Reactivities of acridine compounds in hydride transfer reactions
Abstract
Reactivities of acridine derivatives (10-benzylacridinium ion, 1a+, 10-methylacridinium ion, 1b+, and 10-methyl-9-phenylacridinium ion, 1c+) have been compared quantitatively for hydride transfer reactions with 1,3-dimethyl-2-substituted phenylbenzimidazoline compounds, 2Ha–h. Reactions were monitored spectrophotometrically in a solvent consisting of four parts of 2-propanol to one part of water by volume at 25 ± 0.1 °C. Reduction potentials have been estimated for acridine derivatives by assuming that the equilibrium constants for the reductions of 1a+–c+ by 2Hb would be the same in aqueous solution and accepting −361 mV as the reduction potential of the 1-benzyl-3-carbamoylpyridinium ion. The resulting reduction potentials, E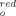 , are −47 mV for 1a+, −79 mV for 1b+, and −86 mV for 1c+. Each of acridine derivatives gives a linear Brønsted plot for hydride transfer reactions. The experimental slopes were compared with those obtained by Marcus theory. This comparison shows that the kinetic data are consistent with a one-step mechanism involving no high-energy intermediates. Copyright © 2007 John Wiley & Sons, Ltd.
, are −47 mV for 1a+, −79 mV for 1b+, and −86 mV for 1c+. Each of acridine derivatives gives a linear Brønsted plot for hydride transfer reactions. The experimental slopes were compared with those obtained by Marcus theory. This comparison shows that the kinetic data are consistent with a one-step mechanism involving no high-energy intermediates. Copyright © 2007 John Wiley & Sons, Ltd.




