Micromapping testicular sperm extraction: A new technique for microscopic testicular sperm extraction in nonobstructive azoospermia
Abstract
Purpose
In microscopic testicular sperm extraction (mTESE) for nonobstructive azoospermia (NOA), sperm can be recovered relatively easily in some cases, and mTESE may be retrospectively considered excessive. However, mTESE is routinely performed in the majority of NOA patients because of the difficulty in predicting tissue status. A minimally invasive and comprehensive sperm retrieval method that allows on-the-spot tissue assessment is needed. We have developed and evaluated a novel sperm retrieval technique for NOA called micromapping testicular sperm extraction (MMTSE).
Methods
MMTSE involves dividing the testis into four sections and making multiple small needle holes in the tunica albuginea to extract seminiferous tubules and retrieve sperm. The sperm-positive group by MMTSE (Group I) underwent additional tissue collection (ATC) via a small incision, whereas the sperm-negative group by MMTSE (Group 0) underwent mTESE.
Results
In total, 40 NOA participants underwent MMTSE. Group I included 15 patients and Group 0 included 25 patients. In Group 1, sperm were recovered from all patients by ATC. In Group 0, sperm were recovered in 4 of 25 cases using mTESE.
Conclusions
MMTSE shows promise as a simple method that comprehensively searches testicular tissue and retrieves sperm using an appropriate method while minimizing patient burden.
1 INTRODUCTION
Nonobstructive azoospermia (NOA) is experienced by approximately 1% of all men and 10%–15% of men diagnosed with infertility. It poses a significant challenge for infertile couples.1 However, with the advent of Intracytoplasmic Sperm Injection (ICSI), it is possible to achieve biological fatherhood, provided that sperm can be retrieved from azoospermic patients.2 Various sperm retrieval methods have been developed, but the overall sperm retrieval rate (SRR) remains around 30%–50% and does not vary significantly among methods.
Conventional Testicular Sperm Extraction (cTESE), introduced by Devroey et al. in 1995, became the fundamental technique for sperm retrieval.3 Since then, several methods have been developed, with Microdissection Testicular Sperm Extraction (mTESE), becoming the standard surgical procedure for NOA sperm retrieval.4, 5 Many reports indicate that mTESE has a higher SRR than cTESE; however, to date, there have been no randomized clinical studies comparing mTESE and cTESE, and the superiority of mTESE in terms of sperm retrieval capacity is still debated.6-8
While mTESE searches the testis extensively to increase the chances of finding sperm, it also carries an increased risk of postoperative complications. Moreover, other methods, such as Fine Needle Aspiration Mapping (FNAP) and Open Testicular Mapping (OTEM), each have their own advantages and disadvantages.9, 10
As a sperm search method, we believe that it is necessary to collect sufficient tissue in a short operation while confirming sperm collection with embryologists. This is because it is important to comprehensively explore the inside of the testis in order to collect higher-quality sperm from testes with high sperm counts and to prevent sperm loss from testes with low sperm counts. Surgery that only needs to be performed once is better for the patient. However, mTESE does not eliminate the risk of complications, such as low testosterone.
A review comparing mTESE and cTESE in cases of NOA found that mTESE SRRs ranged from 42.9% to 63%, whereas those of cTESE were 16.7–45.0%. Thus, in some cases, mTESE with a large incision may not be necessary, even with a preoperative diagnosis of NOA.6 However, if localized sperm collection is performed with only a small incision, it is not possible to know whether the best sperm have been collected.
Conversely, sperm have been found using FNAP, even when they could not be retrieved with mTESE. Jarvis et al.11 found that while sperm recovery with FNAP did not differ by testis location, post-mTESE analysis revealed better sperm recovery near the albuginea, compared to the center of the testis. This result suggests that mTESE may not be able to collect tissues near the albuginea. FNAP has the same sperm collection performance as mTESE, and it is thought to compensate for weaknesses of mTESE. However, EAU guidelines also state that “FNAP cannot be recommended as a primary therapeutic intervention in men with NOA until further RCTs.” Although FNAP is also less invasive, it requires two surgeries to retrieve sperm, which is undesirable for the patient.
In OTAM, a hole is made in the albuginea to collect testicular tissue to search for sperm. If sperm are present, additional tissue is collected and the procedure is concluded, but if there are no sperm, this process is repeated. OTAM is minimally invasive, but sperm quality cannot be assessed, because sperm are collected from the first possible location. Also, if no sperm are found, it is likely that the surgery will take longer.
If the presence, quality, and location of sperm are known before surgery, additional incisions and damage to the testis can be reduced. There are reports suggesting that the SRR may vary depending on factors such as follicle-stimulating hormone (FSH) levels, but there is currently no reliable method for predicting the presence or abundance of sperm.12 The histological appearance of testicular tissue has been proposed as a prognosticator of sperm presence; however, this requires testis dissection for accurate determination.13, 14 New techniques, based on FNAP, adopt the premise that not all cases of NOA require extensive incisions, as in mTESE.
From a retrospective perspective, for patients in whom sperm are relatively easy to find with mTESE, a minimally invasive testicular search that covers a certain area is appropriate at first, even if only a small amount of tissue can be removed. On the other hand, patients with only a small number of sperm in a small area of the testis require as extensive and thorough a sperm search as possible. Furthermore, the surgeon must make every effort to avoid postoperative complications in all cases. Unfortunately, these predictions cannot be made preoperatively, so mTESE is uniformly performed on all NOA patients, and tissue status is determined intraoperatively. In view of existing methods, a minimally invasive and comprehensive sperm retrieval method that allows tissue evaluation on the spot is needed. To achieve this objective, we developed a new sperm collection technique for NOA called MMTSE, which has great potential to meet these requirements. MMTSE can be performed without making a large incision in the testicle, potentially reducing unnecessary large testicular incisions. In addition, areas where tissue is collected with MMTSE include areas that are difficult to reach with mTESE like FNAP, so by combining mTESE and MMTSE, it may be possible to search the testis over a wide area and with minimal invasiveness.
2 MATERIALS AND METHODS
2.1 Patient population
From January 2022 to January 2023, 40 NOA males deemed suitable for mTESE were included in the study, with an average age of 36.2 ± 4.89 years. Underlying causes of NOA in the study population were as follows: idiopathic NOA (22 cases), gr/gr deletion (10 cases), Klinefelter syndrome (2 cases), chromosomal translocation 46,XY,t(11;13)(q23;q12) (1 case), b3b4 deletion (1 case), b2b4 deletion (2 case), AZF indeterminate (1 case), postchemotherapy (2 cases), and spinal injury (1 case). Both 47XXY cases belonged to the gr/gr deletion category as well. The gr/gr deletion is a deletion unique to Japanese people. It is found in approximately 30% of Japanese men and is said to have no effect on SRR. It is generally regarded as equivalent to idiopathic NOA.15, 16
In one case, a testicular tumor was discovered during the initial visit, and sperm retrieval was subsequently performed on the unaffected side following inguinal orchiectomy. Mean testis size was 9.68 ± 2.69 cc for the right testis and 9.55 ± 2.64 cc for the left testis, with no significant lateral difference observed.
All subjects were Japanese. Seven individuals presented with grade 3 left varicocele, 5 patients had grade 2 varicocele on the left side, and 1 had bilateral varicocele. Varicocele surgery was performed in 5 patients with grade 3 varicocele and in the patient with bilateral varicocele. MMTSE was conducted 6 months after varicocele surgery.
Preoperative hormone levels were FSH, 20.4 mIU/mL (2.50–46.2); LH, 9.75 mIU/mL (4.00–38.6); PRL, 12.1 ng/mL (6.1–43.1); E2: 22.9 pg/mL (4.20–49.6); and total testosterone (T–T), 3.91 ng/mL (0.50–13.51). Huang et al. reported that using a combination of FSH >9.2 mIU/mL and right testis size <15 mL, the positive predictive value of NOA was 99.2% and 81.8% for OA.17 In our study, patients with FSH < 9.2 mIU/mL and right testicular size >15 mL were considered OA patients and were excluded. For those who met only one of the conditions, only those without findings predicting OA, such as vasectomy, epididymitis, induration of the epididymis, or vas deferens defects, were included. The rest were NOA and were treated as candidates for MMTSE. There were 3 cases in which only one of the conditions was met. One case was idiopathic NOA with normal testis size, but high FSH (16.5 mIU/mL), and two cases had low FSH levels, but testicular size was around 10 mL. One of these was idiopathic NOA and one was a spinal cord injury patient.
2.2 Surgical method
All surgeries were performed under local anesthesia by the same surgeon. Anesthesia was administered using a mixture of 10 mL of 1% lidocaine and 20 mL of 0.75% ropivacaine. A unilateral testicular block was performed using 5–7 cc of the anesthetic mixture. The incision site was also anesthetized with 5 cc of this solution. The testis was then exposed through a median scrotal incision. Additional anesthesia was administered as needed to manage any pain experienced during the procedure. Once both testes were exposed, MMTSE was performed.
Cases were divided into two groups based on MMTSE results. Group I included cases with sperm recovered by MMTSE and Group 0 included cases without sperm.
Details will be provided hereafter, but after obtaining MMTSE results, additional tissue collection (ATC) was performed in group I, whereas mTESE was performed in group 0.
2.3 The MMTSE method
2.3.1 Collection of testicular tissue
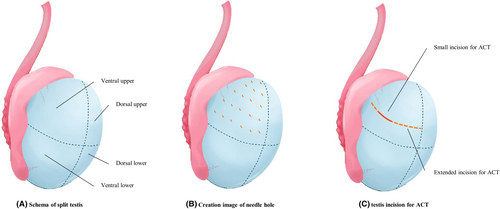
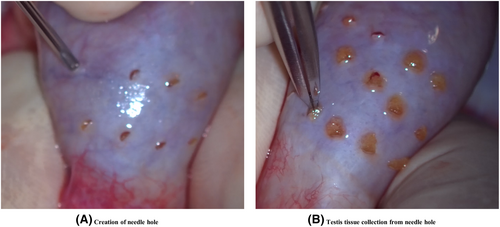
The testis, excluding the epididymis, was divided into four parts: the ventral upper, ventral lower, dorsal upper, and l dorsal lower (Figure 1A). Multiple needle holes were created evenly along the tunica albuginea using an 18-G needle, avoiding areas with prominent blood vessels so as to minimize bleeding (Figures 1B and 2A). The density of needle holes was determined based on the testis surface area. Two-needle holes were made per square centimeter, considering that the epididymis portion accounted for 20% of the total surface area (Table 1).
| Testis volume (cc) | Surface area (cm2) | Surface area 20% removal (cm2) | Total number of punctures | Number of punctures in each site |
|---|---|---|---|---|
| 3 | 10.3 | 8.2 | 16 | 4 |
| 4 | 12.4 | 10.0 | 20 | 5 |
| 5 | 14.4 | 11.6 | 23 | 6 |
| 6 | 16.3 | 13.0 | 26 | 7 |
| 7 | 18.1 | 14.5 | 29 | 7 |
| 8 | 19.8 | 15.8 | 32 | 8 |
| 9 | 21.4 | 17.1 | 34 | 9 |
| 10 | 22.9 | 18.3 | 37 | 9 |
| 11 | 24.4 | 19.5 | 39 | 10 |
| 12 | 25.9 | 20.7 | 41 | 10 |
| 13 | 27.3 | 21.8 | 44 | 11 |
| 14 | 28.7 | 23.0 | 46 | 11 |
| 15 | 30.0 | 24.0 | 48 | 12 |
| 16 | 31.4 | 25.1 | 50 | 13 |
| 17 | 32.7 | 26.1 | 52 | 13 |
| 18 | 33.9 | 27.1 | 54 | 14 |
| 19 | 35.2 | 28.1 | 56 | 14 |
| 20 | 36.4 | 29.1 | 58 | 15 |
- Abbreviation: MMTE, micromapping testicular extraction.
Seminiferous tubules were then pulled out through all needle holes using a micro-claw under a microscope (Figure 2B). Tubule thickness and condition were checked. Tissues collected by MMTSE were grouped by site and submitted to an embryologist as small pieces of testicular tissue for each site. Therefore, specimens from four locations were searched in each testicle. Tissues were immediately minced, and sperm retrieval was performed. After submitting testis tissues from one site, MMTSE tissue collection continued without interruption from the other locations.
No suturing of the needle hole was required. There were several holes that bled, but even if they did, the bleeding stopped on its own within a few minutes. When testicular tissue was collected through the needle hole, most of the tissue was recovered without any remaining tissue, and any tissue that protruded from the needle hole was removed with scissors and added to the collected tissue. No tissue escaped from the needle hole after collection.
2.3.2 Testicular tissue processing and sperm retrieval
All tissue samples were analyzed simultaneously for sperm retrieval (4 samples in unilateral cases and 8 for bilateral cases) in the laboratory next to the operating room by an embryologist, after which sperm retrieval results were reviewed. The search time was up to 5 min for each sample. If no sperm were found, all remaining samples were then searched.
The amount of tissue required for MMTSE is very small, and even if sperm are found, the amount of tissue is insufficient for frozen tissue stock or for multiple ICSI. Therefore, we have to collect more tissue. For Group 1, if MMTSE showed sperm, ATC was performed. If no sperm were found (Group 0), mTESE was used for further searches.
Tissue processing and retrieval employed our usual methods for mTESE.18 Collected testicular samples were placed in 1.5-mL microtubes containing 0.4 mL of culture medium. Samples were then minced using ophthalmologic scissors. After mincing, the tissue suspension was placed in a dish, covered with mineral oil, and analyzed under an inverted microscope at 400× magnification for the presence, morphological quality, and motility of spermatozoa after each collection. Intraoperative microscopic evaluation included assessing the presence or absence of cells, forward motile sperm, tail vibrating sperm, immotile sperm, spermatocytes, and spermatogonia.
2.3.3 ATC for Group I
When sperm were detected by MMTSE, a small transverse incision was made at a site with favorable conditions, such as better sperm motility and morphology. ATC was performed under a microscope while checking the condition of seminiferous tubules, and thicker, healthier tubules were selected. The location and extent of the additional incision were determined based on the condition and density of sperm. In the case of ATC, a 5-mm incision is standard, but if there are few seminiferous tubules in good condition or if the density of sperm in the tissue is low, the incision may be extended to about 20 mm (Figure 1C). If sufficient sperm were obtained through ATC, cryopreservation was performed, and the operation was concluded.
2.3.4 mTESE for Group 0
If no sperm were found during MMTSE, mTESE was performed, as usual, using a lateral incision.
2.4 Statistical evaluation
All statistical analyses were carried out with R, version 4.3.1. Results were compared between groups using the Mann–Whitney test and p < 0.05 was considered significant.
3 RESULTS
3.1 Sperm retrieval
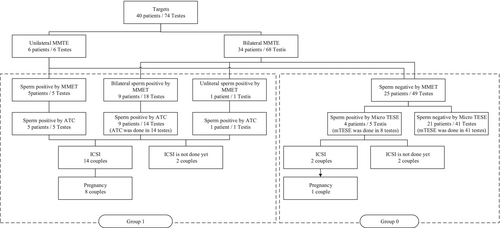
Six patients underwent unilateral MMTSE, whereas 34 patients underwent bilateral MMTSE. In one unilateral case, a testicular tumor was discovered during the first visit and unilateral orchiectomy was given priority. Two cases exhibited severe unilateral atrophy, so only the healthy testis was used. Additionally, at the request of two patients, only one side was treated. In Group I, sperm were found in five testes in five cases in the unilateral group and 19 testes in 10 cases in the bilateral group. ATC was performed, and in all cases, tissues that underwent multiple rounds of ICSI were successfully frozen. In Group 0, mTESE was conducted in the remaining 25 cases, resulting in sperm retrieval in five testes from 4 of these 25 cases. A sample flow chart is shown in Figure 3. The overall final SRR per patient was 47.5% (19/40). In the end, sperm were recovered from 29 testes by mTESE or ATC, and sperm were confirmed by MMTSE in 24 of them. (82.8%) Results of MMTSE, ATC, and mTESE by patient characteristics are shown in Table 2.
| Case number | MMTE | ATC | mTESE | |
|---|---|---|---|---|
| Idiopathic NOA | 22 | 9/22 | 9/9 | 0/13 |
| gr/gr deletion (pure) | 8 | 2/8 | 2/2 | 0/6 |
| 47XXY | 2 | 1/2 | 1/1 | 1/1 |
| b2b4 deletion | 2 | 0/2 | – | 1/2 |
| b2b3 deletion | 1 | 1/1 | 1/1 | – |
| 46,XY,t(11;13)(q23;q12) | 1 | 1/1 | 1/1 | – |
| AZF indeterminate | 1 | 0/1 | – | 0/1 |
| Postchemotherapy | 2 | 0/2 | – | 2/2 |
| Spinal injury | 1 | 1/1 | 1/1 | – |
| Total | 40 | 15/40 | 15/15 | 4/25 |
- Abbreviations: ATC, additional tissue collection; MMTE, micromapping testicular extraction; mTESE, microscopic testicular sperm extraction; NOA, nonobstructive azoospermia.
3.2 ICSI results
ICSI was performed in 14 cases, with the partner's average age being 34.5 ± 4.1 years. Overall patient outcomes were as follows: a 59.2% (100/169) fertilization rate, 48.0% (48/100) blastocyst rate, and 64.3% (9/14) pregnancy rate per transfer. In Group I, the fertilization rate was 59.7% (92/154), the blastocyst rate was 45.6% (42/92), and the pregnancy rate per transfer was 80% (8/10). In Group 0, the fertilization rate was 53.3% (8/15), the blastocyst rate was 75% (6/8), and the pregnancy rate per transfer was 25% (1/4). Statistical comparisons were difficult due to the small number of cases.
3.3 Hormone levels
Before surgery, T–T level was measured, and 1 month after surgery, it was measured again. In Group 1, T–T levels were 3.90 ng/mL (2.66–9.00) before surgery and 3.80 ng/mL (2.00–8.40) afterward. In Group 0, levels were 3.46 ng/mL (0.5–9.04) before surgery and 2.93 ng/mL (0.4–9.91) afterward. We conducted a Mann–Whitney U Test on the results, and the T–T level showed a statistically significant decrease in the MMTSE sperm-negative group (p < 0.05; Table 3).
| All cases | Preoperation | Postoperation 1M |
|---|---|---|
| FSH (mIU/mL) | 20.0 (2.5–46.2) | 25.6 (8.2–64.0)* |
| LH (mIU/mL) | 9.6 (2.1–38.6) | 14.9 (6.2–96.7)* |
| E2 (pg/mL) | 12.1 (6.1–43.1) | 21.7 (5.0–47.3)* |
| Total testosterone (ng/mL) | 3.96 (5.00–9.04) | 3.25 (0.4–9.91)* |
| Group 1 | ||
| FSH (mIU/mL) | 17.4 (2.5–46.2) | 16.8 (8.2–48.5) |
| LH (mIU/mL) | 9.4 (2.09–38.6) | 14.0 (6.2–57.6)* |
| E2 (pg/mL) | 26.1 (5.0–40.9) | 22.2 (5.0–33.5) |
| Total testosterone (ng/mL) | 4.06 (2.66–9.00) | 3.80 (1.00–8.40) |
| Group 0 | ||
| FSH (mIU/mL) | 20.5 (6.3–45.9) | 29.2 (8.7–64.0)* |
| LH (mIU/mL) | 9.7 (4.0–18.7) | 16.6 (6.8–96.7)* |
| E2 (pg/mL) | 22.2 (5.0–47.6) | 20.1 (5.0–47.3)* |
| Total testosterone (ng/mL) | 3.92 (0.50–9.00) | 2.93 (0.40–9.91)* |
- Abbreviation: FSH, follicle-stimulating hormone.
- * p < 0.05.
3.4 Necessary time
Total operation time was 77.3 ± 20.3 min, and the time from the start of MMTSE puncture to confirmation of sperm was 25.6 ± 7.55 min. Comparing Group I and Group 0, operating time was 57.0 ± 15.4 min vs. 82.9 ± 15.4 min, respectively. The time required for MMTSE was 27.3 ± 7.53 min vs. 19.4 ± 3.51 min. Unsurprisingly, operating time was significantly shorter in Group I (p < 0.05).
3.5 Collected tissue amounts
The amount of tissue harvested with MMTSE was 15.52 ± 6.52 mg per site and 62.41 ± 20.56 mg per testis. The amount of tissue collected by ATC when sperm were detected by MMTSE was 159.50 ± 58.11 mg, while the amount of tissue collected during mTESE without sperm retrieval was 224.63 ± 93.93 mg. The amount of collected tissue was significantly less with ATC (p < 0.05).
3.6 Variation in sperm distribution
In Group I, in the same testis, five testes had both positive and negative sites for sperm, using MMTSE. Seven testes displayed variations in sperm motility, and 12 showed no differences.
3.7 Degeneration of testicular tissue
Among the 40 cases, 7 involved 14 testes in which collection from the needle hole was challenging due to degeneration of testicular tissue, but there were no cases in which testicular tissue could not be collected at all. Among these cases, three testes from two patients, including one case of Klinefelter syndrome, had sperm detected with MMTSE, and two cases required mTESE to achieve sperm retrieval.
4 DISCUSSION
4.1 Current sperm retrieval method
NOA is a common condition in male infertility, often with an unidentifiable cause.19 Various methods have been explored to predict sperm retrieval in these cases, but there is currently no reliable method except AZF deletion.20, 21 Multiple sperm retrieval techniques based on mTESE have been reported, but a definitive method has yet to be established. The success of sperm recovery and the quality of testicular tissue are critical factors for achieving successful pregnancies.22
mTESE, the current standard method for sperm collection, involves opening the testis, separating the tissue, and selectively collecting seminiferous tubules while observing them. Sperm retrieval is performed during surgery by an accompanying embryologist. Even if sperm are found, the surgeon may continue the search to obtain better-quality tubules. mTESE requires only one operation and can be completed relatively quickly. Additionally, an adequate amount of tissue can be collected for freezing and later use in ICSI. However, in some reports, it is a more invasive procedure than cTESE, as it requires a larger testicular incision and carries a higher risk of complications due to reduced testicular function.23, 24
FNAP involves systematically acquiring tissue samples from the testis in three dimensions. Sperm are collected through percutaneous needle punctures and aspiration, and collected specimens are stained and subjected to cytological examination. Obtaining results from FNAP can take 1–2 h at a minimum. Since the tissue is fixed, sperm movement cannot be assessed. If sperm are found, an additional extraction is performed using cTESE or mTESE, which may require two surgeries.9 FNAP carries the risk of blind puncture and potential damage to the epididymis, blood vessels, and testicular hematoma.
OTEM, introduced by Vieira et al.,10 has had a significant impact on our practices. This technique involves making a hole in the albuginea of the exposed testis using a 19-G needle without a microscope. Tissue is collected and sperm retrieval is attempted. If sperm are found, a 5-mm incision is made to collect additional tissue. If sufficient sperm are obtained, the operation is concluded. Otherwise, additional holes are made, and a maximum of six samples are collected from each region of the testis (upper, middle, and lower). This multiple sampling prolongs the operation, with up to 36 sampling attempts, if sperm cannot be found.
4.2 What is required of new methods?
- A minimally invasive approach. It is preferable to collect sperm with the smallest incision.25
- High-quality sperm: While finding sperm is the primary goal, obtaining sperm of the highest quality is also important.26, 27
- Simplicity and brevity: The procedure should be straightforward, completed in one operation, and feasible under local anesthesia.
- Sufficient tissue volume: It is crucial to obtain an adequate amount of tissue to be used in multiple ICSI cycles through freezing.
4.3 Regarding our method
4.3.1 Time and simplicity of surgery
Ideally, MMTSE should allow evaluation of testicular histology through separate punctures and collection of sperm from the best site. However, due to limitations of local anesthesia and the need to complete MMTSE, additional sampling, or mTESE within 3 h, certain considerations must be observed. In practice, mTESE is often performed with multiple specimens submitted as a batch. In our study, we divided specimens into four groups per testis to simplify the tissue search process. Each tissue sample was minced and searched within 5 min. The average time required for MMTSE was 25.6 min, and the average operation time was 77.3 min, providing sufficient time for the procedure to be completed under local anesthesia.
In this study, we performed this surgery on patients with various conditions in order to examine tissue conditions for which this surgical technique is applicable. Fortunately, no case in this study failed to collect testis tissue by MMTSE. Although NOA testicular tissue is fragile, it can usually be collected by MMTSE. Nonetheless, in some cases, collecting tissue through a needle hole can be challenging, because of testicular tissue degeneration. Of the seven difficult cases, two had successful sperm retrieval with MMTSE, and two cases required mTESE to achieve sperm retrieval. One of the cases that was sperm-positive by MMTSE involved Klinefelter's syndrome.
4.3.2 For sperm-positive cases
For cases in which sperm can be collected, the goal is to obtain the highest-quality sperm with minimal damage. T–T level deficiency caused by post-mTESE atrophy can result in various unpleasant symptoms, and long-term hormone replacement therapy can inconvenience patients.28 Even in cases in which cTESE successfully retrieves sperm, it does not necessarily yield the best tissue sample from that patient. To address this problem, in MMTSE, tissues are collected from multiple needle holes. In cases in which sperm were successfully retrieved with MMTSE, ATC requires a 5–20 mm incision, in the area where the best sperm were found, and seminiferous tubules are collected under a microscope. The size of the incision depends on the abundance of sperm found during MMTSE. In cases with numerous sperm, a smaller incision was made, and cTESE was used for sperm retrieval. Conversely, when sperm density was low, selective harvesting of seminiferous tubules was performed through a lateral incision up to 2 cm between the equatorial line and the apex of the testis. Although this incision is more damaging to the testis than cTESE, it limits the search to a smaller area of the testis (1/4 of one side of a testicle) and potentially preserves more healthy seminiferous tubules, reducing the risk of complications, compared to mTESE. This time, in order to standardize the surgical technique, an incision was made in the center of the area with the best tissue (Figure 1). However, this surgery is performed under a surgical microscope, and the condition of the seminiferous tubules, such as their thickness and color, can be confirmed. Future studies are required, such as sampling from better locations based on visual differences.
In this study, 15 of 19 cases in which sperm could be recovered avoided mTESE; thus, our objective to recover viable sperm from NOA patients with minimally invasive incisions, and without having to resort to mTESE, has been largely achieved.
4.3.3 For cases with no sperm
When sperm cannot be obtained, considerations regarding where and how long to continue searching become crucial. mTESE, which opens the testis and allows for observation of seminiferous tubules, confirms the absence of sperm. Some reports suggest widening the search by making three-dimensional incisions in the testis to cover a larger area.29 However, while dissecting the testis with a larger incision and many divisions will increase the search area, it will cause more damage to the testis. Nonetheless, cases have been reported in which sperm were found through FNAP, despite unsuccessful retrieval using mTESE.11 This study showed that while sperm recovery with initial FNAP did not differ by location in the testis, post-mTESE analysis revealed better sperm recovery near the albuginea, compared to the center of the testis. In mTESE, the search starts from the incision site, making it easier to search the center of the testis, but technically challenging to approach the area directly under the albuginea. Consequently, insufficient searching in this region may make it easier to find sperm directly under the albuginea, where tissue damage is minimal and normal tissue tends to remain. Our MMTSE technique involves making a needle hole in the tunica albuginea, allowing for direct searching under the albuginea. However, collecting tissue from the center of the testis becomes more challenging. If sperm are not found, the procedure is switched to mTESE. In our study, mTESE successfully retrieved sperm in four cases in which MMTSE was initially negative. This suggests that the location of sperm retention differs from patient to patient, and even when sperm are absent near the tunica albuginea, they may be present in the center of the testis. In mTESE after MMTSE, thorough searching of the testicle center becomes necessary; however, searching just below the albuginea, as in normal mTESE may not be required. Further research is needed to investigate this question. Nevertheless, if damage to the testis in mTESE can be minimized, postoperative complications can potentially be reduced.
4.3.4 Challenge of MMTSE
cTESE is said to have a lower SRR than mTESE, because only a small amount of tissue is collected from one location. Furthermore, mTESE shows clear differences in the state of seminiferous tubules between tissues with and without sperm, even in the same testis. However, as mentioned above, it was reported that this initial FNA mapping showed no differences in the presence of sperm by site. Unfortunately, we did not find any papers showing a bias regarding the presence of sperm in the testes.
From these facts, even if there is no difference in the density of seminiferous tubules depending on the location in the NOA testis where sperm exist, it is necessary to collect seminiferous tubules from a fairly wide range in order to capture seminiferous tubules where sperm exist. For idiopathic NOA and gr/gr deletions, in all cases in which sperm could be retrieved, sperm were detected by MMTSE, and mTESE was avoided. It is thought that these cases have a certain number of good seminiferous tubules. Based on this result, MMTSE may be suitable for cases of idiopathic NOA or gr/gr deletion. However, for patients requiring mTESE in whom no sperm are found during MMTSE, the damage may be greater, although slightly, compared to patients who undergo mTESE only. Therefore, current methods need to be improved. While maintaining the sperm detection rate, it is necessary to minimize the number of holes, determine the search area, and consider the timing of transition to mTESE and the area of testes in which to perform mTESE.
In this study, there were no cases in which sperm were detected by MMTSE, but not by subsequent ATC or mTESE. This means that we have never been able to collect a small number of seminiferous tubules containing sperm by MMTSE yet. However, we believe that with more experience, these results will be useful in determining the suitability of MMTSE, based on the types of cases and tissue conditions.
Our results show that mTESE was required for sperm retrieval, especially in patients with nonidiopathic 47XXY, b2b4 deletion, chromosomal abnormalities, and postchemotherapy. They had low sperm density, and this meant that the amount of tissue randomly collected in MMTSE could not reach good seminiferous tubules. Sperm was detected by MMTSE in one case of 47XXY, but that case had relatively normal seminiferous tubules. Therefore, in nonidiopathic cases, indications and methods of MMTSE need to be considered more carefully.
4.3.5 Limitations
This study was not a randomized or prospective study. It cannot definitively claim that MMTSE is superior to other methods. Sperm retrieval surgery is basically a one-time operation, and for this reason, it would be extremely difficult to conduct prospective studies. To date, no clinical randomized study comparing mTESE in NOA and cTESE has been performed. However, pseudorandomized prospective data indicate that sperm retrieval with NOA is more advantageous in mTESE, especially in histological patterns of patchy spermatogenesis, such as Sertoli cell-only syndrome. In the future, we will consider comparing these methods.
Additionally, due to the limited number of patients with successful sperm retrieval, conclusions regarding the quality of collected sperm cannot be drawn. However, we believe that MMTSE is a valuable, new, minimally invasive method that reduces patient burden and yields higher-quality sperm.
5 CONCLUSIONS
We report MMTSE as a new sperm retrieval method. MMTSE enables brief, minimally invasive, comprehensive searches of testes with NOA, thereby replacing conventional methods.
ACKNOWLEDGMENTS
The authors thank all team members at Kyono ART Clinic for their daily clinical work. I would like to thank my wife, Dr Akiko Tai, for helping me sreate the illustrations for this paper and for her daily support.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest relative to this article.
ETHICS STATEMENT
This study was approved by the Ladies Clinic Kyono Ethics Committee. Ethics committee approval number: 5511-220 924.
HUMAN RIGHTS STATEMENTS AND INFORMED CONSENT
All procedures followed were in accordance with ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and its later amendments. Informed consent was obtained from all patients included in the study.
ANIMAL STUDIES
No experiments with animal subjects were performed by any of the authors in connection with this study.




