A Three-Phase Indirect Electrolysis System for Kilo-scale Generation of C–S Bond Products
Gang Liu
Shandong Provincial Key Laboratory of Chemical Energy Storage and Novel Cell Technology, School of Chemistry & Chemical Engineering, Liaocheng University, Liaocheng, Shandong, 252059 China
Search for more papers by this authorCorresponding Author
Xianqiang Huang
Shandong Provincial Key Laboratory of Chemical Energy Storage and Novel Cell Technology, School of Chemistry & Chemical Engineering, Liaocheng University, Liaocheng, Shandong, 252059 China
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorYingjie Li
Shandong Provincial Key Laboratory of Chemical Energy Storage and Novel Cell Technology, School of Chemistry & Chemical Engineering, Liaocheng University, Liaocheng, Shandong, 252059 China
Search for more papers by this authorShiqi Fu
Shandong Provincial Key Laboratory of Chemical Energy Storage and Novel Cell Technology, School of Chemistry & Chemical Engineering, Liaocheng University, Liaocheng, Shandong, 252059 China
Search for more papers by this authorGuodong Shen
Shandong Provincial Key Laboratory of Chemical Energy Storage and Novel Cell Technology, School of Chemistry & Chemical Engineering, Liaocheng University, Liaocheng, Shandong, 252059 China
Search for more papers by this authorZhen Li
Shandong Provincial Key Laboratory of Chemical Energy Storage and Novel Cell Technology, School of Chemistry & Chemical Engineering, Liaocheng University, Liaocheng, Shandong, 252059 China
Search for more papers by this authorYalin Zhang
Shandong Provincial Key Laboratory of Chemical Energy Storage and Novel Cell Technology, School of Chemistry & Chemical Engineering, Liaocheng University, Liaocheng, Shandong, 252059 China
Search for more papers by this authorQingde Zhang
State Key Laboratory of Coal Conversion, Institute of Coal Chemistry, Chinese Academy of Sciences, Taiyuan, Shanxi, 030001 China
Search for more papers by this authorCorresponding Author
Fei Yu
Jiangsu Collaborative Innovation Centre of Biomedical Functional Materials, Jiangsu Key Laboratory of New Power Batteries, School of Chemistry and Materials Science, Nanjing Normal University, Nanjing, Jiangsu, 210023 China
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Yifa Chen
Guangdong Provincial Key Laboratory of Carbon Dioxide Resource Utilization, School of Chemistry, South China Normal University, Guangzhou, Guangdong, 510006 China
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorGang Liu
Shandong Provincial Key Laboratory of Chemical Energy Storage and Novel Cell Technology, School of Chemistry & Chemical Engineering, Liaocheng University, Liaocheng, Shandong, 252059 China
Search for more papers by this authorCorresponding Author
Xianqiang Huang
Shandong Provincial Key Laboratory of Chemical Energy Storage and Novel Cell Technology, School of Chemistry & Chemical Engineering, Liaocheng University, Liaocheng, Shandong, 252059 China
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorYingjie Li
Shandong Provincial Key Laboratory of Chemical Energy Storage and Novel Cell Technology, School of Chemistry & Chemical Engineering, Liaocheng University, Liaocheng, Shandong, 252059 China
Search for more papers by this authorShiqi Fu
Shandong Provincial Key Laboratory of Chemical Energy Storage and Novel Cell Technology, School of Chemistry & Chemical Engineering, Liaocheng University, Liaocheng, Shandong, 252059 China
Search for more papers by this authorGuodong Shen
Shandong Provincial Key Laboratory of Chemical Energy Storage and Novel Cell Technology, School of Chemistry & Chemical Engineering, Liaocheng University, Liaocheng, Shandong, 252059 China
Search for more papers by this authorZhen Li
Shandong Provincial Key Laboratory of Chemical Energy Storage and Novel Cell Technology, School of Chemistry & Chemical Engineering, Liaocheng University, Liaocheng, Shandong, 252059 China
Search for more papers by this authorYalin Zhang
Shandong Provincial Key Laboratory of Chemical Energy Storage and Novel Cell Technology, School of Chemistry & Chemical Engineering, Liaocheng University, Liaocheng, Shandong, 252059 China
Search for more papers by this authorQingde Zhang
State Key Laboratory of Coal Conversion, Institute of Coal Chemistry, Chinese Academy of Sciences, Taiyuan, Shanxi, 030001 China
Search for more papers by this authorCorresponding Author
Fei Yu
Jiangsu Collaborative Innovation Centre of Biomedical Functional Materials, Jiangsu Key Laboratory of New Power Batteries, School of Chemistry and Materials Science, Nanjing Normal University, Nanjing, Jiangsu, 210023 China
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Yifa Chen
Guangdong Provincial Key Laboratory of Carbon Dioxide Resource Utilization, School of Chemistry, South China Normal University, Guangzhou, Guangdong, 510006 China
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorComprehensive Summary
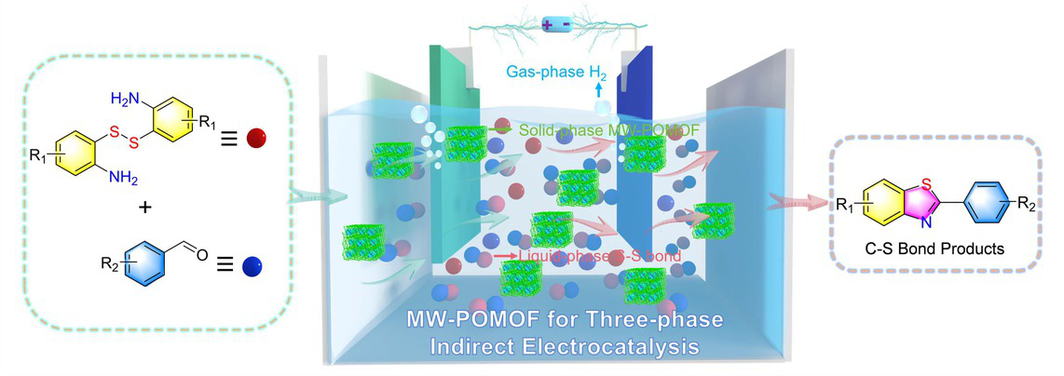
The development of new strategy for environmentally friendly, cost-effective and large-scale electro-synthesis of anticancer drugs is highly desirable to replace high-cost traditional methods and realize high atomic economy. GW 610, an antitumor agent with potent and selective anticancer activity against lung, colon, and breast cancer cell lines in real medical treatment processes, has a market price of ~107 USD/kg and calls for novel methods like electro-synthesis to reduce the cost. Here, for the first time, we design a solid-liquid-gas three-phase indirect electrolysis system based on a kind of microwave-synthesized polyoxometalate-based metal-organic framework (MW-POMOF) that can converse S–S bond substrates into valuable C–S bond products like anticancer drug molecules (e.g., GW 610). Specifically, the solid-phase MW-POMOF as heterogeneous redox mediator exhibits the excellent electrocatalytic efficiency for the formation of liquid-phase C–S bond products (yields up to 95%) coupling with the generation of gas-phase H2 product (~402 μmol·g–1·h–1), resulting in an interesting three-phase indirect electrolysis system. Remarkably, it enables the kilo-scale production (~1 kg in a batch experiment) of GW 610 at one thousandth of the market price (from ~107 to ~3200 USD/kg). This work may inaugurate a new electrocatalytic avenue to explore porous crystalline materials in electrocatalysis field.
Supporting Information
| Filename | Description |
|---|---|
| cjoc202500103-sup-0001-supinfo.pdfPDF document, 4.3 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1 Zhang, S.; Huang, W.-C.; Li, P.; Guo, H.; Poh, S.-B.; Brady, S. W.; Xiong, Y.; Tseng, L.-M.; Li, S.-H.; Ding, Z.; Sahin, A. A.; Esteva, F. J.; Hortobagyi, G. N.; Yu, D. Combating trastuzumab resistance by targeting SRC, a common node downstream of multiple resistance pathways. Nat. Med. 2011, 17, 461–469.
- 2 Theodoropoulos, P. C.; Gonzales, S. S.; Winterton, S. E.; Rodriguez- Navas, C.; McKnight, J. S.; Morlock, L. K.; Hanson, J. M.; Cross, B.; Owen, A. E.; Duan, Y.; Moreno, J. R.; Lemoff, A.; Mirzaei, H.; Posner, B. A.; Williams, N. S.; Ready, J. M.; Nijhawan, D. Discovery of tumor-specific irreversible inhibitors of stearoyl CoA desaturase. Nat. Chem. Biol. 2016, 12, 218–225.
- 3
Yarlagadda, B.; Devunuri, N.; Mandava, V. B. R. Facile synthesis of N-(Benzyl-1H-1,2,3-Triazol-5-yl) (Methyl)-4-(6-Methoxybenzo [d] Thiazol-2-yl)-2-nitrobenzamides via click chemistry. J. Heterocycl. Chem. 2016, 54, 864–870.
10.1002/jhet.2647 Google Scholar
- 4 Vitaku, E.; Smith, D. T.; Njardarson, J. T. Analysis of the structural diversity, substitution patterns, and frequency of nitrogen heterocycles among U.S. FDA approved pharmaceuticals. J. Med. Chem. 2014, 57, 10257–10274.
- 5 Ogawa, Y.; Nonaka, Y.; Goto, T.; Ohnishi, E.; Hiramatsu, T.; Kii, I.; Yoshida, M.; Ikura, T.; Onogi, H.; Shibuya, H.; Hosoya, T.; Ito, N.; Hagiwara, M. Development of a novel selective inhibitor of the Down syndrome-related kinase Dyrk1A. Nat. Commun. 2010, 1, 86.
- 6 Aiello, S.; Wells, G.; Stone, E. L.; Kadri, H.; Bazzi, R.; Bell, D. R.; Stevens, M. F. G.; Matthews, C. S.; Bradshaw, T. D.; Westwell, A. D. Synthesis and biological properties of benzothiazole, benzoxazole, and chromen-4-one analogues of the potent antitumor agent 2-(3,4-Dimethoxyphenyl)-5-fluorobenzothiazole. J. Med. Chem. 2008, 51, 5135–5139.
- 7 Tan, B. S.; Tiong, K. H.; Muruhadas, A.; Randhawa, N.; Choo, H. L.; Bradshaw, T. D.; Stevens, M. F. G.; Leong, C.-O. CYP2S1 and CYP2W1 mediate 2-(3,4-Dimethoxyphenyl)-5-fluorobenzothiazole (GW-610, NSC 721648) sensitivity in breast and colorectal cancer cells. Mol. Cancer. Ther. 2011, 10, 1982–1992.
- 8 Zhang, G.; Liu, C.; Yi, H.; Meng, Q.; Bian, C.; Chen, H.; Jian, J.-X.; Wu, L.-Z.; Lei, A. External oxidant-Free oxidative cross-coupling: A photoredox cobalt-catalyzed aromatic C-H thiolation for constructing C-S bonds. J. Am. Chem. Soc. 2015, 137, 9273–9280.
- 9 Ceylan, M.; Erkan, S.; Yaglioglu, A. S.; Akdogan Uremis, N.; Koç, E. Antiproliferative evaluation of some 2-[2-(2-Phenylethenyl)-cyclopent-3-en-1-yl]-1,3-benzothiazoles: DFT and molecular docking study. Chem. Biodivers. 2020, 17, e1900675.
- 10 Wang, L.; Niu, M.; He, Y.; Tian, C.; Peng, Z.; Jia, J. Synthesis and evaluation of Al18F-NODA complex conjugated 2-(4-aminophenyl)benzothiazole as a potential tumor imaging agent. Bioorg. Med. Chem. Lett. 2020, 30, 127160.
- 11 Wang, K.; Guengerich, F. P. Bioactivation of fluorinated 2-Aryl-benzothiazole antitumor molecules by human cytochrome P450s 1A1 and 2W1 and deactivation by cytochrome P450 2S1. Chem. Res. Toxicol. 2012, 25, 1740–1751.
- 12 Ma, D.; Xie, S.; Xue, P.; Zhang, X.; Dong, J.; Jiang, Y. Efficient and economical access to substituted benzothiazoles: Copper-catalyzed coupling of 2-haloanilides with metal sulfides and subsequent condensation. Angew. Chem. Int. Ed. 2009, 48, 4222–4225.
- 13 Inamoto, K.; Hasegawa, C.; Hiroya, K.; Doi, T. Palladium-catalyzed synthesis of 2-substituted benzothiazoles via a C-H functionalization/ Intramolecular C-S bond formation process. Org. Lett. 2008, 10, 5147–5150.
- 14 Wang, X.; Ye, W.; Kong, T.; Wang, C.; Ni, C.; Hu, J. Divergent S- and C-Difluoromethylation of 2-Substituted Benzothiazoles. Org. Lett. 2021, 23, 8554–8558.
- 15 Li, Z.; Zhou, C.; Ye, R.; Meng, L.-G. Catalyst-free tandem reaction of 2,2’-diaminodiphenyldisulfides, sulfinic acids and aromatic aldehydes: an approach to synthesize unsymmetric thiosulfonates and benzothiazoles. Green Chem. 2022, 24, 3845–3849.
- 16 Xu, L.; Huang, Z.; Yang, M.; Wu, J.; Chen, W.; Wu, Y.; Pan, Y.; Lu, Y.; Zou, Y.; Wang, S. Salting-out aldehyde from the electrooxidation of alcohols with 100 % selectivity. Angew. Chem. Int. Ed. 2022, 61, e202210123.
- 17 Bai, J.; Li, X.; Zhu, Z.; Zheng, Y.; Hong, W. Single-molecule electrochemical transistors. Adv. Mater. 2021, 33, 202005883.
- 18 Qian, X.-Y.; Li, S.-Q.; Song, J.; Xu, H.-C. TEMPO-catalyzed electrochemical C-H thiolation: Synthesis of benzothiazoles and thiazolopyridines from thioamides. ACS Catal. 2017, 7, 2730–2734.
- 19 Elsherbini, M.; Wirth, T. Electroorganic synthesis under flow conditions. Acc. Chem. Res. 2019, 52, 3287–3296.
- 20 Capaldo, L.; Quadri, L. L.; Merli, D.; Ravelli, D. Photoelectrochemical cross-dehydrogenative coupling of benzothiazoles with strong aliphatic C-H bonds. Chem. Commun. 2021, 57, 4424–4427.
- 21 Folgueiras-Amador, A. A.; Qian, X. Y.; Xu, H. C.; Wirth, T. Catalyst- and supporting-electrolyte-free electrosynthesis of benzothiazoles and thiazolopyridines in continuous flow. Chem.- Eur. J. 2017, 24, 487–491.
- 22 Novaes, L. F. T.; Liu, J.; Shen, Y.; Lu, L.; Meinhardt, J. M.; Lin, S. Electrocatalysis as an enabling technology for organic synthesis. Chem. Soc. Rev. 2021, 50, 7941–8002.
- 23 Liu, G.; Chen, Y.; Chen, Y.; Shi, Y.; Zhang, M.; Shen, G.; Qi, P.; Li, J.; Ma, D.; Yu, F.; Huang, X. Indirect electrocatalysis S-N/S-S bond construction by robust polyoxometalate based foams. Adv. Mater. 2023, 35, 202304716.
- 24 Arndt, S.; Weis, D.; Donsbach, K.; Waldvogel, S. R. The ‘Green’ electrochemical synthesis of periodate. Angew. Chem. Int. Ed. 2020, 59, 8036–8041.
- 25 Chakraborty, P.; Mandal, R.; Garg, N.; Sundararaju, B. Recent advances in transition metal-catalyzed asymmetric electrocatalysis. Coord. Chem. Rev. 2021, 444, 214065.
- 26 Gafurov, Z. N.; Kantyukov, A. O.; Kagilev, A. A.; Sinyashin, O. G.; Yakhvarov, D. G. Electrochemical methods for synthesis and in situ generation of organometallic compounds. Coord. Chem. Rev. 2021, 442, 213986.
- 27 Yamamoto, K.; Kuriyama, M.; Onomura, O. Anodic oxidation for the stereoselective synthesis of heterocycles. Acc. Chem. Res. 2019, 53, 105–120.
- 28 Nyman, M.; Burns, P. C. A comprehensive comparison of transition-metal and actinyl polyoxometalates. Chem. Soc. Rev. 2012, 41, 7354.
- 29 Wang, X.; Chang, Z.; Lin, H.; Tian, A.; Liu, G.; Zhang, J. Assembly and photocatalysis of two novel 3D Anderson-type polyoxometalate- based metal-organic frameworks constructed from isomeric bis(pyridylformyl)piperazine ligands. Dalton Trans. 2014, 43, 12272–12278.
- 30 Zhong, B.; Liu, J.; Liu, G.; Zhang, Z.; Chen, Y.; Wang, X. pH-directed polyoxometalate-based supramolecular framework for effectively electrochemical sensing IO3- from glycerol oxidation wastewater. J. Mol. Struct. 2025, 1332, 141679.
- 31 Lu, K.; Liebman Peláez, A.; Wu, L.-c.; Cao, Y.; Zhu, C.-h.; Fu, H. Ionothermal synthesis of five keggin-type polyoxometalate-based metal–organic frameworks. Inorg. Chem. 2019, 58, 1794–1805.
- 32 Coyle, L.; Middleton, P. S.; Murphy, C. J.; Clegg, W.; Harrington, R. W.; Errington, R. J. Protonolysis of [(iPrO)TiMo5O18]3−: access to a family of TiMo5Lindqvist type polyoxometalates. Dalton Trans. 2012, 41, 971–981.
- 33 Huang, X.; Liu, S.; Liu, G.; Tao, Y.; Wang, C.; Zhang, Y.; Li, Z.; Wang, H.; Zhou, Z.; Shen, G.; Xue, Z.; Sun, D. An unprecedented 2-fold interpenetrated open framework built from Zn6 ring seamed trivacant polyoxotungstates used for photocatalytic synthesis of pyridine derivatives. Appl. Catal., B 2023, 323, 122134.
- 34 Horn, M. R.; Singh, A.; Alomari, S.; Goberna-Ferrón, S.; Benages-Vilau, R.; Chodankar, N.; Motta, N.; Ostrikov, K.; MacLeod, J.; Sonar, P.; Gomez-Romero, P.; Dubal, D. Polyoxometalates (POMs): from electroactive clusters to energy materials. Energy Environ. Sci. 2021, 14, 1652–1700.
- 35 Guan, W.; Wang, G.; Li, B.; Wu, L. Organic macrocycle-polyoxometalate hybrids. Coord. Chem. Rev. 2023, 481, 215039.
- 36 Tsunashima, R.; Long, D.-L.; Endo, T.; Noro, S.-i.; Akutagawa, T.; Nakamura, T.; Cabrera, R. Q.; McMillan, P. F.; Kögerler, P.; Cronin, L. Exploring the thermochromism of sulfite-embedded polyoxometalate capsules. Phys. Chem. Chem. Phys. 2011, 13, 7295.
- 37 Li, Z.; Zhang, J.; Jing, X.; Dong, J.; Liu, H.; Lv, H.; Chi, Y.; Hu, C. A polyoxometalate@covalent triazine framework as a robust electrocatalyst for selective benzyl alcohol oxidation coupled with hydrogen production. J. Mater. Chem. A 2021, 9, 6152–6159.
- 38 Dong, C.; Liu, X.; Li, Z.; Guo, K.; Song, C.; Zhang, Y.; Zhang, M.; Ma, F.; Rosei, F.; Zhang, X.; Huang, X. Boosting Electrocatalytic Performance of Sulfide Oxidation on Polyoxomolybdates with Synergistic Effects of CNT-Doped Aerogel Foams. Adv. Funct. Mater. 2025, 35, 2418410.
- 39 Li, Z.; Liu, C.; Geng, W.; Dong, J.; Chi, Y.; Hu, C. Electrocatalytic ethylbenzene valorization using a polyoxometalate@covalent triazine framework with water as the oxygen source. Chem. Commun. 2021, 57, 7430–7433.
- 40 Zhen, N.; Dong, J.; Lin, Z.; Lu, W.; Li, J.; Chi, Y.; Hu, C. A Rhombus-Like Tetrameric Vanadoniobate Containing Pseudo-Sandwich-Type {Li ⊂ V2O8(Nb5O14)2} and Its Electrocatalytic Activity for the Selective Oxidation of Benzyl Alcohol. Inorg. Chem. 2023, 62, 13824–13831.
- 41 Zhu, L.; Huo, A.; Chen, Y.; Bai, X.; Cao, C.; Zheng, Y.; Guo, W. A ROS reservoir based on a polyoxometalate and metal-organic framework hybrid for efficient bacteria eradication and wound healing. Biochem. Eng. J. 2023, 476, 146613.
- 42 Li, X.-X.; Zhao, D.; Zheng, S.-T. Recent advances in POM-organic frameworks and POM-organic polyhedra. Coord. Chem. Rev. 2019, 397, 220–240.
- 43 Thomas-Hillman, I.; Laybourn, A.; Dodds, C.; Kingman, S. W. Realising the environmental benefits of metal-organic frameworks: recent advances in microwave synthesis. J. Mater. Chem. A 2018, 6, 11564–11581.
- 44 Lu, M.; Zhang, M.; Liu, J.; Yu, T.-Y.; Chang, J.-N.; Shang, L.-J.; Li, S.-L.; Lan, Y.-Q. Confining and highly dispersing single polyoxometalate clusters in covalent organic frameworks by covalent Linkages for CO2 photoreduction. J. Am. Chem. Soc. 2022, 144, 1861–1871.
- 45 Sun, C.-Y.; Liu, S.-X.; Liang, D.-D.; Shao, K.-Z.; Ren, Y.-H.; Su, Z.-M. Highly stable crystalline catalysts based on a microporous metal- organic framework and polyoxometalates. J. Am. Chem. Soc. 2009, 131, 1883–18889
- 46 Luo, X.; Li, F.; Peng, F.; Huang, L.; Lang, X.; Shi, M. Strategies for perfect confinement of POM@MOF and Its applications in producing defect-rich electrocatalyst. ACS Appl. Mater. Interfaces 2021, 13, 57803–57813.
- 47 Liu, H.; Gong, L.-G.; Wang, C.-X.; Wang, C.-M.; Yu, K.; Zhou, B.-B. {Cu2SiW12O40}@HKUST-1 synthesized by a one-step solution method with efficient bifunctional activity for supercapacitors and the oxygen evolution reaction. J. Mater. Chem. A 2021, 9, 13161–13169.
- 48 Liu, Y.; Liu, S.; He, D.; Li, N.; Ji, Y.; Zheng, Z.; Luo, F.; Liu, S.; Shi, Z.; Hu, C. Crystal Facets Make a Profound Difference in Polyoxometalate- Containing Metal-Organic Frameworks as Catalysts for Biodiesel Production. J. Am. Chem. Soc. 2015, 137, 12697–12703.
- 49 Zhang, Z.; Liu, Y.; Tian, H.; Ma, X.; Yue, Q.; Sun, Z.; Lu, Y.; Liu, S. Hierarchically Ordered Macro-Microporous Polyoxometalate-Based Metal-Organic Framework Single Crystals. ACS Nano 2021, 15, 16581–16588.
- 50 Ma, F.-J.; Liu, S.-X.; Sun, C.-Y.; Liang, D.-D.; Ren, G.-J.; Wei, F.; Chen, Y.-G.; Su, Z.-M. A sodalite-type porous metal−organic framework with polyoxometalate templates: Adsorption and decomposition of dimethyl methylphosphonate. J. Am. Chem. Soc. 2011, 133, 4178–4181.
- 51 Zhou, J.; Li, J.; Kan, L.; Zhang, L.; Huang, Q.; Yan, Y.; Chen, Y.; Liu, J.; Li, S.-L.; Lan, Y.-Q. Linking oxidative and reductive clusters to prepare crystalline porous catalysts for photocatalytic CO2 reduction with H2O. Nat. Commun. 2022, 13, 4681.
- 52 Liu, G.; Qi, Y.; Li, J.; Chen, Y.; Chen, Y.; Li, Z.; Shen, G.; Ma, D.; Li, Y.; Huang, X. Flowing scalable production of sulfenamides by active site-tuned lacunary polyoxometalate foams. J. Mater. Chem. A 2023, 11, 12258–12265.
- 53 Nguyen, T. B.; Ermolenko, L.; Retailleau, P.; Al-Mourabit, A. Elemental Sulfur Disproportionation in the Redox Condensation Reaction between o-Halonitrobenzenes and Benzylamines. Angew. Chem. Int. Ed. 2014, 53, 13808–13812.
- 54 Chen, Y. X.; Qian, L. F.; Zhang, W.; Han, B. Efficient aerobic oxidative synthesis of 2-Substituted benzoxazoles, benzothiazoles, and benzimidazoles catalyzed by 4-Methoxy-TEMPO. Angew. Chem. Int. Ed. 2008, 47, 9330–9333.
- 55 Qi, Y.; Gu, X.; Huang, X.; Shen, G.; Yang, B.; He, Q.; Xue, Z.; Du, M.; Shi, L.; Yu, B. Microwave-assisted controllable synthesis of 2-acylbenzothiazoles and bibenzo[b][1,4]thiazines from aryl methyl ketones and disulfanediyldianilines. Chin. Chem. Lett. 2021, 32, 3544–3547




