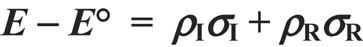Journal list menu
Export Citations
Download PDFs
Contents
Editorials
Research Articles
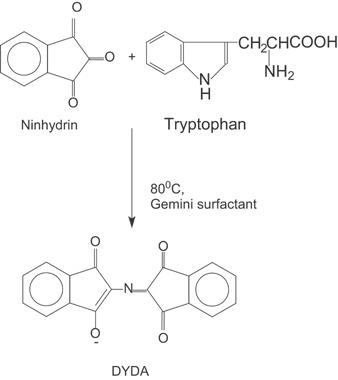
Role of cationic gemini surfactants toward enhanced ninhydrin–tryptophan reaction
- Pages: 440-447
- First Published: 09 May 2007
Cation sitting in aromatic cages: ab initio computational studies on tetramethylammonium–(benzene)n (n=3–4) complexes
- Pages: 448-453
- First Published: 17 April 2007
Our study on TMA-(benzene)n (n = 1–4) complexes revealed a cation could stably sit in an aromatic cage. The more the cage has aromatic monomers, the stronger the binding strength. In details, additivities of both the geometries and the binding energies were observed, providing an easy and accurate way to evaluate the binding between a cationic ligand and an aromatic cage in protein.
Revision of the dual substituent parameter treatment using the DFT-calculated reaction energies
- Pages: 454-462
- First Published: 29 April 2007
Computational studies of the cyclization of thiosemicarbazides
- Pages: 463-468
- First Published: 29 April 2007
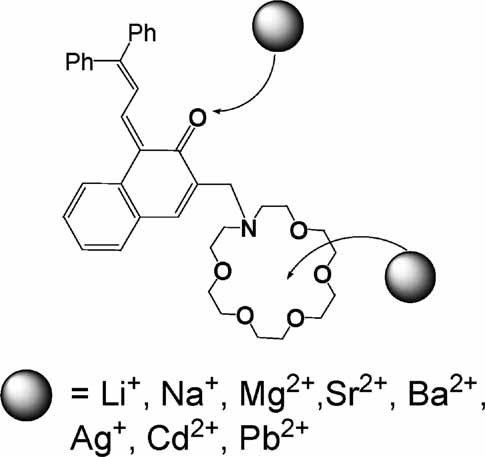
Investigation of cation complexation behavior of azacrown ether substituted benzochromene
- Pages: 469-483
- First Published: 29 April 2007
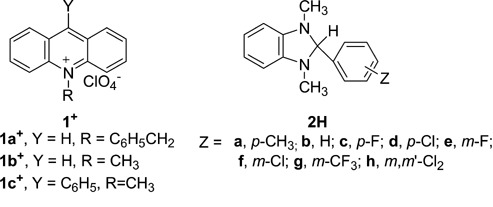
Reactivities of acridine compounds in hydride transfer reactions
- Pages: 484-490
- First Published: 09 May 2007
Energetic and structural characterization of 2-R-3-methylquinoxaline-1,4-dioxides (R = benzoyl or tert-butoxycarbonyl): experimental and computational studies
- Pages: 491-498
- First Published: 09 May 2007
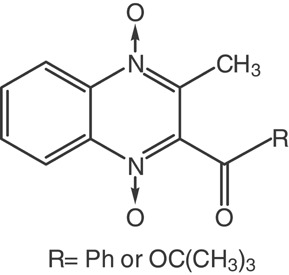
The strength of the NO bonds in two 2-R-3-methylquinoxaline-1,4-dioxides is presented. NO bond dissociation enthalpies are not associated to NO bond lengths. NO bond strength is correlated with the type of atoms in the substituent directly attacted to the quinoxaline ring but not by the group's stereoelectronic characteristics.
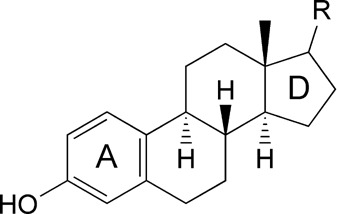
Inclusion complexes of estrone and estradiol with β-cyclodextrin: Voltammetric and HPLC studies
- Pages: 499-505
- First Published: 29 April 2007
Experimental evidence of chiral crown ether complexation with aromatic amino acids
- Pages: 506-513
- First Published: 29 April 2007
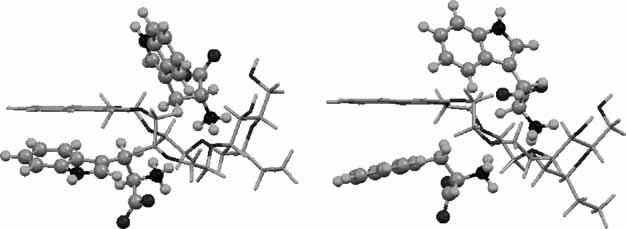
The complexation of L and D enantiomers of phenylglycine, phenylalanine and tryptophan with D-mannonaphto-crown-6-ether was studied by NMR and ITC in methanol solution at 298.15 K. The thermodynamic parameters, Ks, ΔG, ΔH°, and TΔS were determined with the aid of ITC. In contrast to aqueous medium, the chiral discrimination is observed.
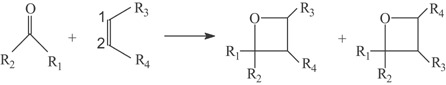
Minimum electrophilicity principle in photocycloaddition formation of oxetanes
- Pages: 514-524
- First Published: 09 May 2007
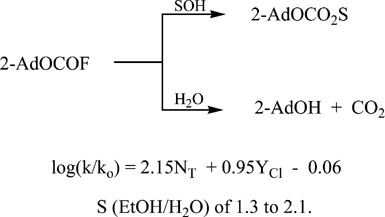
Rate and product studies with 2-adamantyl fluoroformate under solvolytic conditions
- Pages: 525-531
- First Published: 29 April 2007