Journal list menu
Export Citations
Download PDFs
Articles
Ectopic expression of the mitochondrial protein COXFA4L3 in human sperm acrosome and its potential application in the selection of male infertility treatments
- First Published: 29 October 2024
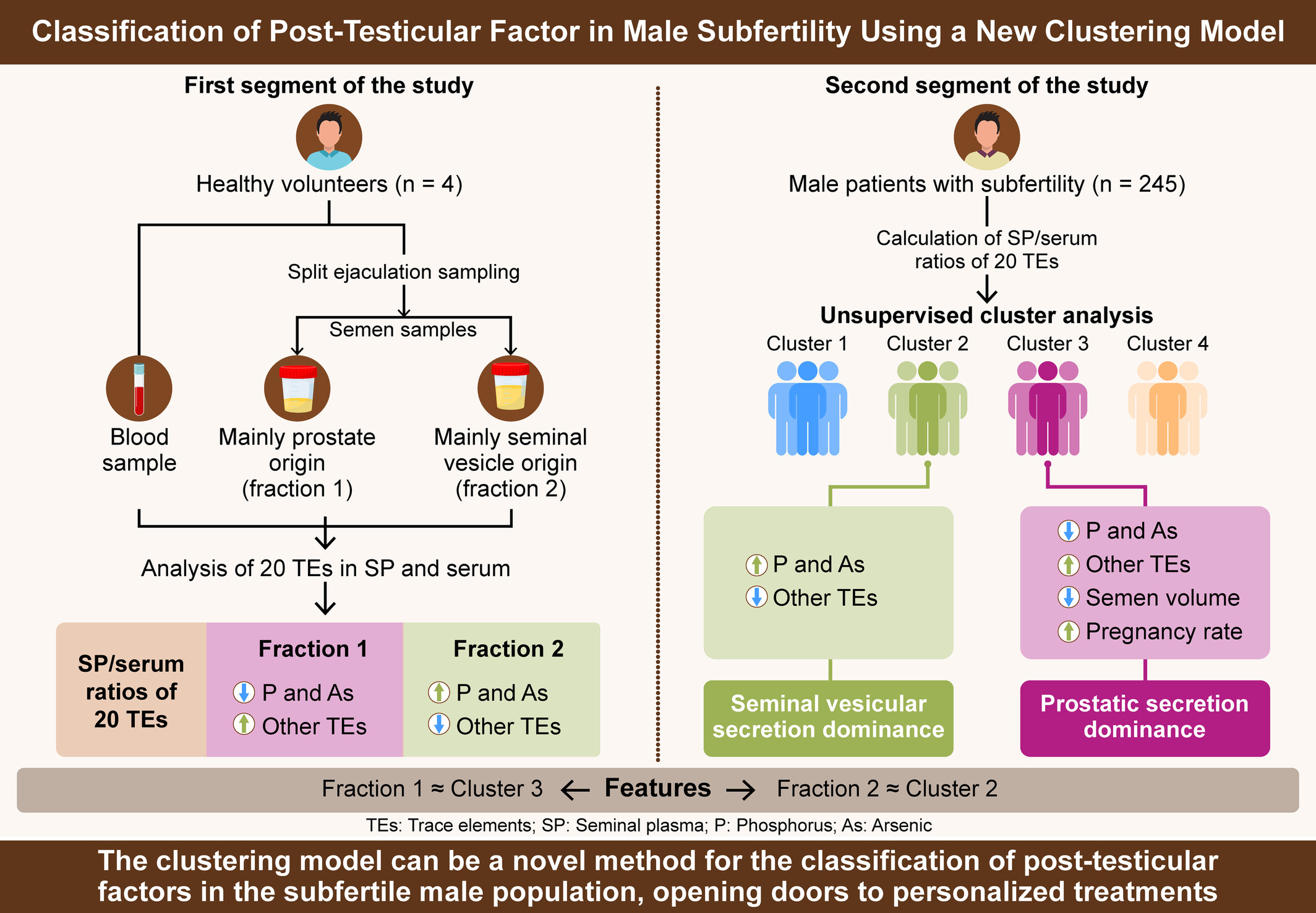
A new clustering model based on the seminal plasma/serum ratios of multiple trace element concentrations in male patients with subfertility
- First Published: 28 May 2024
Differences in clinical outcomes between men with mosaic Klinefelter syndrome and those with non-mosaic Klinefelter syndrome
- First Published: 16 May 2024
Micromapping testicular sperm extraction: A new technique for microscopic testicular sperm extraction in nonobstructive azoospermia
- First Published: 11 March 2024
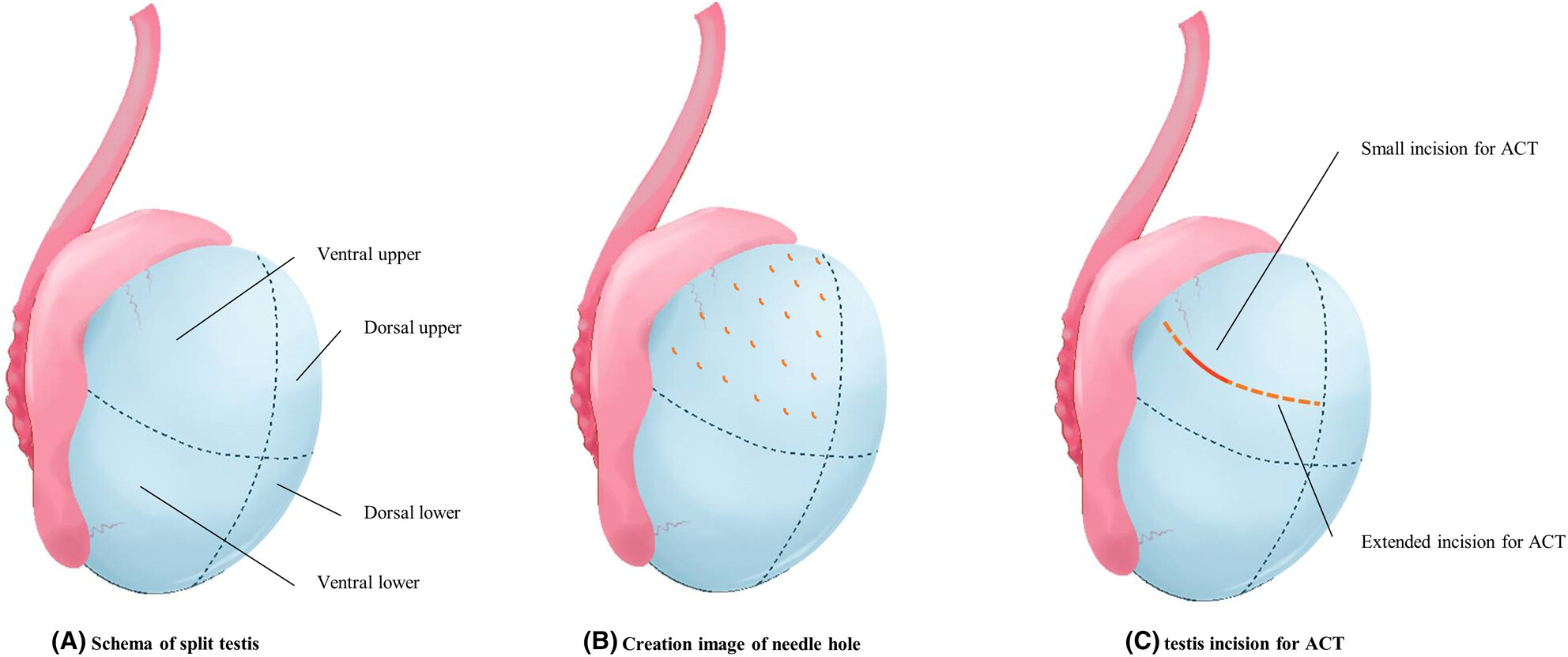
We have developed a novel sperm retrieval technique for nonobstructive azoospermia, termed micromapping testicular extraction (MMTE). MMTE retrieves testicular tissue through multiple small holes made in the albuginea with a needle and searches for sperm. Sperm retrieval rates were comparable to those of micro-TESE. MMTE produced good ICSI results with reduced complications.
Cryptozoospermia: Should we use ejaculated sperm or surgically retrieved sperm for assisted reproductive technology?
- First Published: 26 October 2023
Expression of RSPH6A in the first wave of rat spermatogenesis and oxidative stress conditions: Attenuation by melatonin
- First Published: 02 October 2023
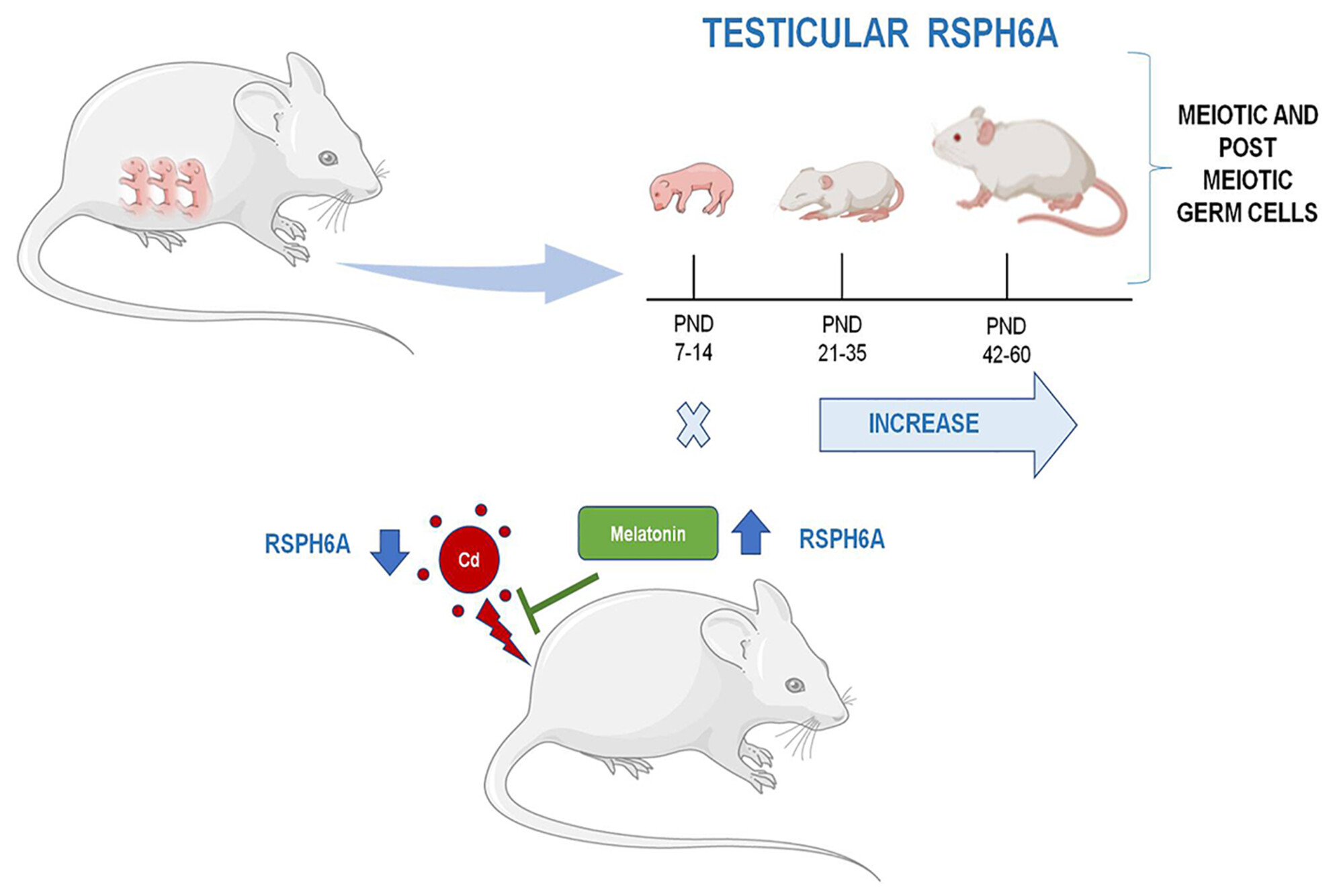
Here is reported, for the first time, the temporal expression and localization of RSPH6A protein during the first wave of rat spermatogenesis. Its expression starts at 21 PND alongside the appearance of ISPC and increases up to 60 PND. The expression and localization of RSPH6A in the testis and epididymal spermatozoa of adult rats treated with cadmium were impaired. Melatonin, given together with Cd, can counteract its damaging effects.
TSNAXIP1 is required for sperm head formation and male fertility
- First Published: 28 June 2023
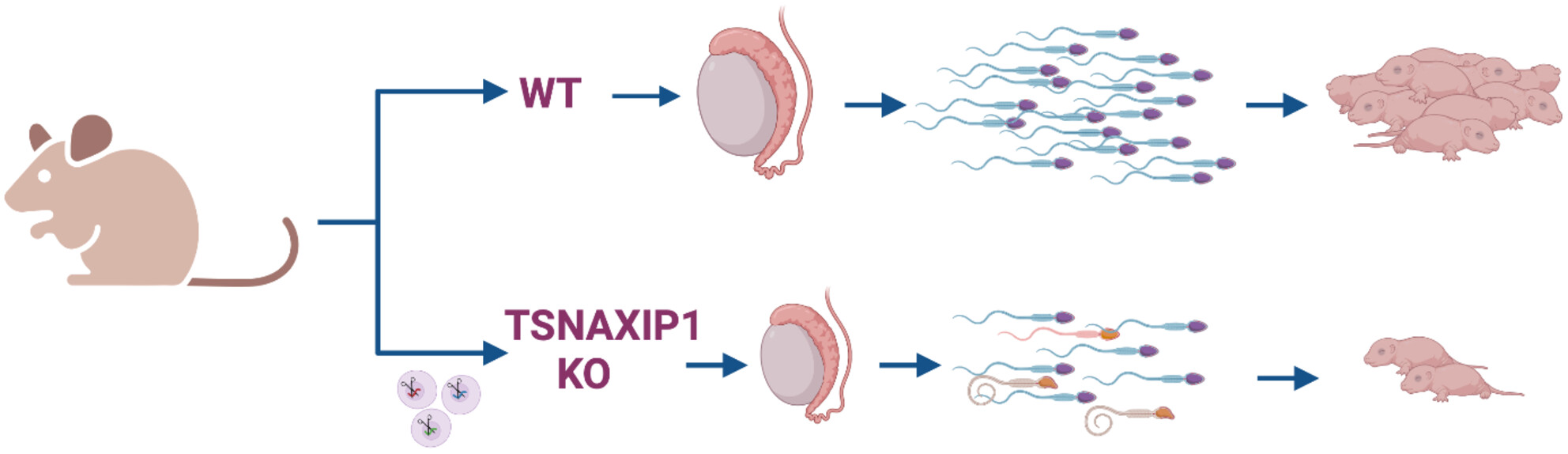
When a testis-expressed gene TSNAXIP1 was disrupted, TSNAXIP1 null male showed sub-fertility and oligospermia. TSNAXIP1 could be essential for the sperm head formation and male fertility, because malformation of sperm head and abnormal connection between sperm head and tail were detected by the lack of TSNAXIP1. Since TSNAXIP1 is highly conserved between mouse and human, TSNAXIP1 might be a causative gene of human infertility.
Prominin-1 deletion results in spermatogenic impairment, sperm morphological defects, and infertility in mice
- First Published: 06 June 2023
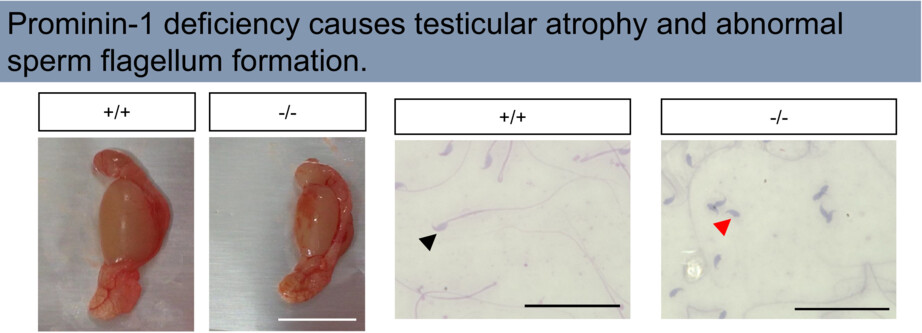
This study reveals a role of Prominin-1 (PROM1) in spermatogenesis. Prominin-1 is a stem cell marker that promotes cell proliferation, migration, and inhibition of apoptosis. PROM1 KO mice showed testicular atrophy and abnormal sperm flagella. Prominin-1 deletion may cause impaired spermatogenesis and flagellum formation.
Evaluation of the efficacy of creatine chemical exchange saturation transfer imaging in assessing testicular maturity
- First Published: 23 February 2023
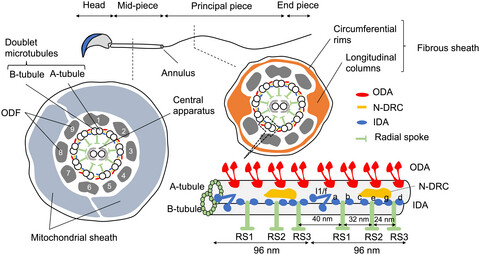
Molecular basis of the morphogenesis of sperm head and tail in mice
- First Published: 23 May 2022
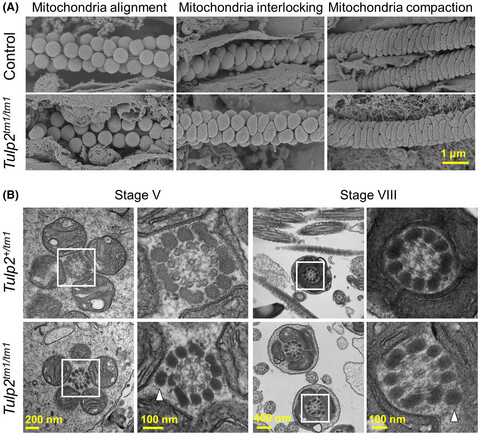
TULP2 deletion mice exhibit abnormal outer dense fiber structure and male infertility
- First Published: 23 May 2022
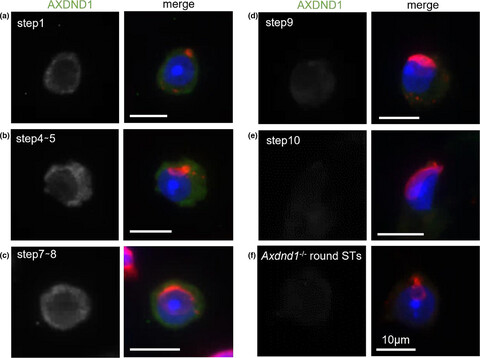
Loss of Axdnd1 causes sterility due to impaired spermatid differentiation in mice
- First Published: 30 March 2022