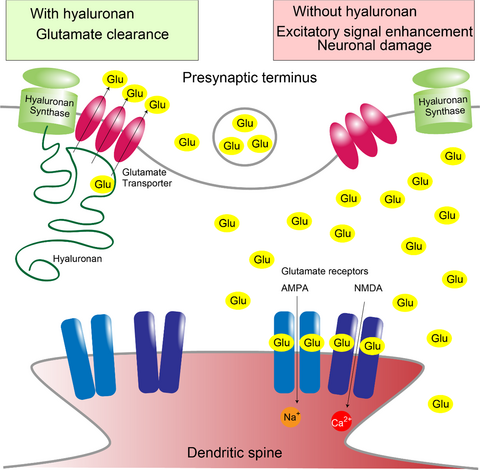
Hyaluronan synthesis supports glutamate transporter activity
Cover Image for this issue: doi: 10.1111/jnc.14516.
Abstract
Hyaluronan is synthesized, secreted, and anchored by hyaluronan synthases (HAS) at the plasma membrane and comprises the backbone of perineuronal nets around neuronal soma and dendrites. However, the molecular targets of hyaluronan to regulate synaptic transmission in the central nervous system have not been fully identified. Here, we report that hyaluronan is a negative regulator of excitatory signals. At excitatory synapses, glutamate is removed by glutamate transporters to turn off the signal and prevent excitotoxicity. Hyaluronan synthesized by HAS supports the activity of glial glutamate transporter 1 (GLT1). GLT1 also retracted from cellular processes of cultured astrocytes after hyaluronidase treatment and hyaluronan synthesis inhibition. A serial knockout study showed that all three HAS subtypes recruit GLT1 to cellular processes. Furthermore, hyaluronidase treatment activated neurons in a dissociated rat hippocampal culture and caused neuronal damage due to excitotoxicity. Our findings reveal that hyaluronan helps to turn off excitatory signals by supporting glutamate clearance.
Cover Image for this issue: doi: 10.1111/jnc.14516.
Abbreviations used
-
- 4MU
-
- 4-methylumbelifenone
-
- AMPA
-
- α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
-
- APV
-
- d-(-)-2-amino-5-phosphonopentanoic acid
-
- BSS
-
- balanced salt solution
-
- CRISPR-Cas9
-
- clustered regularly interspaced short palindromic repeats/CRISPR associated proteins
-
- CSPG
-
- chondroitin sulfate proteoglycan
-
- DIVs
-
- days in vitro
-
- DMEM
-
- Dulbecco’s modified Eagle medium
-
- DNQX
-
- 6,7-dinitroquinoxaline-2,3-dione
-
- EAAT
-
- excitatory amino acid transporter
-
- FBS
-
- fetal bovine serum
-
- FRET
-
- fluorescence resonance energy transfer
-
- Fura-2AM
-
- 1-[6-amino-2-(5-carboxy-2-oxazolyl)-5-benzofuranyloxy]-2-(2-amino-5-methylphenoxy)ethane-N,N,N',N'-tetraacetic acid, pentaacetoxymethyl ester
-
- GLAST
-
- glutamate aspartate transporter
-
- GLT1
-
- glial glutamate transporter 1
-
- HAS
-
- hyaluronan synthases
-
- HRP
-
- house radish peroxidase
-
- m.o.i.
-
- multiplicity of infection
-
- MAP
-
- microtubule-associated protein
-
- MCPG
-
- methyl-(4-carboxyphenyl)glycine
-
- mEGFP
-
- monomeric enhanced green fluorescent protein
-
- PBS
-
- phosphate-buffered saline
-
- RRID
-
- Research Resource Identifier (see scicrunch.org)
-
- TM
-
- transmembrane
-
- TTX
-
- tetrodotoxin
Hyaluronan is a linear nonsulfated glycosaminoglycan. Hyaluronan synthases (HAS) polymerize hyaluronan in the cytoplasm and secrete it into extracellular spaces through their transmembrane (TM) domains (Itano and Kimata 1996). Hyaluronan around neurons buffers ion fluctuation by its hydration and negative charge (Arranz et al. 2014; Morawski et al. 2015). Furthermore, chondroitin sulfate proteoglycans (CSPGs) and link proteins associate with hyaluronan to form perineuronal nets around some neurons (Bignami et al. 1992; Carulli et al. 2006; Kwok et al. 2010; Fowke et al. 2017). Perineuronal nets and neuronal activity affect each other (Reimers et al. 2007; Dityatev et al. 2007; Frischknecht et al., 2014). Expression of CSPGs is enhanced in the hippocampus after rats are trained for memory tasks (Saroja et al. 2014). One CSPG, brevican, contributes to pre–post synapse coupling of fast-spiking neurons and its loss reduced neuronal firing rates (Blosa et al. 2015; Balmer 2016; Sonntag et al. 2018).
During development, perineuronal nets appear toward the end of the critical period and limit neuronal plasticity, and their removal restores ocular dominance plasticity (Pizzorusso et al. 2002) or fear memory erasure (Gogolla et al. 2009). Pathologically, decrease in perineuronal nets was reported in patients with schizophrenia (Mauney et al. 2013) and dementia (Morawski et al. 2014). However, despite the importance of perineuronal nets, their molecular targets to regulate synaptic transmission have not been fully identified.
At excitatory synapses in the central nervous system, L-glutamate (glutamate) is released from presynaptic termini. Glutamate must be promptly removed to terminate the excitatory signals and to prepare for the next signal. Glutamate transporters are responsible for extracellular glutamate clearance. Failure of glutamate clearance in glutamate transporter knockout mice results in epileptic seizures leading to premature death, showing importance of glutamate transporters in controlling excitatory neurotransmission (Rothstein et al. 1996; Tanaka et al. 1997). They localize to finely branched astrocyte processes approaching synapses (Danbolt et al. 1992; Rothstein et al. 1994; Chaudhry et al. 1995) and at the synaptic termini of neurons (Zhou and Danbolt 2014).
Here, we show that hyaluronan synthesis supports the activity and cellular process localization of glutamate transporters. Our study reveals a role of hyaluronan in spatiotemporally restricting glutamate diffusion and avoiding the off-target effects of synaptic glutamate release.
Materials and methods
Materials
Primary antibodies validated by suppliers were used for immunocytochemistry. Chicken polyclonal anti-MAP2 (Research Resource Identifier, RRID:AB_2138153; Abcam, Cambridge, UK, 1 : 1000), rabbit polyclonal GluA2/3 (RRID:AB_90710; Merck Millipore, Burlington, VT, USA, 1 : 1000), mouse monoclonal anti-vGluT1 (RRID: AB_2750766; UC Davis/NIH NeuroMab Facility, Davis, CA, USA, 1 : 1000), and rabbit polyclonal anti-GABA (RRID:AB_306844; Abcam, 1 : 1000). Mouse monoclonal anti-Neurocan antibody 1D1 was deposited to the developmental study hybridoma bank (DSHB) by Margolis, R.U./Margolis, R.K. (RRID:AB_528397, DSHB Hybridoma Product 1D1, 1 : 20). bHABP (BC41; Hokudo, Hokkaido, Japan, 1 : 500) was used to label hyaluronan. Alexa 488 anti-chicken (A21449), Alexa 568 streptavidin (S11226), Alexa 488 anti-rabbit (A11008) and Alexa 647 anti-mouse (A11001) antibodies are all from Thermo Fisher Scientific, Rockford, IL, USA and used at 1 : 3000 dilution. The plasmid pSpCas9(BB)-2A-Puro (PX459) V2.0 (Ran et al., 2013) was a gift from Prof. Feng Zhang (RRID:Addgene_62988, Addgene plasmid #62988).
Animals
All animals were treated in accordance with Japanese national guidelines and regulations, following the Guide for the Care and Use of Laboratory Animals. All experiments were approved either by the Animal Research Committee of the International University of Health and Welfare (18025NA), the National Institute of Health Sciences (544), or the animal resource committee of the Keio University School of Medicine (12069, 13021), and conformed to the regulatory standards. C57BL/6 mice (Sankyo Labo Service Corporation, Tokyo, Japan), CD44-deficient mice on a C57BL/6 background (RRID:IMSR_JAX:005085; Jackson Laboratories, Bar Harbor, ME, USA), or Sprague–Dawley rats (RRID:RGD_10395233; Japan SLC, Shizuoka, Japan) were maintained under specific pathogen-free conditions. One mother and her pups were housed in a mouse or a rat cage (KN-600 or KN-601; Natsume Seisakusho, Tokyo, Japan) at 20–25°C and 40–70% humidity, with free access to food and water on a 12-h light/12-h dark cycle. All animals were killed by decapitation under sevoflurane anesthesia.
Statistical analysis
Statistical analysis was performed after Shapiro–Wilk normality test and one-way analysis of variance in R version 3.5.3 (R Foundation for Statistical Computing, Vienna, Austria) followed by Student’s t-test in Microsoft Excel 2016 (Microsoft, Redmond, WA, USA) for data in pairs, Welch’s t-test in R for pre-normalized data, Tukey’s or Tukey–Kramer’s test in R for more than three data sets. No sample size calculation and test for outliers were performed. Each data point corresponds to an experiment performed using cells from different animals, dates, plates, transfections or passages.
Constructs
All custom-made constructs will be shared upon reasonable request. Recombinant mouse glutamate transporter-1 (GLT1) was cloned using PCR from a mouse brain cDNA library. The deletion construct GLT1-TM contained the amino acid residues 41–504, representing the TM transporter domain of GLT1. Both GLT1 and GLT1-TM were inserted into the monomeric-enhanced green fluorescent protein (pmEGFP)-C1 vector, which was derived from pEGFP-C1 (Clontech, now Takara Bio Inc., Shiga, Japan) by introducing an A206K mutation to monomerize the EGFP (Zacharias et al. 2002) or pmCherry-C1 (Clontech, now Takara Bio Inc.). The PCR primers and restriction enzymes used were as follows.
GLT1: ACTCGAGCATCAACAGAGGGTGCCAACAA and CGAGATCTAATCTCGGTTCTTCAGTTCATGTCGG, XhoI and BamHI for pmEGFP-C1 and pmCherry-C1, Eco47III and BamHI for pENTR11-dual GLT1-TM: ACTCGAGCCCTGGGGAAAAATCTCCTGCTCTCA and CGAATTCAGTCAATGGTGTCCAGCTCAGACT, XhoI and EcoRI for pmEGFP-C1 and pmCherry-C1, Eco47III and BamHI for pENTR11-dual GLAST-TM ACTCGAGCCCTGTTTCGGAATGCCTTCGTTCTG and CGAGATCTAATCTCGGTTCTTCAGTTCATGTCGG, XhoI and BglII for pmEGFP-C1 and pmCherry-C1, AgeI and XbaI for pENTR-dual.
The construct for expressing EGFP-HAS3 has been previously described (Kultti et al. 2006).
Recombinant adenoviruses
To produce recombinant adenoviruses, adenoviral vectors were prepared using the ViraPower™ Adenoviral Gateway™ Expression Kit (K493000, Thermo Fisher Scientific). Lck-mCherry (Zacharias et al. 2002), mEGFP-GLT1, and mEGFP-GLT1-TM were inserted into the pENTR11-dual vector (A10467; Thermo Fisher Scientific) and then transferred to pAv/CMV/DEST-V5 adenoviral vectors (V49320; Thermo Fisher Scientific). The adenoviral vectors were introduced to HEK293A cells (RRID:CVCL_6910; Thermo Fisher Scientific) using Lipofectamine™ LTX Reagent with PLUS™ Reagent (15338030; Thermo Fisher Scientific). Recombinant viruses were amplified by transfection to HEK293A cells, and cells were harvested 3 days after transfection by centrifugation. Cells were resuspended in 2 mL Dulbecco's modified Eagle medium (DMEM, 043-30085; FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan) containing 10% fetal bovine serum (FBS) and lysed by three cycles of freezing and thawing, followed by centrifugation at 1000 g. The resulting viral suspensions contained 108–109 pfu/μL of adenovirus when tittered using HEK293A cells.
Primary mouse astrocyte cultures
Primary astrocyte cultures were prepared from mice and also used to compare wild-type and CD44 knockout. Ten brains of 1-day-old C57BL/6JJ mice of both sexes, 1–2 g body weight for each, were aseptically removed, and the cerebrums were pooled, and then dissociated by trituration and trypsinization. After centrifugation at 400 g for 5 min, the cells were suspended in DMEM with 10% FBS. The residual tissue aggregates were removed by filtration through a cell strainer with a pore size of 40–45 µm. Cell suspensions were transferred to 75-cm2 culture flasks maintained at 37°C in a humidified atmosphere containing 50 mL/L CO2 for 2–5 weeks.
For confocal imaging, astrocytes were transferred to 24-well plates with uncoated glass coverslips at 1 × 105 cells/well. Recombinant adenoviruses were added 2–3 days after plating at ~ 1000 multiplicity of infection (m.o.i.), and the astrocytes were imaged on the next day of infection. To block hyaluronan synthesis, 2 mmol/L 4-methylumbelifenone (4MU; 139-05342; FUJIFILM Wako Pure Chemical Corporation) was added at the time of adenovirus transfection and incubated overnight.
Hippocampal primary neuronal culture
Hippocampal primary neuronal culture was prepared from rats chosen for culture stability. The brains of 1-day-old Sprague–Dawley rats of both sexes, 7–8 g bodyweight, were aseptically removed, and the hippocampi were dissected and pooled. The tissues were dissociated by trituration and trypsinization. After centrifugation at 400 g for 5 min, the cells were suspended in Neurobasal medium (21103049; Thermo Fisher Scientific) supplemented with 20 mL/L B27 supplement (17504044; Thermo Fisher Scientific) and 1 mL/L antibiotic-antimitotic (15240032; Thermo Fisher Scientific) (NB-B27). The residual tissue aggregates were removed by filtration through a cell strainer with a pore size of 40–45 µm. Cell suspensions of 250 µL (24-well plate), 50 µL (96-well plate), or 150 µL (8-well slide chamber) were plated to each well together with the same volume of astrocyte-conditioned medium of a 24-well culture plate (82464; ibidi, Madison, WI, USA), 96-well µ-plate (655986; Greiner Bio-one, Kremsmünster, Austria), or 8-well glass slide chamber (154534; Thermo Fisher Scientific), which were pre-coated with 1 g/L poly-l-lysine in 0.1 mol/L Na-Borate buffer (pH8.5). Half of the medium was replaced with NB-B27 supplemented with 10 µmol/L of cytosine arabinoside on days in vitro (DIV 1). One-third of the medium was changed to fresh NB-B27 once a week until DIV 35. Cultures were maintained at 37°C in a humidified atmosphere containing 50 mL/L CO2.
Recombinant protein expression
1321N1 (RRID:CVCL_0110, European Collection of Authenticated Cell Cultures, Public Health England) and COS-7 (RRID:CRL-1651; American Type Culture Collection, Manassas, VA, USA) cells were cultured in DMEM/10% FBS and plated in 24-well plates containing uncoated glass coverslips at 5 × 104 cells/well. Contamination is reported for 1321N1 cells, the cells are not authenticated, but cloned before use for experimental consistency. Plasmids were introduced using Lipofectamine LTX and Lipofectamine plus (Thermo Fisher Scientific), and the cells were imaged 2 days after transfection. Recombinant adenoviruses encoding mEGFP-GLT1, mEGFP-GLT1-TM, mEGFP-GLAST-TM, or Lck-mCherry were infected at ~ 1000 m.o.i. for expression in astrocytes and in 1321N1 cells and the cells were imaged 2 days after infection.
Confocal microscopy imaging
Glass coverslips with the plated cells were transferred to glass-bottomed culture plates filled with balanced salt solution (BSS) (129 mmol/L NaCl, 4 mmol/L KCl, 1 mmol/L MgCl2, 2 mmol/L CaCl2, 4.2 mmol/L d-glucose, and 10 mmol/L HEPES, adjusted to pH 7.4 with NaOH) for confocal microscope imaging. Live images were obtained using an FV BX61W1 laser scanning confocal microscope system (Olympus, Tokyo, Japan) and Fluoview software (Olympus), with excitation at 488 nm for mEGFP and 559 nm for mCherry. Identity of image files was blinded to the analyst using computer-generated randomization list following simple randomization procedures for cellular process localization analysis. Fixed images were obtained using an A1 plus laser scanning confocal microscope system and NIS Elements software (Nikon, Tokyo, Japan) with excitation at 488 nm for Alexa 488 secondary antibodies and EGFP, 561 mn for Alexa 555 secondary antibodies and mCherry, and 638 nm for Alexa 647 secondary antibodies. A Plan Apo VC 60x WI DIC N2 lens with numerical aperture of 1.2 and a Plan Apo VC 20x DIC N2 lens with numerical aperture of 0.75 were used.
Proteomics
Primary cultures of mouse astrocytes were replated onto a 10-cm culture plate on DIV 30 and cultured for 2 days. Astrocytes were transfected with ~ 1000 m.o.i. recombinant adenovirus encoding mEGFP-GLT1 or Lck-mEGFP, as a negative control. Three days after transfection, cells were rinsed with BSS and then incubated with 0.5 mmol/L 3,3'-dithiobis-sulfosuccinimidyl propionate (21578; Thermo Fisher Scientific), 0.5 mmol/L sulfo-N-κ-maleimidoundecanoyl-oxysulfosuccinimide ester (21111; Thermo Fisher Scientific), or 0.5 mmol/L sulfo-N-γ-maleimidobutyryl-oxysulfosuccinimide ester (22324, Thermo Fisher Scientific) in BSS for 30 min at 25°C. The crosslinking reaction was terminated by incubation with 200 mmol/L Tris (pH 8.0) at 25°C for 30 min. Then, the cells were scraped off from the culture plate, resuspended in a solution containing 20 mmol/L Tris (pH 8.0) and 300 mmol/L NaCl, and collected by centrifugation at 15 000 g for 1 min. Astrocytes not subjected to the crosslinking reaction were lysed by passing 10 times through a 27-G syringe needle. After removing precipitates by centrifugation at 200 g for 10 min, the supernatant was centrifuged at 15 000 g for 10 min to obtain the membrane fraction, as a precipitate. Membrane fractions were solubilized and sonicated in a solution containing 20 mmol/L Tris (pH 8.0), 300 mmol/L NaCl, and 10 mL/L Triton X-100. The solubilized samples were diluted 5 times in 20 mmol/L Tris (pH 8.0)/300 mmol/L NaCl and centrifuged at 15000 g for 10 min.
DSHB-GFP-12A6 monoclonal antibody against GFP was used for immunoprecipitation. DSHB-GFP-12A6 was deposited to the DSHB by DSHB. DSHB-GFP-12A6 hybridoma culture supernatant was mixed with Dynabeads protein G (10003D; Thermo Fisher Scientific) at 25°C for 60 min. After washing, the Dynabeads-anti-GFP conjugate was added to the solubilized supernatant of the membrane fraction described above. After incubation with tube rotation at 4°C for 2 h, the beads were washed twice with 1 mL of 20 mmol/L Tris (pH 8.0), 300 mmol/L NaCl, 1 mL/L Triton X-100 and then washed twice with H2O.
Bound proteins were extracted with guanidine solution (7 mol/L guanidine and 50 mmol/L Tris), reduced by incubating with 5 mmol/L dithiothreitol for 30 min, and alkylated with 10 mmol/L iodoacetamide for 1 h in the dark. Proteins were demineralized, concentrated by methanol/chloroform precipitation, and digested with trypsin (50 mmol/L NH4HCO3, 1.2 mol/L urea, and 0.5 μg trypsin). Liquid chromatography–mass spectrometry was performed using a Q Exactive mass spectrometer (Thermo Fisher Scientific) connected to an HTC-PAL autosampler and a nano-Advance UHPLC (Bruker, Billerica, MA, USA), including a MonoCap C18 nano-flow (0.1 × 150 mm) reversed-phase column and CaptiveSpray Ionization (Bruker). The mass spectrometer was operated in the data-dependent MS mode.
A peak list was generated and calibrated using the MaxQuant software (version1.4.1.2)(Max-Planck Institute for Biochemistry, Martinsried, Germany) (Cox and Mann 2008). Database searches were performed against the complete proteome set of Mus musculus in UniProtKB 2014_03.
Hyaluronidase treatment
Bovine testis hyaluronidase (18240-36; Nakarai Tesque, Kyoto, Japan) or hyaluronidase from Streptomyces hyalurolyticus (H1136; Sigma-Aldrich, St Louis, MO, USA) in solution H (129 mmol/L NaCl, 4 mmol/L KCl, 1 mmol/L MgCl2, 2 mmol/L CaCl2, and 4.2 mmol/L d-glucose; no buffer included) was added dropwise to astrocytes on coverslips to a final concentration of 0.4 mg/mL or 160 U/mL. Where indicated, cells were washed with BSS for 10 min after the hyaluronidase treatment for recovery. Paraformaldehyde at 40 g/L in phosphate-buffered saline (PBS) was used for fixation. Heat inactivation was performed at 95°C for 20 min.
Glutamate uptake assay
Astrocytes in primary culture were plated in a 24-well plate at 1 × 105 cells/mL density. After 2–4 days of incubation, the cells were transfected with recombinant adenovirus encoding mEGFP-GLT1 and assayed on the next day. Where indicated, 2 mmol/L 4 MU were applied at the time of adenovirus transfection. The fluorescence intensity of mEGFP was measured using a fluorescence microscope for standardization. The cells were rinsed twice with solution H. For analysis of hyaluronidase effects, astrocytes were incubated for 1 min in 150 µL of solution H with or without 0.25 mg/mL hyaluronidase and washed and incubated in 150 µL of solution H for 3 min for recovery, where indicated. Then, 150 µL of sodium-containing buffer (5 mmol/L HEPES pH 7.5, 137 mmol/L NaCl, 5.4 mmol/L KCl, 1.26 mmol/L CaCl2, 0.4 mmol/L MgSO4, 0.5 mmol/L MgCl2, 0.64 mmol/L KH2PO4, 5.5 mmol/L d-glucose) containing 200 µM (final concentration, 100 µM) glutamate and 3.7 kBq [3H] glutamate was added and incubated at 25°C for 3 min. Where indicated, 1 000 000 Da hyaluronic acid sodium salt from rooster comb (087-04511; FUJIFILM Wako Pure Chemical Corporation) was added during the incubation. Assays were stopped by removing the assay solution and rinsing three times with 0.5 mL of ice-cold sodium free choline buffer (5 mmol/L HEPES pH 7.5, 137 mmol/L choline chloride, 5.4 mmol/L KCl, 1.26 mmol/L CaCl2, 0.4 mmol/L MgSO4, 0.5 mmol/L MgCl2, 0.64 mmol/L KH2PO4, 5.5 mmol/L d-glucose). Cells were solubilized in 0.2 mL of 0.1 mol/L NaOH, and their radioactivity was measured by liquid scintillation counting.
Surface biotinylation assay
Biotinylation assay was performed using the Cell Surface Protein Isolation Kit (89881; Thermo Fisher Scientific) following the manufacturer’s protocol. Cultured mouse primary astrocytes were transferred to 10-cm plates and transfected with recombinant adenoviruses expressing mEGFP-GLT1, 4 days after plating, at ~ 1000 m.o.i. The next day, cells were rinsed twice with 10 mL BSS, incubated with 3 mL of solution H with or without 0.25 mg/mL hyaluronidase for 3 min, and washed twice with 7 mL PBS containing 0.2% azide at 25°C. Then, 10 mL of PBS/0.2% azide and 12 mg of sulfo-NHS-SS-biotin (21331; Thermo Fisher Scientific) were added and gently shaken at 4°C for 30 min. After addition of 0.5 mL quenching solution, cells were harvested, transferred to a 15-mL tube, and centrifuged at 1000 g for 5 min. The cells were sonicated in 0.12 mL of lysis buffer. After gently shaking at 4°C for 30 min, the solubilized membrane fraction was obtained by centrifugation at 15 000 g for 2 min. The supernatant was mixed with 500 µL of NeutrAvidin resin (31000; Thermo Fisher Scientific) at 25°C for 1 h. After washing the beads three times with 500 µL wash buffer, biotinylated proteins were eluted by incubation with 400 µL sodium dodecyl sulfate polyacrylamide gel electrophoresis sample buffer containing 50 mmol/L dithiothreitol, for 1 h at 25°C. The sample was separated by 8% polyacrylamide gel electrophoresis, transferred to a PDVF membrane (IPVH00010; Merck, Darmstadt, Germany) using Mini Trans-Blot Electrophoretic Transfer Cell (Bio-Rad Laboratories, Hercules, CA, USA) at 30 V for 12 h in 25 mmol/L Tris pH8.3, 192 mmol/L glycine, 200 mL/L methanol, 1g/L SDS. The blotted membrane were blocked for 1 h with 10 g/L skim milk, 2 mL/L Tween 20 in PBS, followed by mouse monoclonal anti-GFP antibody (1E4; Medical and Biological Laboratories, Aichi, Japan, 1 : 2000) and house radish peroxidase-linked anti-mouse IgG (NA931; GE Healthcare, Chicago, IL, USA, 1 : 2000) for 12 h each at 4°C in the same solution. SuperSignal West Femto Maximum Sensitivity Substrate (34096; Thermo Fisher Scientific) was used as substrate, Luminescent Image Analyzer LAS-3000 mini (FUJIFILM Wako Pure Chemical Corporation) was used for signal detection.
FRET analysis
For Fluorescence resonance energy transfer (FRET) analysis, 10 images were obtained without interval and photobleached after acquiring the fifth image at selected circular regions for 10 s, at 100% laser output at 559 nm. For hyaluronidase treatment, COS-7 cells co-expressing mCherry-GLT1 and EGFP-HAS3 that were treated with or without 0.4 mg/mL hyaluronidase for 1 min were fixed with 40 g/L paraformaldehyde in PBS and used for acceptor photobleaching. The average fluorescence intensity of the area used for bleaching was calculated for each image and normalized to the average intensity before bleaching.
HAS gene knockouts by the clustered regularly interspaced short palindromic repeats/CRISPR-associated proteins (CRISPR-Cas9) system
Guide RNA sequences were designed using CRISPRDesign (Hsu et al. 2013). Human astrocytoma 1321N1 cells were transfected with pSpCas9(BB)-2A-Puro (PX459) V2.0 containing the guide RNA sequence to introduce deletions to each of the three HAS genes. Two plasmids with different guide RNA sequences targeting a single HAS gene were co-transfected to cleave the targeted gene at two sites and cause deletion of at least 85 (HAS1_#1 and #2 pair) or 111 (HAS1_#1 pair) amino acid residues of HAS1, 19 (HAS2_#1 and #2 pair) or 66 (HAS2_#2 and #3 pair) amino acid residues of HAS2, and 133 (HAS3_#1 and #3 pair) amino acid residues of HAS3. These sites were designed in the cytoplasmic catalytic domains of HAS proteins so that the successfully targeted products would be dysfunctional, even if genome editing failed to cause a frame shift. After selection of transfected cells using 1 μg/mL puromycin, surviving cells were isolated as colonies. After genomic DNA was prepared from each isolated colony, gene deletion was verified by genomic PCR using primers flanking the targeted regions as listed below. Shortened PCR fragments indicate successful deletion on both alleles (Figure S2). After successful generation of single gene knockout cell lines, secondary mutations were introduced, selected, cloned, and verified by the same method. For generation of triple knockouts of HAS, HAS1/HAS3 double knockout (expressing only HAS2; HAS2-only) cells were transfected with plasmids designed for HAS2 knockout cell generation, selected by puromycin, and used for transfection with a recombinant adenovirus encoding mEGFP-GLT1-TM.
Guide RNA sequences inserted to pSpCas9(BB)-2A-Puro (PX459) V2.0 were as follows: HAS1_#1: GTTGCCGTCCCACACGTACG (6302); HAS1_#2: GCAGAGCCTCTTCGCGTACC (6571); HAS1_#3: gCAGGAGGCCGTAGCGATCGG (6635); HAS2_#1: CTCGCAACACGTAACGCAAT (12551); HAS2_#2: gTCTCACCGGGACCCTTTTCG (12509); HAS2_#3: gCTTGATAGGCAGCGATGCAA (12311); HAS3_#1: gTGTAGTCCACCGAATCGCCG (4444); HAS3_#3: gCACCGGCGCATGCGACGTGC (4031).
The number in parenthesis indicates cleavage sites on NCBI reference sequences of HAS1, NC_00019.10; HAS2, NC_000008.11; HAS3, NC_000016.10.
HAS2_#1, #2, and then HAS1_#1, #3 were used for 1321N1 HAS1-only generation. HAS1_#1, #2, and then HAS3_#3, #1 were used for 1321N1 HAS2-only generation. HAS3_#3, #1, and then HAS2_#1, #2 were used for 1321N1 HAS3-only generation. HAS2_#2, #3 were used to knockout HAS2 in 1321N1 HAS2-only cells.
The deletion was confirmed by genomic PCR using the following primers. The number in parenthesis indicates the primer position on the corresponding NCBI reference sequences listed above.
HAS1_gf: ACGTAGTCCACCGAATCTCCGAGC (6099); HAS1_gr: CATCGCCTTCGCCCTGCTCATC (6722); HAS2_gf: ACACCTCATCATCCAAAGCCTGTTTGC (12215); HAS2_gr: TTGCCCTGGCAGGAATGTGGAGAC (12708); HAS3_gf: TCCTGCCTGACCCTTCATCTCCTGC (3795); HAS3_gr: AACCCAAGGGACCTAGACCAGACC (4570).
Ca2+ imaging
Changes in intracellular calcium concentrations in rat hippocampal neurons were measured by the 1-[6-Amino-2-(5-carboxy-2-oxazolyl)-5-benzofuranyloxy]-2-(2-amino-5-methylphenoxy)ethane-N,N,N',N'-tetraacetic acid, pentaacetoxymethyl ester (Fura-2AM, F015; Dojindo Laboratories, Kumamoto, Japan) Ca2+ imaging method. The culture medium of primary hippocampal cultures, grown in an 8-well glass chamber, was replaced on DIVs 21–35 with Ca2+ imaging buffer (150 mmol/L NaCl, 5.0 mmol/L KCl, 1.8 mmol/L CaCl2, 1.2 mmol/L MgCl2, 25 mmol/L HEPES pH7.4, and 10 mmol/L d-glucose). The cells were loaded with 10 µmol/L Fura-2AM in Ca2+ imaging buffer at 25°C for 45 min and washed with Ca2+ imaging buffer containing 1 µmol/L tetrodotoxin (TTX, 207-15901; FUJIFILM Wako Pure Chemical Corporation). The glass chambers were mounted on an inverted epifluorescence microscope (TE-2000-U; Nikon). The Aquacosmos 1200 software (Hamamatsu Photonics, Shizuoka, Japan) was used for data acquisition and processing. Fluorescent images were obtained by alternating excitation at 340 and 380 nm. The emission signal at 510 nm was collected by a charge-coupled device camera (CRCA-R2; Hamamatsu Photonics), coupled with an image intensifier (GaAsP, C8600; Hamamatsu Photonics). A hyaluronidase solution (0.25 mg/mL hyaluronidase, 150 mmol/L NaCl, 5.0 mmol/L KCl, 1.8 mmol/L CaCl2, 1.2 mmol/L MgCl2, and 10 mmol/L d-glucose, plus 1 µmol/L TTX) was applied by perfusion for 1 min at a speed of 1.5 mL/min. For blocking glutamate receptors, cells were incubated for 1 min in a glutamate receptor antagonist cocktail [0.1 mmol/L α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor and kainite receptor antagonist 6,7-dinitroquinoxaline-2,3-dione (DNQX), 0.1 mmol/L NMDA receptor antagonist d-(-)-2-amino-5-phosphonopentanoic acid (APV), and 0.1 mmol/L metabotropic glutamate receptor antagonist methyl-(4-carboxyphenyl)glycine (MCPG)] in Ca2+ imaging buffer containing 1 µmol/L TTX, followed by hyaluronidase solution containing a glutamate receptor antagonist cocktail of 0.1 mmol/L APV, 0.1 mmol/L DNQX, 0.1 mmol/L MCPG, and 1 µmol/L TTX for 1 min. After each treatment, the cells were washed with Ca2+ imaging buffer including 1 µmol/L TTX.
Neuronal toxicity of hyaluronidase treatment and immunocytochemistry
Hippocampal primary cultures were incubated for 30 min at 25°C in solution H with 0.25 mg/mL hyaluronidase, glutamate receptor antagonist cocktail of 0.1 mmol/L APV, 0.1 mmol/L DNQX, 0.1 mmol/L MCPG, or 0.1 mmol/L glutamate where indicated. Hippocampal primary cultures were fixed with 40 g/L paraformaldehyde for 30 min at 25°C. Cells were immunostained using chicken polyclonal anti-MAP2 and Alexa 488 anti-chicken antibody. The images were obtained using an A1 microscope (Nikon). The length of MAP2-positive fibers was automatically analyzed using the ImageJ (Schneider et al. 2012) based FIJI 1.52 (Schindelin et al. 2012) software and the skeletonize function.
Results
Process localization of glutamate transporters is hyaluronan dependent
Rodent GLT1 or human excitatory amino acid transporter 2 are the major subtypes of glutamate transporters in the cerebral cortex and hippocampus, followed by rodent glutamate aspartate transporter (GLAST) or human excitatory amino acid transporter 1 (Pines et al. 1992). These glutamate transporters have a trimeric transmembrane (TM) transporter domain with both N- and C-terminal tails at the cytoplasm. As we showed in a previous study, mEGFP-GLT1 localized to cellular processes of cultured astrocytes (Fig. 1a and b). The TM transporter domains of the two glutamate transporters (mEGFP-GLT1-TM and mEGFP-GLAST-TM), with all TM helices and their connecting loops, but without cytoplasmic tails, localized to cellular process tips. This result shows that cellular process tip localization is a common feature of trimeric TM transporters, while the cytoplasmic tails of GLT1 facilitate plasma membrane delivery (Hayashi and Yasui 2015).
To identify recruiters of glutamate transporters to cellular processes, we proteomically analyzed chemically crosslinked mEGFP-GLT1 immunoprecipitates. CD44, a hyaluronan-binding TM protein (Aruffo et al. 1990) was identified in the immunoprecipitates. If CD44 mediates process localization, hyaluronidase treatment should affect the localization of glutamate transporters. Strikingly, mEGFP-GLT1 retracted from cellular processes to cell bodies within 2 min of bovine testis hyaluronidase treatment. The cellular processes visualized by membrane marker Lck-mCherry, remained intact (Fig. 1a and b). Furthermore, the TM domain fragments of both GLT1 and GLAST retracted from the process tips after the hyaluronidase treatment (Fig. 1c and d). Biotinylated hyaluronan-binding protein (bHABP) did not label astrocyte processes (Figure S1a) but detected hyaluronan deposits in the neuronal culture, which was removed by 1 min hyaluronidase treatment (Figure S1b). Hyaluronidase from Streptomyces hyalurolyticus caused minor retraction of mEGFP-GLT1 from 2 out of 22 astrocytes, indicating weaker activity compared to bovine testis hyaluronidase (Figure S1c).
The retraction of mEGFP-GLT1 upon hyaluronidase treatment was reversible, as it returned to the cellular processes within 2 min after hyaluronidase washout. Hyaluronan synthesis inhibition by 4MU abolished the cellular process localization of mEGFP-GLT1 and was not further affected by hyaluronidase treatment (Fig. 1c and d). Taken together, hyaluronan is responsible for the cellular process localization of glutamate transporters, and rapidly restored after hyaluronidase washout by hyaluronan resynthesis. Short hyaluronan chains at astrocyte processes are not sufficiently long or stable to be labeled by bHABP, but they determine the process localization of glutamate transporters.
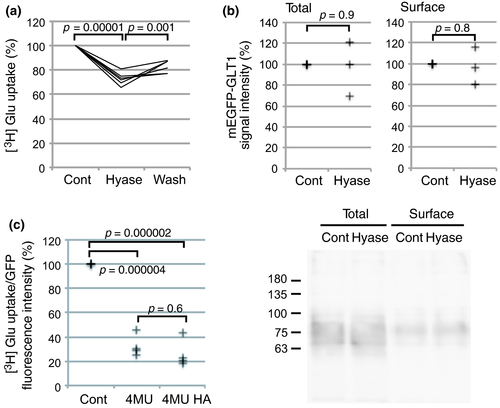
Glutamate uptake is supported by hyaluronan synthesis
Glutamate transporters are responsible for removal of extracellular glutamate. Hyaluronidase treatment of primary cultured astrocytes expressing mEGFP-GLT1 reduced [3H] glutamate uptake (Fig. 2a), while the amount of cell surface mEGFP-GLT1 was not affected (Fig. 2b). The decrease in glutamate uptake through mEGFP-GLT1 showed a sign of recovery in 3 min after hyaluronidase washout, but did not reach statistical significance. Hyaluronan synthesis dependency was confirmed by the significantly reduced glutamate uptake after preincubation with 4 MU (Fig. 2c). Importantly, supplementing exogenous hyaluronan to cells treated with 4MU failed to restore glutamate uptake. Taken together, hyaluronan must be synthesized constantly in the vicinity of GLT1 to maintain its activity. Exogenously applied hyaluronan, not anchored to the plasma membrane, could not compensate for the lack of endogenous hyaluronan synthesis.
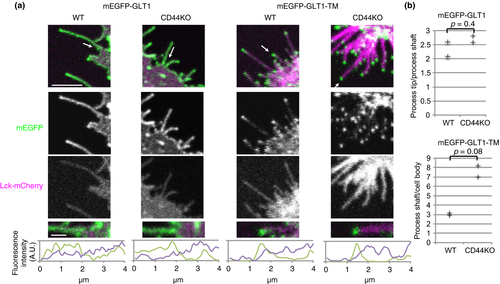
CD44 is not required for process localization of glutamate transporters
Next, we studied which protein recruits glutamate transporters to cellular processes in a hyaluronan-dependent manner. CD44 was the primary candidate because it was the only hyaluronan-binding protein identified in our proteomic analysis of mEGFP-GLT1 immunoprecipitates. Unexpectedly, even in astrocytes prepared from CD44 knockout mice, both mEGFP-GLT1 and mEGFP-GLT1-TM localized to cellular processes and to process tips, respectively (Fig. 3). This constitutes clear evidence that CD44 is not required for process localization of glutamate transporters. Their process localization was stronger, but did not reach statistical significance in CD44 knockout cells, and may reflect loss of competition with CD44 for hyaluronan.
HAS3 interacts with GLT1 in a hyaluronan-dependent manner
The next candidate recruiters of glutamate transporters to cellular process tips were hyaluronan synthases. HAS polymerized hyaluronan at their cytoplasmic catalytic domains and then secreted the product to extracellular spaces through their TM domains (Itano and Kimata 1996). They also localize to cellular processes, with HAS3 showing the strongest localization (Kultti et al. 2006; Törrönen et al. 2014). EGFP-HAS3 localized to the cellular processes of COS-7 cells and colocalized with mCherry-GLT1 at cellular processes when co-expressed (Fig. 4a), suggesting that HAS might recruit GLT1 to cellular processes.
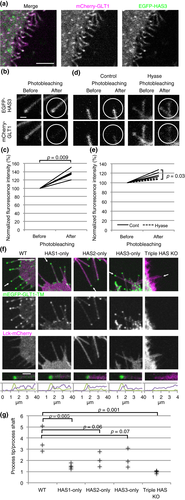
Next we measured FRET between EGFP-HAS3 and mCherry-GLT1. FRET occurs only when two molecules are in close proximity to form a complex (Karpova et al. 2003). Photobleaching mCherry-GLT1 significantly increased the fluorescence intensity of EGFP-HAS3, a signature of FRET (Fig. 4b and c). Hyaluronidase treatment significantly reduced FRET signaling, indicating that the interaction between the two proteins is hyaluronan dependent (Fig. 4d and e).
To identify which of the three HAS proteins is responsible for recruiting GLT1 to cellular processes, we performed serial knockouts using the CRISPR-Cas9 technique (Ran et al. 2013). Guide RNA sequences were designed (Hsu et al. 2013) to delete a part of each HAS gene including its TM and catalytic domain. Deletion in HAS genes of both alleles of human astrocytoma 1321N1 cells was confirmed by genomic PCR (Figure S2). Each of the intact HAS proteins maintained mEGFP-GLT1-TM at process tips (Fig. 4f and g). Among the three subtypes, HAS1-only cells showed significantly reduced process tip localization of mEGFP-GLT1-TM, reflecting weak localization to process tips of HAS1 (Törrönen et al. 2014). In contrast, the loss of HAS1-3 completely impaired this localization. Taken together, we found that all HAS proteins are capable of recruiting GLT1 to cellular process tips.
Hyaluronan degradation excites neurons and leads to excitotoxicity
While astrocytes strongly express glutamate transporters (Rothstein et al. 1994; Schmitt et al. 1997), neurons also express glutamate transporters. For example, neuronal GLT1 contributes to synaptosomal glutamate uptake (Petr et al. 2015). Perineuronal nets are frequently associated with cell bodies of parvalbumin-positive GABAergic neurons (Härtig et al. 1992; Yamada and Jinno 2013) which also express excitatory amino-acid carrier 1 subtype of glutamate transporters (Holmseth et al. 2012).
We studied whether glutamatergic synapses bear hyaluronan in hippocampal neurons prepared from neonatal rats. Hyaluronan deposits labeled by bHABP were observed around vGluT1 vesicular glutamate transporter positive excitatory synapses to an extent similar to that in GABA positive inhibitory synapses (Figure S3a and b).
Based on our results, we speculated that hyaluronan help to turn off excitatory synaptic transmission by supporting glutamate clearance. Therefore, we monitored activity in dissociated rat hippocampal neurons treated with hyaluronidase. Neurons were loaded with the intracellular Ca2+ indicator Fura-2AM, while their synchronized bursts were suppressed by TTX. Ca2+ concentration in neuronal cell bodies rose immediately after hyaluronidase application and decreased after its washout (Fig. 5a and b). An antagonist cocktail for α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid, kainate, N-methyl-d-aspartate, and metabotropic glutamate receptors almost completely blocked the hyaluronidase effect. These results indicate that hyaluronidase treatment evokes glutamatergic responses. The same neurons successfully responded to another dose of hyaluronidase after washout of the glutamate receptor antagonists.
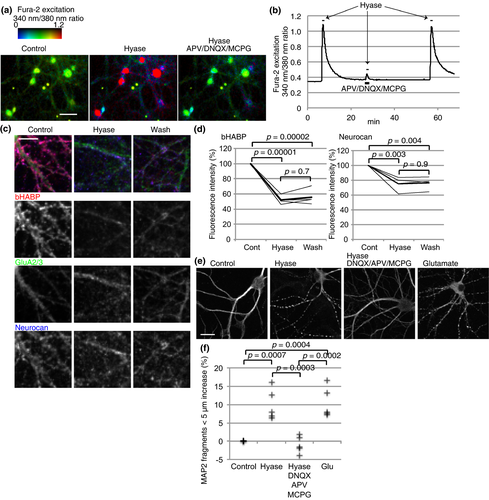
Hyaluronidase treatment removed bHABP labeled hyaluronan around AMPA receptor GluA2/3 positive dendrites. A sign of recovery of hyaluronan was observed after hyaluronidase washout (Fig. 5c and d), consistent with rapid recovery of process localization and activity of glutamate transporters observed in astrocytes. Neurocan, a CSPG, was also removed from neurons. Taken together, we speculate that after hyaluronan removal, spontaneous releases of glutamate resulted in excitatory signal due to decreased glutamate transporter activity, and short chains of hyaluronan actively synthesized by neurons restored glutamate clearance after hyaluronidase washout.
Neuroprotective role of hyaluronan
The Ca2+ response of neurons prompted us to study the excitotoxic effects of prolonged hyaluronidase treatment. We used microtubule-associated protein 2 (MAP2) staining of neuronal dendrites as an indicator of neuronal damage (Park et al. 1996). Control, healthy neurons showed continuous MAP2-positive dendrites, while a 10-min incubation with glutamate increased MAP2 beads, reflecting glutamate excitotoxicity (Fig. 5e and f). Importantly, 10 min of hyaluronidase treatment also increased MAP2 beads. The glutamate receptor antagonist cocktail described above blocked this excitotoxicity. Although hyaluronidase from Streptomyces hyalurolyticus failed to induce significant neuronal damage (Figure S3c), heat inactivation and addition of hyaluronan significantly reduced the neuronal damage (Figure S3d). These results indicate that reduction in glutamate uptake by hyaluronidase treatment caused neuronal damage through activation of glutamate receptors. In all, hyaluronan synthesis supports glutamate uptake by glutamate transporters and thereby helps to turn off excitatory neurotransmission and protects neurons from excitotoxicity.
Discussion
In this study, we characterized a novel physiological role of hyaluronan of supporting glutamate clearance by glutamate transporters. Hyaluronan is the backbone of perineuronal nets and synthesized by HAS at the plasma membrane and supports glutamate transporter GLT1 activity (Fig. 6). While astrocytes are not labeled by bHABP, process localization and activity of glutamate transporters are still affected by hyaluronan synthesis and degradation. Although bHABP does not label short hyaluronan chains, these short hyaluronan chains anchored to HAS around glutamate transporters appears to be sufficient to maintain their activity. The difference in action of hyaluronidase from bovine testis and Streptomyces hyalurolyticus we observed could be attributed to whether these hyaluronidases are able to trim short hyaluronan chains anchored to HAS so that newly synthesized hyaluronan do not reach glutamate transporters. We previously reported that an extracellular loop connecting the transport subdomain and trimerization subdomain of GLT1 is required for cellular process localization (Hayashi and Yasui 2015). We speculate that the interaction between the extracellular loop of GLT1 and a short chain of hyaluronan anchored to neighboring hyaluronan synthase affects conformational change between subdomains for substrate transport.
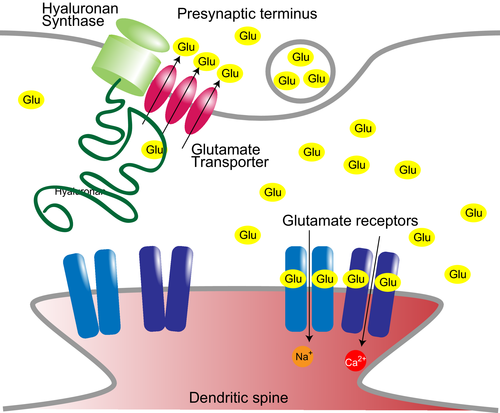
Hyaluronan forms the backbone of perineuronal nets (Kwok et al. 2010) to which CSPGs bind. Parvalbumin-positive GABAergic neurons are frequently associated with preineuronal nets (Härtig et al. 1992; Yamada and Jinno 2013) and also express excitatory amino-acid carrier 1 subtype of glutamate transporters (Holmseth et al. 2012). In astrocytes, knockdown of tenascin R, a perineuronal nets component, reduces glutamate uptake through downregulation of GLAST (Okuda et al. 2014). These evidences indicate that perineuronal nets may affect expression and/or stability of glutamate transporters through regulation of hyaluronan synthesis or degradation.
In our study, hyaluronidase treatment-activated glutamate receptors and raised neuronal intracellular calcium concentration. We speculate that spontaneous release of glutamate from neurons leads to excitatory signals when glutamate clearance by both neurons and astrocytes is reduced. There are other synaptic molecules regulated by hyaluronan, such as molecular diffusion of AMPA receptors (Frischknecht et al. 2009), L-type voltage-dependent calcium channels (Kochlamazashvili et al. 2010), or TRPV1 channels (Caires et al. 2015), and hyaluronidase treatment also affects their functions. The hyaluronidase effect we observed in neurons could be a combination of effects on these molecules and glutamate transporters.
We focused on the short-term effects of hyaluronan removal in this study because transient Ca2+ signaling can cause many indirect long-term effects (Cohen and Greenberg 2008). To support our study, several studies on the long-term effects of hyaluronan deprivation have reported findings consistent with our results. For example, epileptiform activity of primary hippocampal cultured neurons was observed after long-term hyaluronidase treatment (Vedunova et al. 2013). Long-term treatment by hyaluronidase or chondroitinase affected synapse density and the electrophysiological responses of neurons in culture (Pyka et al. 2011; Vedunova et al. 2013). These changes can be the consequence of the long-term effects of proteins regulated by hyaluronan including glutamate transporters. In addition, there is a HAS knockout mice study reporting episodic epilepsy indicating enhanced excitatory neurotransmission (Arranz et al. 2014). Our study indicated that reduction in glutamate uptake due to lack of HAS resulted in the epileptic phenotype. Our results also explain why HAS knockout mice had a milder phenotype compared to prematurely deceased GLT1 knockout mice (Tanaka et al. 1997). The remaining HAS subtype of the single or double HAS knockout mice should be capable of supporting glutamate transporters for their survival.
Our findings show the potential of hyaluronan to protect neurons from excitotoxicity by supporting glutamate clearance. Several studies have been conducted on the neuroprotective effect of perineuronal net components, such as hyaluronan and its derivatives (Morawski et al. 2004; Wakao et al. 2011; Suttkus et al. 2014; Vinukonda et al. 2016), or CSPGs (Okamoto et al. 1994). Our study indicates that the glutamate transporter is a strong candidate of the target of action unidentified in their studies. Such temporal control of glutamate signaling may allow high frequency firing by rapid clearance of glutamate to prevent glutamate receptor saturation. Coincidentally, perineuronal nets are prominently observed around the soma of parvalbumin-positive neurons (Härtig et al. 1992), which are often characterized as fast-spiking neurons.
It is known that both abnormal perineuronal nets and excitatory–inhibitory imbalance are involved in psychiatric diseases (Ramocki and Zoghbi 2008; Mauney et al. 2013). Fast-spiking parvalbumin-positive neurons, which tend to have thick perineuronal nets, are active when animals are focused (Kim et al. 2016). Therefore, abnormal perineuronal nets and excitatory–inhibitory imbalance observed in patients with psychiatric diseases may be related to hyaluronan and glutamate uptake. Our finding that hyaluronan controls excitatory signals suggests a possible mechanism of how hyaluronan affect excitatory and inhibitory balance, which is required for healthy tuning of brain function.
Acknowledgments and conflict of interest disclosure
This work was supported by the Mochida Memorial Foundation for Medical and Pharmaceutical Research, Takeda Science Foundation, Keio Gijuku Academic Development Funds, Ichiro Kanehara Foundation, JSPS KAKENHI Grant number 17K07344, and a Grant-in-Aid for Scientific Research on Innovative Areas (Comprehensive Brain Science Network) from the Ministry of Education, Science, Sports and Culture of Japan and International University of Health and Welfare. We thank Profs. Katsuya Miyake and John Heuser (International University of Health and Welfare), Drs. Keiko Matsuda, Osamu Nagano, Eiji Sugihara, Yoshinori Yukutake, Profs. Hideyuki Saya and Kenji Tanaka (Keio University), Prof. Hidetoshi Tozaki-Saitoh, and Dr. Tomohiro Yamashita (Kyushu University) for sharing research recourses; Dr. Zhi Zhou (Keio University) and Dr. Tetsushi Sakuma (Hiroshima University) for technical advice on CRISPR-CAS9, Dr. Michiko Yanagisawa and Prof. Kohichi Tanaka (Tokyo Medical and Dental University) for technical advice on the glutamate uptake assay, and Prof. Yu Yamaguchi (Sanford-Burnham Medical Research Institute) for helpful discussions. The monoclonal antibody against GFP, 12A6, was developed by and obtained from the Developmental Studies Hybridoma Bank, created by the NICHD of the NIH and maintained at The University of Iowa, Department of Biology, Iowa City, IA 52242. The authors declare that they have no competing interests.
All experiments were conducted in compliance with the ARRIVE guidelines.