P2), LCPP team, Bat 308F, Université Claude Bernard Lyon 1 CPE Lyon, CNRS, UMR, 5265, 43 Bd du 11 novembre 1918, F-69616 Villeurbanne, France
E-mail: [email protected], [email protected]Search for more papers by this authorAbstract
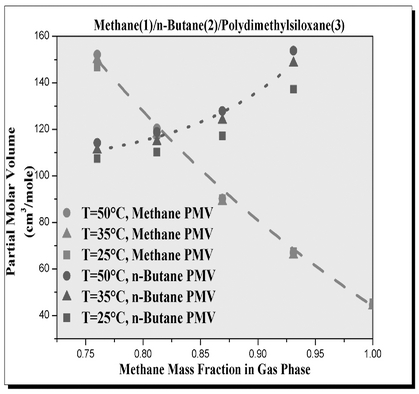
Experimental mixed-gas sorption/dilation data and mixture densities estimated by the fitted Sanchez–Lacombe equation of state have been used to estimate the partial molar volumes (PMV) of gases and polymers in multicomponent mixtures (i.e., ternary) at conditions of industrial relevance. The method developed estimates accurately the PMV and volumetric thermal expansion coefficients of various highly soluble gases and polymers in multicomponent mixtures over a wide range of temperatures, pressures, and gas phase compositions. A comparison of solubility, volumetric thermal expansion coefficients, and PMVs of the gases involved in the studied ternary mixtures reveal that, irrespective of the polymer nature, co-solvent effect is caused by the gas with higher solubility in the polymer phase and higher thermal expansion coefficient, which provides an explanation to the occurrence of co-solubility effects in multicomponent gases/polymer mixtures. It has also been shown that the PMV behavior of gases in the ternary mixtures with polymers is different from their PMV behavior in the corresponding binary gas/polymer mixtures, and that the PMV of a gaseous penetrant in a multicomponent system depends on its gas phase concentration.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| macp201500170-sup-0001-S1.pdf169.1 KB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1Y. Kamiya, K. Terada, K. Mizoguchi, Y. Naito, Macromolecules 1992, 25, 4321.
- 2B. J. Banaszak, D. Lo, T. Widya, W. H. Ray, J. J. de Pablo, A. Novak, J. Kosek, Macromolecules 2004, 37, 9139.
- 3M. A. Bashir, V. Monteil, V. Kanellopoulos, M. Al-haj Ali, T. F. L. McKenna, Ind. Eng. Chem. Res. 2013, 52, 16491.
- 4B. Bonavoglia, G. Storti, M. Morbidelli, Ind. Eng. Chem. Res. 2005, 45, 1183.
- 5B. Bonavoglia, G. Storti, M. Morbidelli, A. Rajendran, M. Mazzotti, J. Polym. Sci. B Polym. Phys. 2006, 44, 1531.
- 6B. D. F. Claudio, P. Ribeiro, J. Poly Sci: Part B: Poly. Phys. 2010, 48, 456.
- 7M. G. De Angelis, T. C. Merkel, V. I. Bondar, B. D. Freeman, F. Doghieri, G. C. Sarti, J. Polym. Sci. B Polym. Phys. 1999, 37, 3011.
10.1002/(SICI)1099-0488(19991101)37:21<3011::AID-POLB11>3.0.CO;2-V CAS Web of Science® Google Scholar
- 8H. Durchschlag, P. Zipper, Ultracentrifugation 1994, 94, 20.
- 9I. M. Klotz, R. M. Rosenberg, Chemical Thermodynamics. Basic Concepts and Methods, John Wiley & Sons, Inc., Hoboken, NJ, USA 2008.
10.1002/9780470285237 Google Scholar
- 10Y. Kamiya, Y. Naito, K. Terada, K. Mizoguchi, A. Tsuboi, Macromolecules 2000, 33, 3111.
- 11A. Rajendran, B. Bonavoglia, N. Forrer, G. Storti, M. Mazzotti, M. Morbidelli, Ind. Eng. Chem. Res. 2004, 44, 2549.
- 12C. P. Ribeiro, B. D. Freeman, Macromolecules 2008, 41, 9458.
- 13J. Ribeiro, B. D. Freeman, Polymer 2010, 51, 1156.
- 14B. J. Banaszak, R. Faller, J. J. de Pablo, J. Chem. Phys. 2004, 120, 11304.
- 15M. K. Kozlowska, U. Domanska, M. Lempert, M. Rogalski, J. Chrom. A 2005, 1068, 297.
- 16A. Tsuboi, P. Kolar, T. Ishikawa, Y. Kamiya, H. Masuoka, J. Polym. Sci. B Polym. Phys. 2001, 39, 1255.
- 17R. D. Raharjo, B. D. Freeman, E. S. Sanders, J. Memb. Sci. 2007, 292, 45.
- 18M. A. Bashir, M. A. Ali, V. Kanellopoulos, J. Seppala, Fluid Phase Equilib. 2015, 388, 107.
- 19M. A. Bashir, M. Al-haj Ali, V. Kanellopoulos, J. Seppälä, Fluid Phase Equilib. 2013, 358, 83.
- 20C. McCabe, A. Galindo, M. N. Garcia-Lisbona, G. Jackson, Ind. Eng. Chem. Res. 2001, 40, 3835.
- 21A. Novak, M. Bobak, J. Kosek, B. J. Banaszak, D. Lo, T. Widya, W. Harmon Ray, J. J. de Pablo, J. Appl. Polym. Sci. 2006, 100, 1124.
- 22J. S. Yoon, C. Y. Chung, I. H. Lee, Euro. Poly. J. 1994, 30, 1209.
- 23J. S. Yoon, H. S. Yoo, K. S. Kang, Euro. Poly. J. 1996, 32, 1333.
- 24Y. Kamiya, Y. Naito, T. Hirose, K. Mizoguchi, J. Polym. Sci. B Polym. Phys. 1990, 28, 1297.
- 25A. B. Michaels, R. W. Hausslein, J. Polym. Sci., C Polym. Symp. 1965, 10, 61.
- 26V. Kanellopoulos, D. Mouratides, P. Pladis, C. Kiparissides, Ind. Eng. Chem. Res. 2006, 45, 5870.
- 27V. Kanellopoulos, D. Mouratides, E. Tsiliopoulou, C. Kiparissides, Macro. React. Eng 2007, 1, 106.
- 28C. Kiparissides, V. Dimos, T. Boultouka, A. Anastasiadis, A. Chasiotis, J. Appl. Polym. Sci. 2003, 87, 953.
- 29M. Minelli, M. G. De Angelis, M. G. Baschetti, F. Doghieri, G. C. Sarti, C. P. Ribeiro, Proc. Eng. 2012, 44, 347.
10.1016/j.proeng.2012.08.411 Google Scholar
- 30H. Orbey, C. P. Bokis, C. C. Chen, Ind. Eng. Chem. Res. 1998, 37, 4481.
- 31I. C. Sanchez, R. H. Lacombe, J. Phys. Chem. 1976, 80, 2352.
- 32E. Neau, Fluid Phase Equilib. 2002, 203, 133.
- 33I. C. Sanchez, R. H. Lacombe, Macromolecules 1978, 11, 1145.
- 34M. A. Bashir, M. Al-haj Ali, V. Kanellopoulos, J. Seppälä, E. Kokko, S. Vijay, Macro. React. Eng. 2013, 7, 193.
- 35S. Jiang, L. An, B. Jiang, B. A. Wolf, Macromol. Chem. Phys. 2003, 204, 2265.
- 36V. Nitsche, K. Ohlrogge, K. Sturken, Chem. Eng. Technol. 1998, 21, 925.
- 37P. Costas, I. C. Sanchez, Polymer 1992, 33, 5090.
- 38H. Lin, B. D. Freeman, Macromolecules 2005, 38, 8394.
- 39J. E. Mark, Polymer Data Handbook, Oxford University Press, London, UK 1999.
- 40V. A. Kusuma, B. D. Freeman, M. A. Borns, D. S. Kalika, J. Membr. Sci. 2009, 327, 195.
- 41V. A. Kusuma, S. Matteucci, B. D. Freeman, M. K. Danquah, D. S. Kalika, J. Membr. Sci. 2009, 341, 84.
- 42H. Lin, E. V. Wagner, J. S. Swinnea, B. D. Freeman, S. J. Pas, A. J. Hill, S. Kalakkunnath, D. S. Kalika, J. Membr. Sci. 2006, 276, 145.
- 43S. Kelman, H. Lin, E. S. Sanders, B. D. Freeman, J. Membr. Sci. 2007, 305, 57.
- 44H. Lin, B. D. Freeman, J. Membr. Sci. 2004, 239, 105.
- 45H. Lin, B. D. Freeman, J. Mol. Struct. 2005, 739, 57.