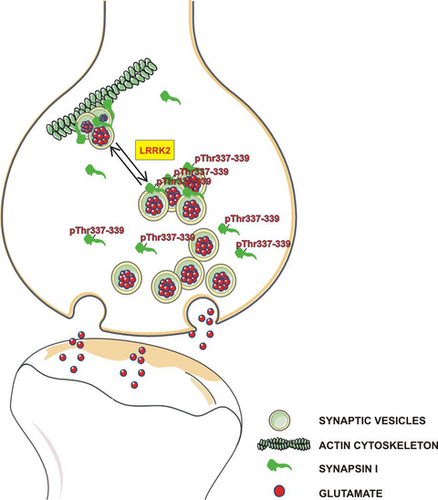
Leucine-rich repeat kinase 2 phosphorylation on synapsin I regulates glutamate release at pre-synaptic sites
Data Availability Statement: The datasets generated and analyzed during this study are available from the corresponding author on reasonable request.
Abstract
Leucine-rich repeat kinase 2 (LRRK2) is a large multidomain scaffolding protein with kinase and GTPase activities involved in synaptic vesicle (SV) dynamics. While its role in Parkinson’s disease has been largely investigated, little is known about LRRK2 physiological role and until now few proteins have been described as substrates. We have previously demonstrated that LRRK2 through its WD40 domain interacts with synapsin I, an important SV-associated phosphoprotein involved in neuronal development and in the regulation of neurotransmitter release. To test whether synapsin I is substrate for LRRK2 and characterize the properties of its phosphorylation, we used in vitro kinase and binding assays as well as cellular model and site-direct mutagenesis. Using synaptosomes in superfusion, patch-clamp recordings in autaptic WT and synapsin I KO cortical neurons and SypHy assay on primary cortical culture from wild-type and BAC human LRRK2 G2019S mice we characterized the role of LRRK2 kinase activity on glutamate release and SV trafficking. Here we reported that synapsin I is phosphorylated by LRRK2 and demonstrated that the interaction between LRRK2 WD40 domain and synapsin I is crucial for this phosphorylation. Moreover, we showed that LRRK2 phosphorylation of synapsin I at threonine 337 and 339 significantly reduces synapsin I-SV/actin interactions. Using complementary experimental approaches, we demonstrated that LRRK2 controls glutamate release and SV dynamics in a kinase activity and synapsin I-dependent manner. Our findings show that synapsin I is a LRRK2 substrate and describe a novel mechanisms of regulation of glutamate release by LRRK2 kinase activity.
Abbreviations used
-
- anova
-
- analysis of variance
-
- CaMKI,
-
- CaMKII Ca2+/calmodulin-dependent I and II kinases
-
- Cat. N.
-
- catalog number
-
- Cdk5
-
- cyclin-dependent kinase-5
-
- c-Src
-
- pp60c-src
-
- DMEM
-
- Dulbecco’s modified Eagle’s medium
-
- DTT
-
- dithiothreitol
-
- eEPSCs
-
- evoked excitatory post-synaptic currents
-
- FBS
-
- fetal bovine serum
-
- HEK293T
-
- Human embryonic kidney
-
- KO
-
- knock out
-
- LRRK2
-
- leucine-rich repeat kinase 2
-
- MAPK/Erk
-
- mitogen-activated protein kinase
-
- PD
-
- Parkinson’s disease
-
- PEI
-
- polyethylenimine
-
- PKA
-
- cAMP-dependent kinase
-
- RP
-
- reserve/recycling pool
-
- RRID
-
- research resource identifiers
-
- RRP
-
- readily releasable pool
-
- SEM
-
- standard error of mean
-
- SV
-
- synaptic vesicle
Leucine-rich repeat kinase 2 (LRRK2) is a multidomain protein (286 kDa), containing a serine-threonine kinase domain, a Ras of complex proteins GTPase domain (Greggio, 2012; Taymans, 2012) and four domains involved in protein or membrane interaction: the armadillo, ankyrin, leucine-rich repeats, and WD40 domains (Cookson, 2010; Davies et al., 2013). Mutations in the LRRK2 gene cause autosomal dominant Parkinson’s disease (PD) with clinical and pathological phenotypes similar to those of the idiopathic disease (Paisán-Ruíz et al., 2004; Zimprich et al., 2004). Unfortunately, little is known on the role of mutant LRRK2 in the pathogenesis of PD. The pathogenic G2019S mutation located in the kinase domain of LRRK2 is the most frequent variant identified in both familial and apparently sporadic PD cases (Goldwurm et al., 2005). This mutation induces a significant increase in LRRK2 kinase activity in vitro and in vivo (West et al., 2005; Greggio et al., 2006; Sheng et al., 2012). Interestingly, an increase in LRRK2 kinase activity may be toxic for neurons (Greggio et al., 2006; Smith et al., 2006). In this regard, LRRK2 seems to be involved in various neuronal processes, including cytoskeletal dynamics (Kett et al., 2012; Caesar et al., 2013; Civiero et al., 2015) and vesicular trafficking (Matta et al., 2012; Cirnaru et al., 2014; Belluzzi et al., 2016). Recently, we have demonstrated that LRRK2 is associated with synaptic vesicles (SV) through its WD40 domain (Piccoli et al., 2014). This domain is also able to interact with various proteins involved in neurotransmitter release, including synapsin I (Piccoli et al., 2014).
The synapsins are major SV-associated phosphoproteins that play important roles in neuronal development and synaptogenesis, as well as synaptic transmission and plasticity in mature neurons (Hilfiker et al., 1999; Baldelli et al., 2006; Cesca et al., 2010; Valente et al., 2012). Synapsins are known to interact with the actin cytoskeleton and SVs at pre-synaptic terminals, mediating clustering of SVs and their reversible attachment to the cytoskeleton (Cesca et al., 2010; Messa et al., 2010; Rizzoli, 2014). Synapsins are phosphorylated by multiple serine-threonine kinases, including cAMP-dependent kinase, Ca2+/calmodulin-dependent kinase I and II, mitogen-activated kinase, cyclin-dependent kinase-5 (PKA, CaMKI, CaMKII, MAPK/Erk, and Cdk5, respectively) at distinct serine residues (Cesca et al., 2010); furthermore synapsins are also phosphorylated on tyrosine by pp60c-src (c-Src) (Onofri et al., 2007). While phosphorylation on serine residues causes the dissociation of the synapsins from SVs and the transition of SVs from the reserve/recycling pool (RP) to the readily releasable pool (RRP) to promote exocytosis (Chi et al., 2001, 2003; for review, see Cesca et al., 2010), tyrosine phosphorylation induces the association of synapsins to SV and actin.
Here, we show that synapsin I is a robust substrate for LRRK2 kinase activity. Specifically, LRRK2 phosphorylates synapsin I at threonine residues located in domain C, a highly conserved region involved in binding to both SVs and actin cytoskeleton. We thus considered the possibility that threonine phosphorylation of synapsin I is involved in the regulation of neurotransmitter release by affecting SV mobility and demonstrated that this phosphorylation decreases synapsin I association with SVs and actin filaments. Moreover, using specific inhibitors for LRRK2 kinase activity and LRRK2 G2019S–BAC transgenic mice, we showed that LRRK2 phosphorylation on synapsin I plays a pivotal role in the regulation of neurotransmitter release and SV dynamics. Taken together our results suggest that pathogenic LRRK2 mutations, such as the G2019S mutation, may alter SV dynamics and neurotransmitter release by causing synapsin I hyperphosphorylation.
Methods
Animals
Synapsin I knock out (KO) mice were generated by homologous recombination (Chin et al., 1995) and were fully backcrossed on a C57BL/6J background for over 10 generations (Charles River, Calco, Italy). After the injection of the embryonic stem cells, the C57BL/6 blastocysts were implanted in pseudopregnant females. Male chimeric mice were re-crossed to C57BL/6 females and the genotypes of the littermates verified by genomic Southern blot. Synapsin I KO mice were subsequently bred in homozygosis (Chin et al., 1995). Non-transgenic wild-type (WT) and LRRK2 BAC hG2019S mice, back-crossed on a C57BL/6J strain, were obtained from Mayo Clinic (Jacksonville, FL, USA) through a collaboration with Dr. Heather Melrose (Melrose et al., 2010). All the mice used in this study were adult male (2–3 months) of about 25 g. Sprague–Dawley male rats (research resource identifiers, RRID:RGD_1566457) of 125–150 g were from Charles River (Calco, Italy). Animals were housed with free access to water and food (max four mice for cages with a floor space of almost 330 cm2 and three rats for cages with a floor space of almost 800 cm2). All experiments on animals were carried out in accordance with the guidelines established by the European Community Council (Directive 2010/63/EU of September 22, 2010 ) and approved by the Italian Ministry of Health (authorization 793/2016-PR and 365 D.lgs 116/92-art.7, IRCCS San Martino-IST, PROT. n. 0005278/14). Transgenic mice could be shared upon reasonable request under material transfer agreement.
Plasmids and constructs
pCHMWS 3xFlag-tagged LRRK2 WT, K1906M and G2019S constructs were previously described (Civiero et al., 2012). For LRRK2 GST-WD40 domain and GST alone, bacterial cells were transformed to ampicillin resistance by electroporation with constructs containing pGEX-2T in frame with sequences encoding for WD40 domain. Rat synapsin I cDNA was cloned into pEGFP using the Xho1/KPN1 restriction sites (Clontech, CA, USA; kind gift of Dr H.-T. Kao, Brown University, Providence, RI, USA). Synapsin I mutants were generated using the QuickChange mutagenesis kit [Agilent Technologies, CA, USA; catalog number (Cat. N. 200523)] according to the manufacturer’s instructions. Plasmids were validated by restriction analysis and DNA sequencing.
Cell culture and transfection
Human embryonic kidney (HEK293T) or COS-7 cells were maintained in Dulbecco’s modified Eagle’s medium (Thermo Fisher, Waltham, MA USA) supplemented with 10% fetal bovine serum (Thermo Fisher) at 37°C and 5% CO2.
For transfections, linear polyethylenimine (PEI, Polysciences, Warrington, PA, USA) was used with a ratio DNA : PEI 1 : 2 in OPTI-MEM (Thermo Fisher). After 20 min of incubation to permit the formation of the DNA/PEI complexes, the mix was added directly to the cells in Petri dishes of 15 cm2. The cells were used after 24–48 h.
Protein purification and phosphorylation assays
Protein purification
For LRRK2 GST-WD40 domain or GST purification, large-scale cultures of Luria broth with ampicillin (100 µg/mL) were grown at 37°C to log phase and induced with isopropyl β-D-thiogalactopyranoside (100 µM) for 3–5 h.
Bacteria were then lysed and GST or GST-WD40 domain fusion protein were purified by affinity chromatography on glutathione-Sepharose and dialyzed against 25 mM Tris-HCl, 50 mM NaCl, pH 7.4 (Onofri et al., 1997). Synapsin I was obtained from bovine brain under non-denaturing conditions as described (Bähler and Greengard, 1987).
Actin was prepared from an acetonic powder of rabbit skeletal muscle in buffer A (0.2 mM CaCl2, 0.2 mM ATP, 0.5 mM NaN3, 0.5 mM β-mercaptoethanol, 2 mM Tris-HCl, pH 8.0), purified by gel filtration on a Sephadex G-150 column and stored in liquid nitrogen (Spudich and Watt, 1971; MacLean-Fletcher and Pollard, 1980). SVs were prepared from male rats through the step of controlled-pore glass chromatography (Huttner et al., 1983).
In order to purify GFP-labeled WT and mutated synapsin I, COS-7 were processed with GFP-Trap_A kit (Chromotek, Planegg-Martinsried, Germany; Cat. N. gtak-20) according to manufacturer’s protocol. Cells were solubilized (20 mM Tris-HCl pH 7.5, 150 mM NaCl, 1 mM EDTA, 0.5% Tween 20 or 1% Triton X-100, 2.5 mM sodium pyrophosphate, 1 mM beta-glycerophosphate, 1 mM Na3VO4, Sigma protease inhibitor cocktail) and centrifuged for 20 min at 10 000 g. The lysates were incubated with GFP trap agarose beads for 2 h at 4°C on a rotator. After several washes with the wash buffer from the kit, when necessary, proteins were eluted with the elution buffer.
For LRRK2-K1906M, LRRK2-WT, or LRRK2-G2019S, HEK293T were lysed as previously described and centrifuged for 30 min at 14 000 g. Afterward, lysates containing 3xFlag-tagged protein were incubated with anti-Flag M2 agarose beads (Sigma-Aldrich, St. Louis, MO, USA; Cat. N. A2220) for 2 h at 4°C on a rotator. After extensive washing (two washes with Tris-HCl 20 mM, NaCl 500 mM, Tween 0.5%; two washes with Tris-HCl 20 mM, NaCl 300 mM, Tween 0.5%; two washes with Tris-HCl 20 mM, NaCl 150 mM, Tween 0.5%; two washes with Tris-HCl 20 mM, NaCl 150 mM, Tween 0.1%; two washes with Tris-HCl 20 mM, NaCl 150 mM, Tween 0.02%), proteins were eluted in elution buffer (20 mM Tris-HCl pH 7.5, 150 mM NaCl, 0.02% Tween 20 or 0.02% Triton X-100 and 150 ng/µL of 3xFlag peptide) by shaking for 30–40 min at 4°C.
COS-7 and HEK293T used for the purifications were from ATCC (Sesto San Giovanni, MI, Italy). These cell lines are not listed as a commonly misidentified cell line by the International Cell Line Authentication Committee and they are not be authenticated. COS-7 and HEK293T have been maintained in culture for maximum 8–10 passages.
Phosphorylation assays
For kinase assays, synapsin I was phosphorylated (Syn P) or not (Syn DP) in vitro by LRRK2 in kinase buffer (25 mM Tris-HCl pH 7.5, 2 mM dithiothreitol (DTT), 10 mM MgCl2 containing 100 μM [γ-32P]ATP or [γ-33P]ATP, 1 µCi/sample) using purified recombinant LRRK2-K1906M, LRRK2-WT, LRRK2-G2019S, or LRRK2970-2527 (Invitrogen, Waltham, MA, USA; Cat. N. PV4873) at 1 : 50 LRRK2 : synapsin I ratio for various times at 30°C, as described (Civiero et al., 2012). No dephosphorylating pre-treatment was performed in order to eliminate the other physiologically phosphorylated sites of Synapsin I. LRRK2 inhibitors were used at 150 nM to 1 µM (PF-06447475, Sigma Aldrich; Cat. N. PZ0248), 1–3 µM (IN-1, Tocris, Bristol, UK; Cat. N. 4273) or 0.2 µM (GSK2578215A, Reith et al., 2012; Tocris, Bristol, UK; Cat. N. 4629). 33P incorporation was also analyzed in the presence of the LRRK2 GST-WD40 domain or GST alone (1 µM).
Cysteine-specific cleavage of synapsin I
Phosphorylated synapsin I was subjected to cysteine-specific cleavage as described (Schiebler et al., 1986; Bähler et al., 1989). Briefly, after a reduction step in 10 mM DTT, samples were denatured by extensive dialysis against 200 mM Tris-HCl pH 8.0, 6 M guanidine-HCl, 0.1 mM DTT, 0.1 mM EDTA and added with 5 mM NTCB. The samples were incubated at 37°C in the dark for 72 h in order to complete the chemical cleavage. Finally, after a dialysis against 4 M urea, 5 mM Na phosphate pH 7.0, the fragments were resolved by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) on 7.5–15% gradient gels and blotted onto nitrocellulose. The identification of peptides was based on electrophoretic mobility after immunostaining with specific anti-peptide polyclonal antibodies (Vaccaro et al., 1997). The LRRK2-phosphorylated peptide was identified by analyzing 32P radioactivity. In particular the membranes with the radioactive samples were exposed to photographic films with an intensifying screen at −80°C. After 1–3 days of exposure, the films developed and quantified by densitometric analysis.
Sample preparation for mass spectrometry analysis
200 µL of 100 mM (NH4)HCO3/50% CH3CN were used to destain the gels until colorless, the gels were then washed with 200 µL 100% CH3CN and then with 200 µL 100 mM (NH4)HCO3/1 mM CaCl2 and moisturized with 80 µL 100 mM (NH4)HCO3, 1 mM CaCl2 in 30% CH3CN. The samples were digested overnight at 37°C with 1 µg trypsin and the peptides were collected by centrifugation. The gels were washed three times with 50 mM (NH4)HCO3 pH 9 in 40% CH3CN. The supernatants were dried and the sample suspended in 50 µL 2% CH3CN with 0.1% HCOOH.
NanoLC setup
The peptide separation was performed with Dionex Ultimate 3000 RSLC nanoSystem (Thermo Scientific, Waltham, MA, USA). The sample was first loaded from the sample loop into a trapping column, then the peptides from the trapping column were pushed in the separation column (50 cm × 75 µm ID, PepMap C18, 2 µm particles, 100 Å pore size; Thermo Scientific) and eluted with increasing organic solvent 2–60% solution B (80% CH3CN and 20% H2O, 0.1% HCOOH) in 80 min at a flow rate of 250 nL/min.
Mass spectrometer setup
The mass spectrometer LTQ-Orbitrap Velos Pro was operated in positive ionization mode. Single MS survey scans were performed in the Orbitrap, registering the masses between 350 and 1650 m/z with a resolution of 60 000. The maximum ion injection time and the automatic gain control were set to 250 ms and to 1 000 000 ions, respectively. The polydimethylcyclosiloxane background ions were used as internal standard calibration of spectra.
The experiments were done in data-dependent acquisition mode, for each MS scan was allowed a maximum of 10 MS/MS. The minimum MS signal for triggering MS/MS was set to 3000 ions, with the most abundant ion signal selected for MS/MS using an isolation window of 2 Da. The exclusion duration was set to 30 s with a mass tolerance of ± 5ppm. Collision-induced dissociation (CID) in ion trap was chosen as fragmentation method. CID was done with a target value of 5000 ions, a maximum ion injection time of 150 ms, normalized collision energy of 35%, a Q-value of 0.25 and an activation time of 10 ms. The analysis of phosphopeptides on a linear ion trap mass spectrometer was performed by Multi-Stage Activation. The neutral loss species at 97.97, 48.99, or 32.66 m/z below the precursor ion, exceeded a threshold of 500 ion count, were activated for 30 ms during fragmentation (pseudo-MS3).
Data analysis
Thermo Scientific Proteome Discoverer software version 1.4 was used to process the raw data files. Peak list files were screened using the SEQUEST search engine on the UniProt human database and trypsin was set as digestion enzyme. Percolator was used to filter the peptide list; the peptide mass deviation was set to 10 ppm and a minimum of six amino acids per identified peptide and a maximum of 1% FDR were required. For the assignment of phosphorylation sites in peptides, the algorithm PhosphoRS was used. The Database search parameters were mass tolerance precursor 20 ppm, mass tolerance fragment CID 0.8 Da with dynamic modification of deamidation (N, Q), oxidation (M), phosphorylation (S, T, Y), and static modification of alkylation with IAM (C). Proteins were grouped by applying the maximum parsimony rule. The computational methods used to determine the absolute protein quantification were the average of the three most abundant peptides.
Synaptic vesicle-binding assays
The binding of purified synapsin I to synapsin I-depleted SVs was performed as previously described (Schiebler et al., 1986). Synapsin I-depleted SVs (10 μg/sample) were incubated for 1 h at 0°C with increasing concentrations (100–800 nM) of synapsin I which had been phosphorylated by LRRK2 in a buffer containing 220 mM glycine, 30 mM NaCl, 5 mM Tris-HCl, 4 mM HEPES (pH 7.4), 0.22 mM NaN3, and 100 μg/mL bovine serum albumin. After incubation, SV-bound synapsin I was separated by high-speed centrifugation (400 000 g for 30 min) through a 10% (w/w) sucrose cushion. Aliquots of the resuspended pellets were subjected to SDS-PAGE followed by 32P radioactivity analysis (as described above) and immunoblotting with synapsin I-specific antibodies, to follow the phosphorylated and dephosphorylated forms of synapsin I, respectively. The recovery of SVs, used to correct the amounts of bound synapsin I, was determined by quantitative immunoblotting with anti-synaptophysin antibodies.
Actin-binding assays
After the polymerization for 3 h at 25°C with 90 mM KCl, 2 mM MgCl2, G-actin (5 μM) was incubated with Synapsin I (600 nM), phosphorylated or not by LRRK2 as described, in a buffer containing 12.5 mM NaCl, 0.6 mM ATP, 100 mM KCl, 1.2 mM MgCl2, 1.5 mM 2-mercaptoethanol, 6 mM HEPES, 8 mM Tris-HCl (pH 7.4). After 1 h, samples were subjected to high-speed centrifugation (400 000 g for 30 min at 4°C) for recovery of total F-actin (Bähler and Greengard, 1987). The actin pellets were resuspended in SDS-sample buffer and subjected to SDS-PAGE, as described. The amount of synapsin I bound to actin filaments was determined by 32P-radioactivity analysis (as described above) and immunoblotting with synapsin I-specific antibodies.
Pull-down assays
GFP-labeled WT and T337-339A synapsin coupled to GFP-Trap as described above were incubated with an extract of cortical rat brain rapidly dissected out after carbonarcosis and decapitation. Incubation was made for 3 h at 4°C under gentle rotation in the absence of detergents in order to preserve SV structure. After the incubation period, the samples were extensively washed with a detergent-free buffer (50 mM Tris, 150 mM NaCl) and the bound proteins eluted in Laemmli sample buffer and subjected to SDS-PAGE and immunoblotting against actin and synaptophysin (Onofri et al., 1997). Synaptophysin was used as marker of SV recovery.
Preparation of purified synaptosomes
Following cervical dislocation, WT or synapsin I KO male mice were decapitated, brains were quickly removed and the cerebral cortex or striatum dissected out at 4°C. After an homogenization step in 0.32 M sucrose, buffered at pH 7.4 with Tris–HCl, using a glass-teflon tissue grinder (clearance 0.25 mm, 12 up–down strokes in about 1 min), samples were centrifuged (5 min, 1000 g at 4°C) to eliminate nuclei and debris. The supernatant was gently stratified on a discontinuous Percoll gradient (6%, 10%, and 20% v/v in Tris-buffered sucrose) and after a centrifugation at 33 500 g for 5 min at 4°C the synaptosomal fraction (layer between 10% and 20% Percoll) was collected and resuspended in physiological HEPES-buffered medium (NaCl 140 mM; KCl 3 mM; MgSO4 1.2 mM; NaH2PO4 1.2 mM; HEPES 10 mM; glucose 10 mM; pH 7.4; Nakamura et al., 1993).
Release experiments
After the incubation with 0.03 µM [3H] D-ASP (37°C for 15 min), identical aliquots of synaptosomal suspension were layered on microporous filters placed at the bottom of a set of parallel superfusion chambers (Superfusion System; Ugo Basile, Comerio, Varese, Italy) maintained at 37°C and superfusion was started at a flow rate of 0.5 mL/min (Zappettini et al., 2010). The collection of the samples started after 36 min of superfusion to equilibrate the system and followed the scheme: two 3-min samples (t = 36–39 min and t = 45–48 min; basal release) before and after one 6-min sample [t = 39–45 min; K+-evoked release]. A 90 s period of depolarization was applied at t = 39 min of superfusion. Depolarization was performed using 15 mM KCl, substituting for an equimolar concentration of NaCl. IN-1 3 µM or PF-06447475 1 µM was added 9 min before depolarization. Collected fractions and superfused synaptosomes were counted for radioactivity by liquid scintillation counting. The efflux of radioactivity in each fraction was expressed as a percentage of the total radioactivity present in synaptosomes at the onset of the fraction collection (fractional rate). In order to calculate the depolarization-evoked neurotransmitter overflow the transmitter content of the basal release was subtracting from that of the K+-evoked release.
Preparation of cortical cultures
For cortical autaptic culture, mice were killed by CO2 inhalation, and 17/18-day embryos (E17-E18) were removed immediately after a caesarean section. Cortices were dissected from the rest of the brain, stripped of meninges and cut into small pieces that were dissociated through enzymatic digestion using 0.125% Trypsin solution for 20 min at 37°C and then triturated using a fire-polished Pasteur pipette as previously described (Valente et al., 2016). No antimitotic pharmacologic agents were added to avoid glial cells proliferation. Primary cultures of hippocampal neurons were grown as autaptic cells as described before (Bekkers and Stevens, 1991) with slight modifications. To do this we have used the protocol proposed by Allen (Allen 2006). Briefly, dissociated neurons were plated at very low density (20 cells/mm2) on microdots (having a diameter of about 40–300 μm) obtained by spraying a mixture of poly-d-lysine (0.1 mg/mL) and collagen (0.25 mg/mL) on dishes. Dishes were pre-treated before with a solution of 0.15% agarose. Both glial cells and single neurons grown as autaptic cells were present under this culture condition. Neuron cultures for immunofluorescence and SypHy assay were prepared from mouse cortexes obtained from embryonic day 15.5–16.5 mice (C57BL/6J) as previously indicated (Corti et al., 2008; Pischedda et al., 2018). GSK-2578215A compound (Reith et al., 2012) or DMSO were added to culture media at the concentrations indicated through the text.
Patch-clamp recordings, data acquisition, and analysis
Patch-clamp recordings were performed in whole-cell configuration in autaptic neurons grown on microislands, as previously described (Valente et al., 2012). Patch pipettes were pulled from thick borosilicate glass capillaries (Hilgenberg, Mansfield, Germany), to a final resistance of 3–4 MΩ and filled with the standard internal solution. Evoked excitatory post-synaptic currents (eEPSCs) were recorded using a double EPC-10 amplifier (HEKA Electronic, Lambrecht, Germany) by superfusing the whole-cell clamped cell with a Tyrode solution containing (in mM): 140 NaCl, 2 CaCl2, 1 MgCl2, 4 KCl, 10 glucose, 10 HEPES (pH 7.3 adjusted with NaOH). On the day of experiments (11 DIV), d-(-)-2-amino-5-phosphonopentanoic acid, and (+)-bicuculline (d-AP5; 50 μM and bicuculline 30 μM; Tocris, Bristol, UK; Cat. N. 0106 and 0130) were added to the Tyrode solution to block N-methyl-d-aspartate receptors and GABAA receptors, respectively. The standard internal solution was composed of (in mM): 126 K gluconate, 4 NaCl, 1 MgSO4, 0.02 CaCl2, 0.1 BAPTA, 15 glucose, 5 HEPES, 3 ATP, 0.1 GTP (pH 7.2 adjusted with KOH). Experiments were carried out at 25°C. Neurons under whole-cell recordings were voltage clamped at −70 mV. Action potentials were evoked by a rapid (0.5 ms) depolarization of the cell body to + 40 mV at frequency of 0.1 Hz. eEPSCs were acquired at 10–20 kHz and filtered at one fifth of the acquisition rate with an 8-pole low-pass Bessel filter. Electrophysiological recordings having leak currents > 100 pA or series resistance > 10 MΩ were discarded. The acquisition of data was performed using PatchMaster programs (HEKA Elektronic, Lambrecht, Germany) and stored on a computer for offline analysis executed with FitMaster programs (HEKA Elektronic). eEPSCs were inspected visually, and only those that were not contaminated by spontaneous activity were used.
SypHy assay
We infected DIV four primary neurons with viruses expressing sypHy, a fusion construct of synaptophysin and super ecliptic pHluorin (Granseth et al., 2006). At DIV 14 neurons were treated with DMSO (control) or GSK2578215A (0.2 µM, 2 h). Syphy positive boutons were assayed in a stimulation chamber on the stage of a Zeiss Axiovert 200M equipped with a mono-chromator (Poly V) and a cooled CCD camera (PCO, Imago QE), both from TILL photonics (Gräfelfing, Germany). The assay was carried out as described previously (Belluzzi et al., 2016). Briefly, cells were submerged in 500 μl of KRH buffer (125 mM NaCl, 5 mM KCl, 1.8 mM CaCl2 2.6 mM MgSO4 5 mM Hepes, pH 7.2) in the presence of APV (2mM; Sigma-Aldrich) and CNQX (2 mM; Sigma-Aldrich). SypHy was excited at 475 nm and its fluorescence emission collected at 525 nm using a 60×, 1.1 NA water immersion objective. Images were acquired every second for 200 s using TillVision software (TILL Photonics). At frame 30, cells were stimulated with 40 action potential (AP, 20Hz) then at frame 70 with 300 AP (20 Hz). Total fluorescence was measured upon incubation with 50 mM NH4Cl. Quantitative measurements of the fluorescence intensity at individual boutons were obtained by averaging a selected area of pixel intensities using ImageJ (National Institute of Health, Bethesda, MD, USA). Net fluorescence changes (ΔF) were obtained by subtracting the average intensity of the first 15 frames (F0) from the intensity of each frame (Ft) for individual boutons and normalized F0 (ΔF/F0). The fluorescence increase and decay, reflect exo- and endocytosis, respectively.
Immunofluorescence
We transfected GFP-synapsin I WT and GFP-synapsin I T337-339A into WT and LRRK2 BAC hG2019S cortical neurons at DIV 10 using Lipofectamine 2000 (Invitrogen). We processed cell at DIV 14 for immunocytochemistry. We fixed neurons in 4% paraformaldehyde and 4% sucrose at 25°C. We applied mouse anti-synaptophysin antibody (1 : 400; Sigma-Aldrich) in GDB buffer (30 mM phosphate buffer, pH 7.4, containing 0.2% gelatin, 0.5% Triton X-100, and 0.8 M NaCl) overnight at 4°C. We applied secondary antibodies (1 : 1000, Cy-3 coupled, anti-mouse; ) (Thermo Scientific) in GDB buffer for 2 h at 25°C. Cover slips were mounted with prolonged reagent (Thermo Scientific) and observed with Zeiss Observer Z1 microscope equipped with an Apotome module using a plan-Apochromat 63×/1.40 Oil objective, pixel size 0.102 μm × 0.102 μm. The obtained images provide an axial resolution comparable to confocal microscopy (Schaefer et al., 2004; Garini et al., 2005). Colocalization studies were performed on the single plan generated by optical sectioning elaborated by Apotome module. Acquired images were analyzed with ImageJ software using the colocalization analysis plugin to detect colocalization between GFP-synapsin I and synaptophysin. Transfected neurons were randomly imaged from three different cell preparation.
Antibodies, SDS-PAGE, and western blotting analysis
SDS-PAGE was performed according to Laemmli (1970), and samples were subjected to SDS polyacrylamide gel electrophoresis and blotted onto nitrocellulose membranes (Whatman, Sigma-Aldrich). When required, 50 µM Phos-tag™ Acrylamide (Wako Pure Chemical Corporation, Neuss, Germany, Cat. N. AAL-107) was added to the separating gel, accordingly to manufacturer protocol (Kinoshita et al. 2006). Membranes were blocked for 1 h in 5% non-fat dry milk in Tris-buffered saline (TBS; 10 mM Tris, 150 mM NaCl, pH 8.0) plus 0.1% Triton X-100 and incubated overnight at 4°C or for 2 h at 25°C with the following primary antibodies: anti-Flag M2 (1 : 10 000; Sigma-Aldrich; Cat. N. F7425); anti-synapsin I (1 : 5000; 10.22 made in our laboratories; 1 : 1000 G304, G143, G309 generated in Paul Greengard’s laboratory, The Rockefeller University, New York, NY, USA); anti-LRRK2 (1 : 1000, C41-2; Abcam, Cambridge, UK; RRID: AB_2713963); anti-LRRK2 pSer935 (1 : 1000, UDD2 10(12); Abcam, Cambridge, UK; RRID:AB_2732035); anti-synaptophysin (1 : 10 000; G111 generated in Paul Greengard’s laboratory, The Rockefeller University, New York, NY, USA); anti-actin (1 : 1000; Sigma-Aldrich; RRID:AB_476730); anti-GFP (1 : 2000; Thermo Scientific; RRID:AB_221569). After several washes with TBS 1× plus 0.1% Triton X-100, membranes were incubated for 1 h at 25°C in the same buffer of the primary antibodies with peroxidase-conjugated anti-mouse (1 : 5000; BioRad, Hercules, CA, USA; RRID: AB_11125547) or anti-rabbit (1 : 3000; BioRad; RRID: AB_2713963) antibodies. After three washes with TBS 1× plus 0.1% Triton X-100 and two washes detergent-free, bands were revealed with the ECL chemiluminescence detection system (Thermo Scientific). The membranes with the radioactive samples (32P) exposed on photographic films and the immunoblots were quantified by densitometric analysis (Quantity One software; Bio-Rad). The extent of synapsin I phosphorylation was calculated as the ratio between phosphorylation-specific and total synapsin I immunoreactivities assessed in the same samples.
Custom-made materials could be shared upon reasonable request.
Statistical analysis
For each experiment the controls were tested in the same session of the treated samples. The final number of experiments per condition is listed in figures legend. All quantitative data are expressed as means ± SEM. Statistical analysis was carried out by two-tailed unpaired t-test, one-way or two-way anova followed by the post hoc Bonferroni or Tukey’s multiple comparison test as indicated in the text and figure captions. Significance was set at p < 0.05. The normal distribution of the data was assessed by the Kolmogorov–Smirnov test. No sample size calculation, no randomization, and no blinding were performed. No test for outliers has been applied.
Results
LRRK2 phosphorylates synapsin I both in vitro and cellular model
We have previously shown that LRRK2 interacts with synapsin I through its WD40 domain (Cirnaru et al., 2014; Piccoli et al., 2014). In view of this interaction, we explored the possibility that synapsin I could act as a substrate for LRRK2 and be phosphorylated on serine/threonine residues. To this aim, we performed in vitro experiments, in which purified flag-tagged LRRK2-WT, kinase dead LRRK2-K1906M or pathogenic LRRK2-G2019S variant was incubated with purified synapsin I at a LRRK2 : synapsin I ratio of 1 : 50 under kinase assay conditions (Civiero et al., 2012). As shown in Fig. 1 we observed that synapsin I was robustly phosphorylated by LRRK2 (LRRK2-WT and LRRK2 G2019S p < 0.0001 vs. LRRK2-KM; LRRK2 G2019S p < 0.0001 vs. LRRK2-WT, one-way anova/Bonferroni’s tests). The observed stoichiometry of phosphorylation was 0.02097 ± 0.00013 mol of phosphate/mol of synapsin I with LRRK2-K1906M, 0.03883 ± 0.00122 with LRRK2-WT and 0.11076 ± 0.00199 with LRRK2-G2019S (Fig. 1a and b). Moreover, taking advantage of the Mn2-Phos-tag™ SDS-PAGE technology, we demonstrated the phosphorylation of synapsin Ia by LRRK2 occurs also in cultured cortical neurons as the synapsin from the cells treated at 12 DIV with the LRRK2 kinase inhibitor PF-06447475 (1 µM; 2 h) is down-shifted (Fig. 1c). To better characterize the properties of synapsin I as substrate we used LRRK2970–2527, an artificial truncated variant characterized by higher constitutive activity (Martin et al., 2014) and 32P incorporation was analyzed as a function of incubation time and substrate concentration. Synapsin I phosphorylation increased progressively with time with a time constant (τon) of 35 ± 6 min (Fig. 2a and b) and reached a stoichiometry of 0.4 mol of phosphate/mol of synapsin I, consistent with a higher activity of the truncated LRRK2 protein. The rate of 32P incorporation in synapsin I was constant during the first hour and then declined afterwards (Fig. 2a and b). LRRK2 phosphorylation of synapsin I followed a simple Michaelis–Menten kinetics characterized by a Km of 62 ± 11 nM (Fig. 2c and d).
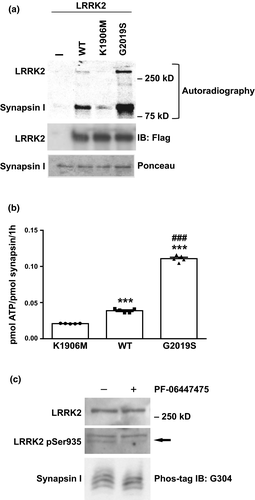

We also demonstrated, by competitive assays, that the interaction between LRRK2 WD40 domain and synapsin I is crucial for synapsin I phosphorylation by LRRK2; indeed, GST-WD40 significantly reduced the ability of LRRK2-WT to phosphorylate synapsin I (49 ± 6% vs. Ctrl; p < 0.0001, one-way anova/Bonferroni’s tests), whereas GST alone was ineffective (95 ± 3% vs. Ctrl p > 0.9999; Fig. 2e and f). On the contrary not significant difference in the levels of phosphorylation of LRRK2 were observed after incubation with GST-WD40 (p = 0.3253, one-way anova/Bonferroni’s tests).
LRRK2 phosphorylates synapsin I in the highly conserved C domain
To obtain information on the location of the phosphorylation site(s), synapsin I phosphorylated by LRRK2 was subjected to cysteine-specific chemical cleavage at Cys223, Cys360, and Cys370, obtaining α, β, and γ fragments of 30, 15, and 40–35 kDa, respectively (Fig. 3a) (Vaccaro et al., 1997). The bulk of phosphate incorporation was found in a central region, encompassing residues Syn223–360/370 containing several threonine/serine residues (Fig. 3b). To finally identify the phosphorylation site(s), we performed MS analysis on purified synapsin I phosphorylated by LRRK2 and the only phosphorylated peptide identified in the central region was 329TSVSGNWKTNTGSAMLEQIAMSDRYK354 (Fig. 3c).
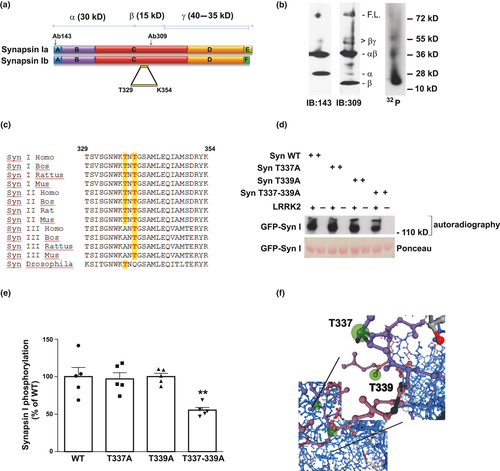
The MS data were subsequently validated by site-direct mutagenesis and in vitro kinase assays. WT and phospho-deficient synapsin I mutants T337A, T339A, and T337-339A were expressed in COS-7 cells, purified and subsequently incubated in vitro with LRRK2970–2527 under phosphorylation conditions. The analysis showed that, while the single mutants were phosphorylated by LRRK2 to the same extent of WT synapsin I (T337A 97 ± 8%, p > 0.9999; T339A 100 ± 4%, p > 0.9999), the double mutant (T337-339A) displayed an 32P incorporation of 55 ± 4% respect to WT synapsin I (Fig. 3d and e; p = 0.0058 vs. WT, one-way anova/Bonferroni’s tests). Interestingly, these sites belong to one of the most evolutionary conserved synapsin I regions and the phosphorylated threonines are conserved in all synapsin isoforms and orthologues from Drosophila to human (Fig. 3c) (Kao et al., 1999). Structural analysis of the two putative phosphorylation sites revealed that threonine337 and threonine339 are sufficiently exposed to the external environment and therefore accessible for phosphorylation (Fig. 3f; modified from Swissmodel). As the double mutation was not able to completely abolish the phosphorylation by LRRK2 on synapsin I, we also tested S341, another largely conserved residue identified by MS analysis in the same peptide. Synapsin S341A was phosphorylated at similar extent as the WT isoform, suggesting it is not a major phosphorylation site (data not shown).
LRRK2 phosphorylation of synapsin I decreases the interactions of synapsin I with synaptic vesicles and actin
Phosphorylation of synapsin I is known to modulate its interactions with SVs and/or actin (Cesca et al., 2010). Thus, we tested whether phosphorylation of synapsin I by LRRK2 may also alter these interactions. In order to study the impact of LRRK2 phosphorylation on synapsin I-SV interaction, we incubated SVs, which had been depleted of synapsin I (Huttner et al., 1983), with increasing concentrations of purified bovine synapsin I (100–800 nM) previously phosphorylated by LRRK2. Interestingly, the binding isotherms demonstrated that LRRK2-phosphorylated synapsin I had a significantly decreased ability to bind SVs (Fig. 4a and b; Syn DP Bmax = 3872 ± 84 fmol/µg prot, Syn P Bmax = 2722±279 fmol/µg prot; p = 0.0043 vs. Syn I DP Student’s t-test. Syn DP Kd = 107±17 nM, Syn P Kd = 352±99 nM; p = 0.0398 vs. Syn I DP Student’s t-test).
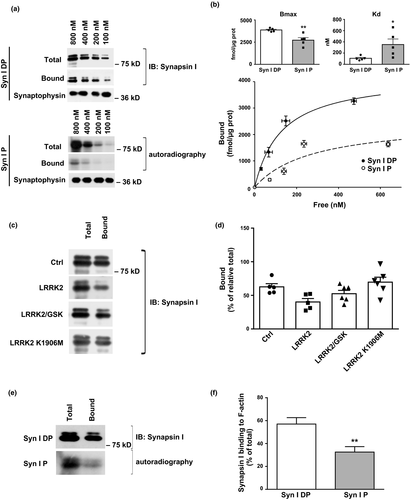
To exclude the possibility that the binding of LRRK2 to synapsin I, rather than LRRK2 kinase activity on synapsin I, might affect the ability of synapsin I to interact with SVs by steric hindrance, we performed binding experiments by either blocking LRRK2 kinase activity with the GSK2578215A inhibitor (0.2 µM) or using kinase-dead LRRK2-K1906M. The experiments demonstrated that the presence of LRRK2 per se, in the absence of kinase activity, does not affect the binding of synapsin I to SVs emphasizing that synapsin I phosphorylation by LRRK2 is responsible for the observed effects on SV binding (Fig. 4c and d; Synapsin I binding 62.66 ± 4.02% Synapsin I binding with GSK 52.45 ± 5.01%, Synapsin I binding in the presence of LRRK2 K1906M 69.70 ± 7.40%. All data were analyzed vs. relative Ctrl; LRRK2 GSK p = 0.7662, LRRK2 K1906M p > 0.9999 vs. Ctrl). Given the low stoichiometry of phosphorylation by LRRK2, the SV-bound immunoreactive synapsin I showed only a trend to a decrease (40.21 ± 5.07, p = 0.1078 vs. Ctrl). Given the importance of actin as cytoskeletal regulator of SV trafficking (for review, see Cingolani and Goda, 2008), we also tested the effect of LRRK2 phosphorylation of synapsin I on the interaction of synapsin I with actin filaments. Purified synapsin I was phosphorylated by LRRK2 as described above and subsequently incubated with preformed actin filaments. Noteworthy, LRRK2 phosphorylation of synapsin I significantly impaired synapsin I binding to actin filaments (Syn I DP actin binding 57 ± 6% of total, Syn P actin binding 33 ± 5% of total; p = 0.0023 vs. Syn I DP Student’s t-test), thus mimicking the effects of PKA, MAPK/Erk or CaMKII phosphorylation of synapsin I (Fig. 4e and f). Finally, in order to evaluate the contribution of the two phosphorylation sites on synapsin I-SV and actin interaction, we performed pull-down experiments. Our data show that synapsin I carrying the T337-339A mutation in COS-7 cells is less phosphorylated (Fig. 5a) and has higher affinity for actin and SVs compared to the WT (Syn I T337-339A actin binding 154 ± 16, p = 0.027 Student’s t-test; synaptophysin binding 143 ± 10% vs. Syn I WT, p = 0.022 vs. Syn I WT Student’s t-test; Fig. 5b and c).
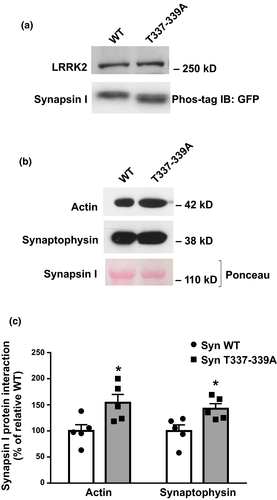
LRRK2 synapsin I phosphorylation modulates glutamate release
To evaluate the functional role of synapsin I phosphorylation by LRRK2 in the control of neurotransmitter release, we performed release experiments in nerve terminals isolated from WT and synapsin I KO mice upon inhibition of LRRK2 kinase activity. First we confirmed that phosphate incorporation in LRRK2 and synapsin I was indeed significantly reduced in the presence of PF-06447475 and IN-1, two inhibitors of LRRK2 kinase activity (Fig. 6a and b; LRRK2 32P incorporation: PF 0.15 µM 54.01 ± 2.26%, PF 1 µM 26.86 ± 3.34%, IN-1 1 µM 61.65 ± 3.18% IN-1 1 µM 21.99 ± 2.64%; p < 0.0001 vs. relative control one-way anova/Bonferroni’s tests. Synapsin I 32P incorporation: PF 0.15 µM 28.34 ± 3.26%, PF 1 µM 10.60 ± 1.61%, IN-1 1 µM 37.89 ± 5.55% IN-1 1 µM 11.53 ± 2.47%; p < 0.0001 vs. relative control one-way anova/Bonferroni’s tests). Next, we pre-labeled synaptosomes with [3H]D-Aspartate, a non-metabolizable marker of the glutamatergic transmission (Marte et al., 2010) and induced SV exocytosis via KCl stimulation (15 mM) in the presence or absence of LRRK2 inhibitors. Interestingly, we found that the inhibition of LRRK2 kinase activity caused a significant decrease in glutamatergic transmission in cortical (Fig. 6c; WT overflow Ctrl 2.17 ± 0.06, IN-1 1.28 ± 0.10, PF 1.40 ± 0.09; Syn I KO overflow Ctrl 1.34 ± 0.06, IN-1 1.32 ± 0.06, PF 1.17 ± 0.08. p < 0.0001 IN-1 and PF vs. relative Ctrl; two-way anova/Bonferroni’s tests) and striatal (Fig. 6d; WT overflow Ctrl 2.26 ± 0.11, IN-1 1.64 ± 0.15, PF 1.61 ± 0.11; Syn I KO overflow Ctrl 1.46 ± 0.09, IN-1 1.57 ± 0.12, PF 1.53 ± 0.16. p = 0.0015 IN-1, p = 0.0009 PF vs. relative Ctrl two-way anova/Bonferroni’s tests) synaptosomes prepared from WT mice. As already shown, glutamate release is reduced in synapsin I KO mice (Fassio et al., 2011); (p < 0.0001 anova/Bonferroni’s tests). Strikingly, the inhibition of LRRK2 activity by either drugs had virtually no effect in synaptosomes prepared from synapsin I KO mice (Fig. 6c and d; p > 0.9999 vs. relative Ctrl).
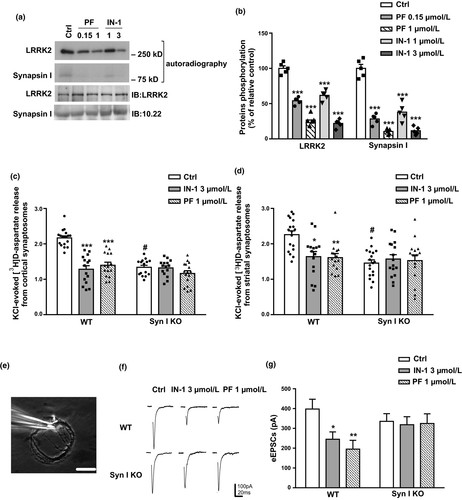
The effect of LRRK2 on the glutamatergic transmission, was also confirmed under physiological conditions using patch-clamp recordings in autaptic cortical neurons (Fig. 6e) maintained for 12–14 DIV. Neurons from WT and Syn I KO mice (E18) were treated with vehicle (Ctrl) or with the LRRK2 inhibitors IN-1 (3 µM) or PF-06447475 (1 µM) for 1 h (Fig. 6f). Notably, both LRRK2 inhibitors significantly reduced evoked excitatory post-synaptic currents (eEPSCs) in WT autaptic neurons (WT eEPSCs Ctrl 397.65 ± 49.67 pA, IN-1 245 ± 37.18 pA, PF 195.31 ± 44.38 pA; Syn I KO eEPSCs Ctrl 335.58 ± 38.86 pA, IN-1 318.69 ± 40.10 pA, PF 324.63 ± 48.98 pA. p = 0.0438 IN-1, p = 0.0060 PF vs. relative Ctrl two-way anova/Bonferroni’s tests), whereas this effect was virtually absent in Syn I KO neurons (Fig. 6g, p = 0.9586 IN-1, p = 0.9824 PF vs. relative Ctrl). All together, the data indicate that synapsin I is the final common pathway for the LRRK2-mediated the regulation of glutamatergic transmission.
Synapsin I phosphorylation on T337 and T339 has a key role in the functional impact of LRRK2 G2019S mutation
To further determine the role of LRRK2 kinase activity at the pre-synapse, we performed dynamic SV assays taking advantage of the Synaptophysin-pHluorin (sypHy) assay in primary cortical culture from WT and BAC hG2019S mice treated or not with LRRK2 kinase inhibitor GSK2578215A (GSK, 0.2 µM, 2 h), a brain penetrant, selective LRRK2 inhibitor (IC50 10 nM; Reith et al., 2012). Quantitative immunoblotting revealed that LRRK2 protein was expressed at similar levels in WT and BAC mice (Figure S1).
sypHy is a pH-sensitive fluorescent reporter that, by analogy with the original synaptopHluorin (synaptobrevin-pHluorin), is quenched in the acidic intracellular space of the SV and will only become fluorescent upon SV fusion, when the contents of the SV is exposed to the more basic pH of the extracellular space (Granseth et al., 2006). At the onset of the stimulus, exocytosis caused a rapid increase in sypHy fluorescence, which after cessation of the stimulus, slowly returned to baseline (Fig. 7a). The first stimulus, 40 AP, is predicted to mobilize SV belonging to the RRP, whereas 300 AP are sufficient to trigger the fusion of SV belonging to the total recycling pool. We measured a significant increase in SV fusion (increased fluorescence) in BAC hG2019S neurons compared to WT cells upon either 40 or 300 AP stimuli (ΔF40/F0 and ΔF300/F0, respectively, Fig. 7b and c. ΔF40/F0: WT 0.040 ± 0.007; WT GSK 0.013 ± 0.003; G2019S 0.066 ± 0.005; G2019S GSK 0.016 ± 0.004, p = 0.0039 WT vs. WT GSK, p < 0.0001 G2019S vs. G2019S GSK, p = 0.0004 G2019S vs. WT anova/Bonferroni’s tests. ΔF300/F0: WT 0.096 ± 0.017; WT GSK 0.037 ± 0.006; G2019S 0.165 ± 0.015; G2019S GSK 0.038 ± 0.007 p = 0.0232 WT vs. WT GSK, p < 0.0001 G2019S vs. G2019S GSK, p = 0.0009 G2019S vs. WT anova/Bonferroni’s tests). Interestingly, such increase was completely abolished in the presence of GSK. These data suggest that increased kinase activity augments SV fusion in BAC hG2019S mice. Synapsin I is a major molecular determinant of SV dynamics via direct binding to SV. Thus we wondered whether LRRK2 phosphorylation on synapsin I might alter synapsin I interaction with SV. To this aim, we expressed GFP-synapsin WT in DIV 4 cortical neurons prepared from WT or BAC hG2019S embryonic mice. At DIV 14 we processed the culture for imaging purposes and decorated the culture with an antibody against synaptophysin, a well-established SV marker. We noticed that GFP-synapsin I WT was distributed in synaptophysin positive cluster once expressed in WT neurons (Fig. 7d and e). Instead, GFP-synapsin I co-localization with synaptophysin was robustly reduced in BAC hG2019S. To appreciate the functional relevance of LRRK2-mediated phosphorylation on T337-339, we studied the intracellular distribution of GFP-synapsin I T337-339A. Interestingly, GFP-synapsin I T337-339A demonstrated a strong co-localization with synaptophysin in both WT and BAC hG2019S cultures. These data suggest that LRRK2 phosphorylation on T337-339 alters synapsin I subcellular localization. (% of co-localization with synaptophysin: GFP Synapsin I WT in WT 57 ± 4, GFP Synapsin I T337-339A in WT 50 ± 6, GFP Synapsin I WT in BAC hG2019S 15 ± 3, GFP Synapsin I T337-339A in BAC hG2019S 37 ± 5; p < 0.0001 GFP Synapsin I WT in BAC hG2019S vs. GFP Synapsin I WT in WT, p = 0.0077 GFP Synapsin I T337-339A in BAC hG2019S vs. GFP Synapsin I WT in BAC hG2019S).
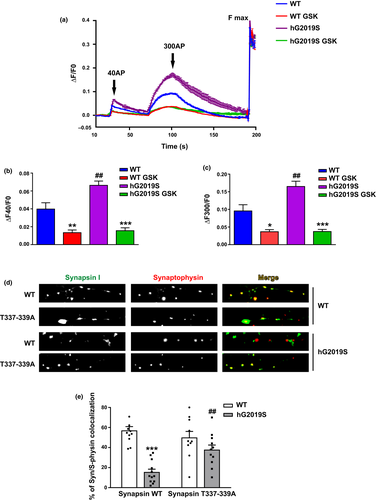
Discussion
Understanding the detailed molecular mechanisms by which LRRK2 affects neuronal function is an important goal in the physiology of synaptic transmission as well as in the pathogenesis of LRRK2-related diseases. We have previously demonstrated that LRRK2 is associated with SVs (Cirnaru et al., 2014) and is able to interact with several pre-synaptic proteins, namely synapsin I (Piccoli et al., 2014) and NSF (Belluzzi et al., 2016). Since both synapsin I and LRRK2 have been implicated in the regulation of neurotransmitter release (Cesca et al., 2010; Cirnaru et al., 2014), we investigated in detail the physical and functional interactions between LRRK2 and synapsin I. Importantly, we found that synapsin I is phosphorylated by LRRK2 both in vitro and in cultured cortical neurons.
In agreement with the consensus sequence predicted for LRRK2 kinase activity (Nichols et al., 2009; Martin et al., 2014), we identified threonine337 and threonine339, highly conserved residues located in the central domain C, as the major LRRK2 sites in synapsin I. The fact that, after double mutagenesis, synapsin I phosphorylation by LRRK2 is decreased but not abolished, suggests that these threonines are not the only LRRK2 phosphorylation sites in synapsin I. Furthermore, the observation that a reduction in the phosphorylation is observed only in the case of the double mutant indicates that the absence of either one of the two sites may be compensated by an increased phosphorylation stoichiometry on the other one. Moreover, as previously reported (Martin et al., 2014), we showed that the LRRK2 WD40 domain is crucial for both binding of LRRK2 to synapsin I and for synapsin I phosphorylation as the addition of this domain strongly inhibited synapsin I phosphorylation. The fact that the WD40 domain did not affect LRRK2 kinase activity suggests that it predominantly acts as a competitor for the interaction with synapsin I, without disrupting the formation of LRRK2 dimer.
The finding that synapsin I is phosphorylated by LRRK2 in the highly conserved C domain, prompted us to investigate whether phosphorylation modulated the molecular interactions of synapsin I in nerve terminals and had an effect on pre-synaptic function. In fact, the highly conserved C domain is a region of key importance in synapsin I function. It includes a major SV-binding site, with sequences that penetrate the phospholipid bilayer (mapped to synapsin I278-327; (Benfenati et al., 1989a, 1989b; Cheetham et al., 2001), a major actin-binding site (mapped to synapsin I223-360; (Bähler et al., 1989) as well as dimerization-tetramerization sequences (Esser et al., 1998; Brautigam et al., 2004) involved in synapsin oligomerization (Hosaka and Südhof, 1999) and a binding site for ATP (Esser et al., 1998; Orlando et al., 2014). The possibility that phosphorylation on the threonines identified affects the molecular interactions of synapsin I was addressed in complementary models taking advantage of independent assays. LRRK2 phosphorylation of synapsin I was found to decrease the association of synapsin I with SVs and actin filaments in vitro. Accordingly, we noticed that LRRK2-mediated phosphorylation on T337-339 affects synapsin I-SV colocalization in cortical neuron. Noteworthy, we reported a kinase-dependent increase in SV fusion in a murine model of G2019S mutation.
These effects are similar to the inhibitory effect on SV and actin binding reported for PKA/CaMKI, CaMKII, and MAPK/Erk phosphorylation of synapsin I (Schiebler et al., 1986; Benfenati et al., 1989a, 1992; Jovanovic et al., 1996; Hosaka et al., 1999) and opposite to those of tyrosine phosphorylation of synapsin I by c-Src (Messa et al., 2010). The loss of the modulation of synapsin I binding to SVs under conditions of inhibition of LRRK2 activity confirms that the effect is dependent on phosphorylation of synapsin I, and not a mere consequence of the LRRK2-synapsin I association.
Since the binding of synapsin I to SVs and actin filaments collectively participates in the formation of SV clusters and their anchorage to the actin cytoskeleton, LRRK2 phosphorylation of synapsin I might decrease the recruitment of SVs to the RP and favor the transition of SVs from the RP to the RRP. This hypothesis is consistent with the decrease in the depolarization-evoked glutamate release after inhibition of LRRK2 activity observed in wild-type synaptosomes and primary cortical neurons, but not in the synapsin I KO background (Cirnaru et al., 2014). Although the evidence that LRRK2 silencing increases SV motility and evoked EPSC amplitude after single-pulse stimulation seems to contradict our findings (Piccoli et al., 2011), it is crucial to underlie that in our experiments LRRK2 was catalytically inhibited, while preserving its role in the pre-synaptic protein interaction network. Moreover, the observation that LRRK2 activity inhibition is ineffective in the absence of synapsin I strongly supports the hypothesis that synapsin I plays a key role in the regulation of glutamate release as a downstream effector of LRRK2.
Glutamate mediates excitatory synaptic transmission, having a primary role in brain physiology (Fonnum, 1984). In particular, through glutamatergic projections, the cerebral cortex exerts an important control mechanism on the basal ganglia. It is known that excessive glutamatergic neurotransmission is involved in the pathogenesis of neurodegenerative diseases such as PD (Kim et al., 2011). Although PD is characterized by the progressive loss of dopaminergic neurons (Albin et al., 1995), data from the literature showed a parallel increase in the levels of extracellular glutamate (Robinson et al., 2003; Touchon et al., 2005; Meredith et al., 2009) and an increased expression of glutamate receptors in both experimental models of PD and PD patients (Weihmuller et al., 1992; Ulas et al., 1994; Sanchez-Pernaute et al., 2008). Furthermore, some reports showed a key role for LRRK2 in post-synaptic signaling (Sweet et al., 2015), whereas many others described alterations at the pre-synaptic level (Piccoli et al., 2011; Volta et al., 2015; Beccano-Kelly et al., 2015). In this regard, recent papers demonstrate an elevation in dopamine and glutamate neurotransmission in LRRK2 G2019S knock-in (KI) young, but not in adult mice (Volta et al., 2017) and a different sensibility to the pharmacological stimulation of dopamine D2 receptor in G2019S KI respect to WT mice (Tozzi et al., 2018). Moreover, in this paper no alteration of the glutamatergic transmission has been described in G2019S KI striatal slices.
This result, however, is only apparently in conflict with our data in striatal synaptosomes as it could be because of the different model employed as well as to the different technique used. In superfused synaptosomes we analyze only and exclusively the release of glutamate from the nerve terminals while in slices all the effect are the results of multiple factors acting in a complex circuitry.
All together, these observations suggest that the effects of LRRK2 and its PD-linked mutations on the regulation of neurotransmitter release are still controversial and request further investigations. Our data, in addition to the identification of synapsin I as a novel LRRK2 substrate, support a model in which LRRK2 can modulate SV availability and glutamate release in a kinase-dependent manner through phosphorylation of synapsin I. It is tempting to speculate that synapsin I represents a checkpoint that links LRRK2 kinase activity to the regulation of excitatory neurotransmission.
Acknowledgments and conflict of interest disclosure
We thank Paul Greengard (The Rockefeller University, New York, NY, USA) for the kind gift of the anti-synapsin I and anti-synaptophysin antibodies. This study was supported by the Michael J Fox Foundation (to GP, FO and EG). The support of Telethon-Italy (Grant no. GGP12237 to EG, FO, and GP) is also acknowledged. The authors declare that prof. Elisa Greggio is an editor for Journal of Neurochemistry.
Data availability statement
The datasets generated and analyzed during this study are available from the corresponding author on reasonable request.