Journal list menu
Export Citations
Download PDFs
Issue Cover
Issue Cover (August 2019)
- First Published: 26 July 2019
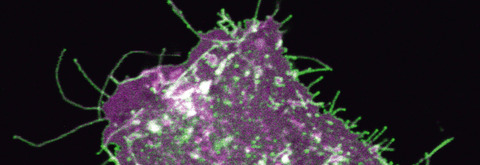
Front cover:
At excitatory synapses, glutamate is removed by glutamate transporters to turn off the signal and prevent excitotoxicity. Hyaluronan is synthesized, secreted, and anchored by hyaluronan synthases (HAS) at the plasma membrane. Cellular process localization of glutamate transporters is hyaluronan synthase dependent, while a major hyaluronan receptor CD44 is not necessary. Hyaluronan synthesized by hyaluronan synthase interacts with glutamate transporters and supports glutamate uptake. Our findings reveal that hyaluronan synthesis helps to turn off excitatory signals.
Image Content: Confocal microscopy image of an astrocyte in CD44 knockout mice primary culture. mEGFP-labeled glutamate transporter GLT1 (green) localizes to astrocyte cellular processes. Lck-mCherry labels cellular membrane (magenta).
Read the full article ‘Hyaluronan synthesis supports glutamate transporter activity’ by M. K. Hayashi, T. Nishioka, H. Shimizu, K. Takahashi, W. Kakegawa, T. Mikami, Y. Hirayama, S. Koizumi, S. Yoshida, M. Yuzaki, M. Tammi, Y. Sekino, K. Kaibuchi, Y. Shigemoto-Mogami, M. Yasui, K. Sato (J. Neurochem. 2019, vol. 150 (3), pp. 249–263) on doi: 10.1111/jnc.14791
Issue Information
REVIEW
Current status and potential role of circular RNAs in neurological disorders
- Pages: 237-248
- First Published: 16 May 2019
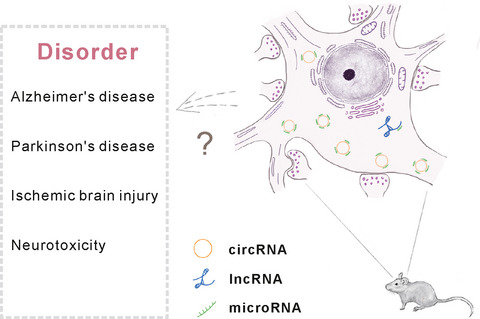
Circular RNAs (circRNAs) are an emerging class of non-coding RNAs which are enriched in the mammalian brain. Interaction between circRNAs and other non-coding RNAs such as microRNAs (miRNAs) and long non-coding RNAs (lncRNAs) is essential in modulating brain functions in rodent models. In this review we discuss the possible functional role of circRNA regulation within the brain and wish it may shed new light on the further studies of some neurodisorders (Alzheimer’s disease, Parkinson’s disease, ischemic brain injury, and neurotoxicity) in human.
ORIGINAL ARTICLES
Signal Transduction & Synaptic Transmission
Hyaluronan synthesis supports glutamate transporter activity
- Pages: 249-263
- First Published: 12 June 2019
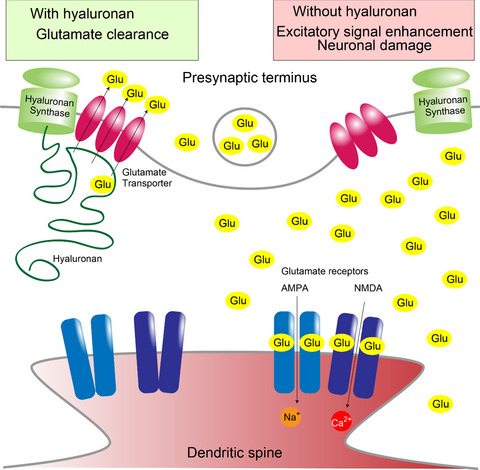
At excitatory synapses, glutamate is removed by glutamate transporters to turn off the signal. Hyaluronan is synthesized, secreted, and anchored by hyaluronan synthases at the plasma membrane. We show that hyaluronan is synthesized to support glutamate uptake by glutamate transporters. Hyaluronan synthases and glutamate transporters form a hyaluronan dependent complex, and are recruited to cellular process tips. These findings reveal that hyaluronan helps to turn off excitatory signals by supporting glutamate clearance for healthy excitatory signal tuning.
Cover Image for this issue: doi: 10.1111/jnc.14516.
Leucine-rich repeat kinase 2 phosphorylation on synapsin I regulates glutamate release at pre-synaptic sites
- Pages: 264-281
- First Published: 30 May 2019
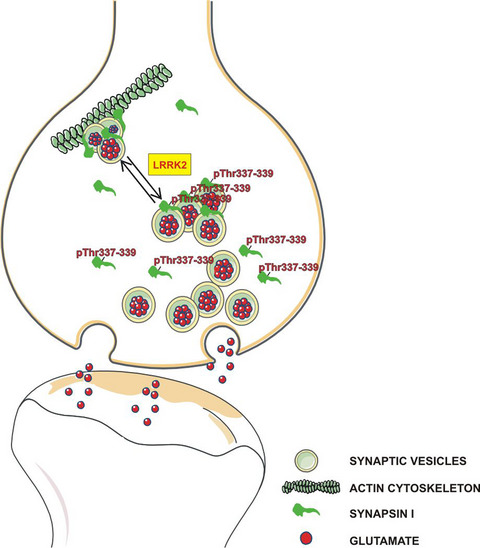
Leucine-rich repeat kinase 2 (LRRK2) is a multidomain protein extensively studied being mutated in familial Parkinson's disease. Because its kinase activity is a highly promising pharmacological target for the treatment of the disease, the identification of LRRK2 substrates is crucial to understand the consequence of LRRK2 kinase inhibition. Here we demonstrated that synapsin I is phosphorylated by LRRK2 at threonine 337 and 339. Functionally, we provided several evidences that LRRK2-dependent phosphorylation of synapsin I impacts glutamate release and synaptic vesicles dynamics. Since LRRK2 mutations display increased kinase activity, our results may imply that mutant LRRK2 impair synaptic vesicle dynamics via aberrant phosphorylation of synapsinI.
Neuroinflammation & Neuroimmunology
Metabolomics-driven identification of adenosine deaminase as therapeutic target in a mouse model of Parkinson's disease
- Pages: 282-295
- First Published: 23 May 2019
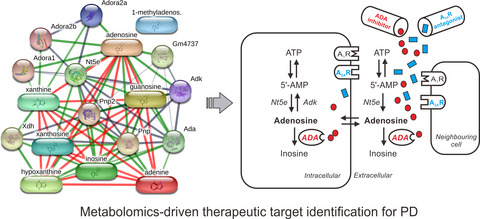
Parkinson’s disease (PD) is a common degenerative disorder. Currently, no treatment strategy can slow or halt PD progression. Based on metabolomics analysis, adenosine and adenosine deaminase (ADA) were screened out as the most promising therapeutic targets for PD. Adenosine augmentation might be a rational approach to slow PD progression. Our verification study indicated for the first time that ADA inhibitor protected against dopaminergic neurodegeneration induced by the neurotoxin MPTP. Additive antiparkinsonian effects were found when ADA inhibitor was used in combination with adenosine A2A receptors (A2AR) antagonist. This study shed a light on the feasibility of metabolomics-driven therapeutic target identification.
Plasma kallikrein-kinin system contributes to peripheral inflammation in temporal lobe epilepsy
- Pages: 296-311
- First Published: 17 June 2019
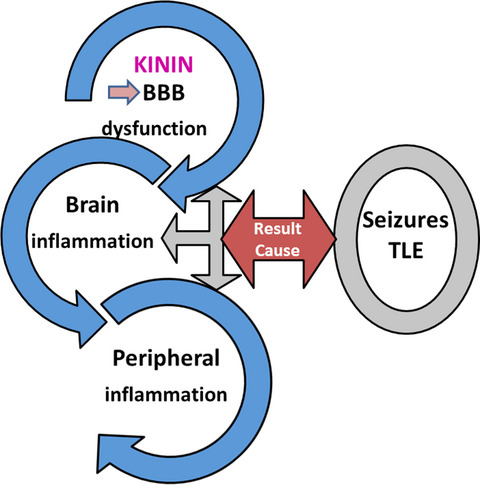
Temporal lobe epilepsy (TLE) is a chronic disease that involves a disruption of blood brain barrier (BBB), linking central and peripheral systems in TLE patients, resulting in a vicious cycle, which affects the brain excitability by activation of the plasma kallikrein-kinin system. The circulation of bradykinin or related peptides in the blood or tissues, as a result of this system activation, could play a role in the interaction between central and peripheral inflammation, maintaining or even aggravating the epileptic seizure.
Open Science: This manuscript was awarded with the Open Materials Badge
For more information see: https://cos.io/our-services/open-science-badges/
Molecular Basis of Disease
Pink1 regulates FKBP5 interaction with AKT/PHLPP and protects neurons from neurotoxin stress induced by MPP+
- Pages: 312-329
- First Published: 08 February 2019
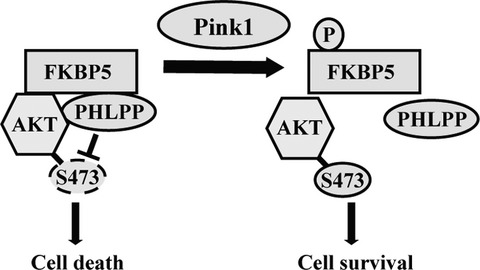
Mutations in PD-associated genes such as Pink1 have been shown to contribute to dysregulation of numerous cellular processes. We identified that Pink1 endogenously interacts with a novel interacting partner FKBP5 and directly phosphorylates it. We provide evidence that Pink1 regulates AKT Ser473 phosphorylation in response to MPP+-induced mitochondrial stress and FKBP5 negatively regulates this phosphorylation and sensitizes cultured neurons to cell death. Our results suggest a model in which Pink1 regulates AKT through phosphorylation of FKBP5 and its interaction with AKT and PHLPP; a potential mechanism by which Pink1-FKBP5 pathway may contribute to neuronal death in PD.
Open Science: This manuscript was awarded with the Open Materials Badge
For more information see: https://cos.io/our-services/open-science-badges/
Viral vector-mediated Cre recombinase expression in substantia nigra induces lesions of the nigrostriatal pathway associated with perturbations of dopamine-related behaviors and hallmarks of programmed cell death
- Pages: 330-340
- First Published: 12 February 2019
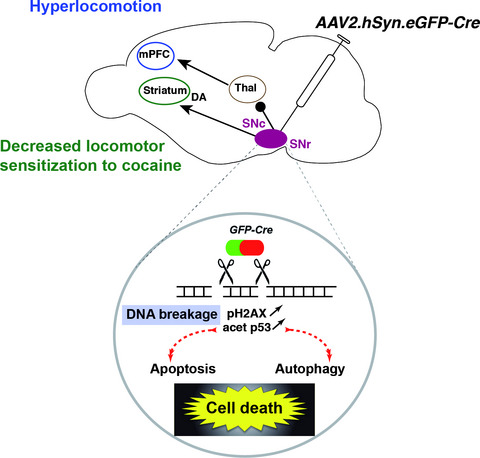
Cre/loxP recombination is a widely used approach to study gene function in vivo, using mice models expressing the Cre recombinase under the control of specific promoters or through viral delivery of Cre-expressing constructs. Our experiments show that AAV-mediated Cre recombinase expression in the substantia nigra (SN) provokes lesions of the SN pars compacta and reticulata and perturbations of dopamine-related behaviors by inducing DNA breakage, increased apoptosis, and perturbation of autophagy. These observations underscore the need for careful control of Cre toxicity when using this tool to study gene function in the brain.





