Effect of chitosan-based melatonin composite coating on the quality of minimally processed pomegranate aril-sacs during cold storage
Abstract
The current study evaluated the potential of chitosan-based melatonin (CH-MT) composite coating to maintain quality and delay senescence of minimally processed pomegranate aril-sacs during refrigerated storage. Mature pomegranate fruit (cv. Wonderful) without physical defects were processed into aril-sacs. Subsequently, the following treatments were applied; distilled water (control), CH 0.5% (w/v), CH+0.1 mM MT, CH+0.4 mM MT, CH+0.8 mM MT, and CH+1 mM MT. Each treatment was applied by immersing the aril-sacs in the coating solution for 3 min and allowed to dry at room temperature. Aril-sacs were then packed in polyethene terephthalate punnets (three to four aril-sacs/punnet) and stored at 5°C and 85% RH for 21 days. Aril color, weight loss, respiration rate, ascorbic acid content, total anthocyanins, browning index, and antioxidant capacity were monitored during storage at 3 days intervals. The study results showed that the CH-MT coating treatments significantly (p < 0.05) delayed the increase in aril-sacs weight loss and respiration rate. These treatments also retained higher aril chroma, ascorbic acid, anthocyanin content, DPPH-radical scavenging activity, and aril-sacs antioxidant power (FRAP). In addition, surface browning development was significantly suppressed in the coated aril-sacs compared with control sacs. However, the effectiveness of CH-MT treatments in maintaining the quality of aril-sacs was concentration-dependent, with CH+0.8–1 mM MT treatments showing the best results. Therefore, CH+0.8–1 mM MT treatments are recommended to suppress the development of physiological disorders and maintain the quality of minimally processed pomegranate aril-sacs.
Novelty Impact Statement
This study focuses on the development and application of chitosan-melatonin composite coating (CH-MT) as an active and functional formulation to maintain quality and extend the storage life of minimally processed pomegranate aril-sacs. The results showed that incorporating melatonin with chitosan improved the effectiveness of chitosan base coating. This was demonstrated by the CH-MT coating's enhanced retention of ascorbic acid, anthocyanins, radical scavenging activity, and antioxidant power. Further, delaying the increase in aril-sacs surface browning, weight loss, and respiration rate. The study findings are valuable in the commercial adoption of safe preservation technologies to maintain the quality and maximize the storability of minimally processed products.
1 INTRODUCTION
Pomegranate (Punica granatum L.) is globally recognized as a “super fruit” owing to its countless health and functional benefits (Fawole and Opara, 2013a; Fawole et al., 2012; Mditshwa et al., 2013; Opara et al., 2009). Increasing awareness of the functional benefits of consuming pomegranate has increased the global demand for fruit and opened up more market opportunities to meet consumer demand (Fawole and Opara, 2013a). However, despite pomegranate's reported nutritional and medicinal benefits, extracting the arils from the fruit remains a labor-intensive and time-consuming process. This makes the pomegranate fruit less convenient for consumption (Aindongo et al., 2014a; Caleb et al., 2013). Thus, minimal processed and fresh-cut pomegranate products present a suitable alternative to enhance the consumption of the fruit and higher commercial value (Aindongo et al., 2014a; Sau et al., 2021).
The growing demand for minimally processed pomegranate products has prompted the formulation of pomegranate aril-sacs (Aindongo et al., 2014a), an alternative to minimally processed arils. Processing to aril-sacs offers an advanced alternative product whereby arils remain attached to the peel, thus, minimizing aril damage and dehydration, which results in prolonged storage life of the produce. According to Aindongo et al. (2014a), the transpiration rate (RT) of “Bhagwa” pomegranate arils was, on average, 8.34 and 5.29 g/kg/day for aril-sacs. The high TR of the arils is due to the larger surface area exposed compared with aril-sacs, where some arils are protected and covered with the membrane and peel. This highlights the advantage of processing pomegranate fruit into aril-sacs as an alternative to arils. However, the quality of minimally processed fresh horticultural produce, including aril-sacs, deteriorates rapidly during storage due to tissue damage as a result of processing. Consequently, this results to the accelerated ripening rate and senescence (Antunes et al., 2012; Kawhena et al., 2020). This limits consumer appeal and marketability of minimally processed products. Therefore, proper handling, low-temperature storage, and other postharvest technologies offer the possibility to preserve quality and prolong storage life of minimally processed pomegranate aril-sacs.
The increasing consumer awareness of fresh, quality, and safe food has prompted the agri-food industry to develop sustainable postharvest technologies as alternatives to plastic and chemical use for the quality management of fresh horticultural produce. Edible coatings constitute an ideal natural-based postharvest strategy reported to effectively preserve quality and prolong the storage life of various minimally processed fresh-cut products (Antunes et al., 2012; Guroo et al., 2021; Kawhena et al., 2020; Wu et al., 2021). Edible coatings provide selective permeability to gas and water vapor, thus, reducing the rate of metabolic process, resulting in reduced ripening rate and delayed senescence of fresh produce.
Chitosan (poly-β-[1, 4]-2-amino-2-deoxy-d-glucan; CH) is one of the most used poly-saccharide-based coating materials due to its wide range of properties, such as ubiquity, biodegradability, non-toxicity, ability to form films, and carrier of active ingredients. However, the practicality of CH is limited because of its low antimicrobial and antioxidant properties (Jiao et al., 2019). Nonetheless, the functionality of CH can be expanded by incorporating antioxidants, antimicrobials, bacteriostatic agents, essential oils and other properties to enhance quality preservation and provide more protection for food products (Arroyo et al., 2020; Chen et al., 2014; Jiao et al., 2019; Lo'ay and Taher, 2018; Molamohammadi et al., 2019; Nair et al., 2020; Sayyari et al., 2016; Shi et al., 2018; Sinha et al., 2021; Wu and Yang, 2016; Zhang et al., 2015; Zhao et al., 2020).
Among the various natural antioxidants, melatonin (N-acetyl-5-methoxytryptamine; MT) has recently emerged as an alternative biosafe treatment to maintain postharvest quality and prolong the shelf life of whole and minimally processed fruits and vegetables (Sharafi et al., 2021; Zhang et al., 2018; Zhao et al., 2020). MT is an indolic compound with many biological actions, such as regulating plant growth processes, including rooting, flowering, and photosynthetic efficiency (Arnao and Hernandez-Ruiz, 2018). Moreover, MT has the ability to act as a bio-stimulant in plants against stress due to its strong scavenging capacity against free radicals.
In postharvest management, exogenous MT treatments have been reported to preserve quality, alleviate the incidence of physiological disorders and prolong the storability of fresh produce by enhancing antioxidant enzymes activity (Aghdam and Fard, 2017; Du et al., 2021; Liu et al., 2020; Onik et al., 2021; Sharafi et al., 2021; Sun et al., 2020; Zhang et al., 2018; Zhao et al., 2020). More studies have also reported an induced accumulation of flavonoids (Sharafi et al., 2021; Zhang et al., 2018), proline (Aghdam et al., 2019; Liu et al., 2020), and phenolics (Sharafi et al., 2021; Sun et al., 2020), which strengthen the free radicals scavenging system capacity of the produce (Sharafi et al., 2021), thus, maintaining quality. In line with these advantages, there is a need to develop various formulations of coatings with MT to improve the coatings' functionality for better packaging. Therefore, the purpose of this study was to develop CH-MT composite edible coating as an active and functional formulation for maintaining quality and delaying senescence of minimally processed pomegranate aril-sacs.
2 MATERIALS AND METHODS
2.1 Plant material and chemicals
Mature pomegranate fruit ~17.4°Brix (cv. Wonderful) were harvested from Ubali pomegranate farm located in Kameelfontein, Pretoria, South Africa, during the 2020/2021 season. Immediately after harvest, the fruits were transported to the postharvest and agro-processing research laboratory at the University of Johannesburg (PARL-UJ) and evaluated for uniform size, color, and free of external defects. Subsequently, the fruit was stored overnight at 15°C to remove field heat, and then disinfected with 0.01% NaClO. For processing, individual fruit were carefully cut into four to five sections to obtain intact aril-sacs. All processing procedures were conducted in cool laboratory room and using sterilized knives to avoid physiological stress and contamination of the fruit.
Chitosan (ChitoPlant®) was purchased from ChiPro GmbH, Bremen, Germany. All the other chemicals were purchased from Sigma-Aldrich Co., except for sodium hydroxide and ferric chloride, which were acquired from Merck Millipore Co.
2.2 Treatments and experimental design
The treatment formulations used in this study were: Control, CH-0.5% w/v, CH+0.1 mM MT, CH+0.4 mM MT, CH+0.8 mM MT, and CH+1 mM MT. Each of the treatment formulation contained; Tween-20 (0.1% w/v), glycerol (0.1% w/v), and pure canola oil (0.05% w/v). Coatings were applied by dipping the aril-sacs for 3 min in the prepared solution. The control aril-sacs were treated with distilled water. Subsequently, aril-sacs were left allowed to dry at ambient temperature, then packaged into transparent polyethene terephthalate (PET) punnets (11.5 × 4.5 cm) (Zibo Containers) disinfected with 70% ethanol (Figure 1, Figures S1 and S2). The aril-sacs were then stored in refrigerated storage (5 ± 0.5°C and 85 ± 5 RH) for 21 days. Refrigerator temperature and RH were monitored with the Ebro EBI300 data logger (EbroElectronic, EBI300 data logger). For quality evaluation, five punnets per treatment, each containing approximately three and four aril-sacs (120–150 g/punnet), were used as replicates which were sampled every 3 days.
2.3 Physiological responses
2.3.1 Weight loss
2.3.2 Respiration rate
The respiration rate of pomegranate aril-sacs was assessed following the method of Fawole and Opara (2013b) with slight modifications. Briefly, 2 aril-sacs were incubated in a 300 ml airtight container at ambient temperature for 2 h and this was done in five replicates. After the incubation period, carbon dioxide gas production was measured inside the container using an infrared gas analyzer (Dansensor® CheckPoint 3). Results were expressed as ml∙CO2/kg·h.
2.3.3 Browning index
2.4 Physico-chemical properties
2.4.1 Aril texture
The texture of arils was assessed using the method of Fawole and Opara (2013c) using an Agrosta texture meter (Agrosta® texture analyzer) fitted with a 35 mm compression probe. The instruments operating settings were: 1 mm/s probe speed and 0.30 N force. Texture (N) was tested using 20 arils per treatment.
2.4.2 Color attributes
2.4.3 Total soluble solids and titratable acidity
TA content was estimated using a titratable acidity meter (ThermoFisher Scientific, Orion-Star T910 pH Titrator). Briefly, 2 mL of pomegranate juice (PJ) was diluted with 90 mL of double distilled water and titrated to pH 8.2 with 0.1 M sodium hydroxide (NaOH). The results were expressed as citric acid (%) equivalent. TSS was estimated using a digital handheld refractometer (Refractometer PAL-1, Atago), calibrated to zero using double distilled water.
2.5 Phytochemical contents and antioxidant properties
2.5.1 Ascorbic acid content
Ascorbic acid content was quantified according to the method described by Kawhena et al. (2020). Briefly, 500 μl of PJ was extracted with 4.5 ml of HPO3 (1%) and sonicated in ice for 10 min using the ultrasonic bath (Labotech SonicClean, model 705). Subsequently, 250 μl was mixed with 2250 μl of 2,6-dichlorophenolindophenol and kept in the dark for 30 min. Thereafter, the absorbance was recorded at 515 nm using the UV–Vis Spec (Spectrum instruments, SP-UV 300, UV–VIS Spec). The results were calculated as follows; milligram of ascorbic acid equivalent per 100 ml of crude PJ.
2.5.2 Total anthocyanin content
2.5.3 DPPH radical-scavenging activity
The DPPH radical-scavenging activity was quantified using the method of Fawole and Opara (2013a). Briefly, 30 μl of the extracted PJ was mixed with 1470 μl of methanol, and then 1500 μl DPPH mixture (0.1 mM) was added. The mixture was thoroughly mixed and kept in the dark for 30 min. Thereafter, the absorbance was recorded at 517 nm using the UV–Vis Spec (Spectrum instruments, SP-UV 300, UV–VIS Spec). DPPH radical-scavenging activity was calculated as follows; ascorbic acid (mM) equivalent per 100 ml of crude PJ.
2.5.4 Ferric reducing antioxidant power (FRAP)
FRAP was quantified following the method of Benzie and Strain (1996). Briefly, a FRAP consisting of C2H3O21− (300 mM), TPTZ, and FeCl3 20 mM (10:1:1) was prepared on each day of analyses. 75 μl of PJ extract was diluted with 1425 μL of the FRAP solution and kept in the dark for 30 min. Thereafter, the absorbance was recorded at 517 nm using the UV–Vis Spec (Spectrum instruments, SP-UV 300, UV–VIS Spec). FRAP was calculated as follows; Trolox (mM) equivalent per 100 ml of crude PJ.
2.6 Statistical analysis
Two-way anova was performed using GenStat statistical software (GenStat for windows 18th Edition, VSN International). Principal component analysis (PCA) and heat map analysis were performed using XLSTAT software (XLSTAT version April 1, 2020). The graphical presentations were mapped out using GraphPad Prism software (GraphPad for windows version 5.04 Software).
3 RESULTS AND DISCUSSION
3.1 Weight loss and respiration rate
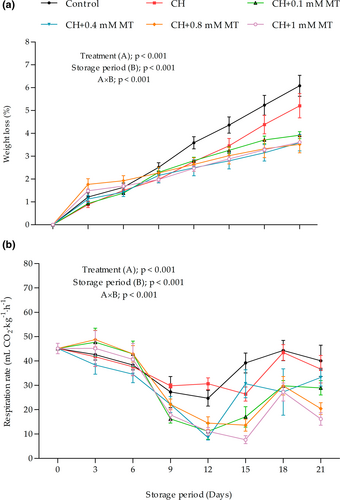
Figure 1a presents the trend of aril-sacs weight loss, which was evaluated as one of the key parameters affecting fresh produce quality during postharvest storage (Maguire et al., 2010). The results showed a significant (p < 0.001) interaction effect between treatment and storage period. The weight loss of aril-sacs increased progressively (p < 0.001) with the advancement of storage, regardless of the treatment. Notably, no significant differences (p > 0.05) were observed between the treatments until day 9 of storage. However, on the 12th day of storage, control aril-sacs showed a sharp increase in weight loss. Another sharp rise in weight loss of aril-sacs was later observed on day 18 of storage for CH treatment. This indicates that CH treatment alone can delay the aril-sacs weight loss for up to 18 days. This is a 6-day extension compared with the control treatment. On day 21 of storage, the maximum weight loss was measured in control aril-sacs (5.21%) followed by the CH-treated sacs (3.92%), while the minimum was measured in CH+0.8 mM MT-treated aril-sacs.
The weight loss of fresh produce is primarily due to moisture loss due to transpiration. This is greatly influenced by factors such as respiration, RH, and temperature, resulting in a high rate of metabolic processes. Thus, the observed delayed weight loss in CH-treated aril-sacs could result from CH's good film-forming properties (Cazón et al., 2017), resulting in reduced O2 permeability, which leads to reduced respiration rate. Further, the semi-permeable layer of CH on surfaces serves as a barrier against evaporative water loss (Cazón et al., 2017; Guroo et al., 2021). Thus, the enhanced effectiveness of the CH+0.8–1 mM MT treatments, respectively, in inhibiting aril-sacs weight loss might have resulted from the synergistic effect of CH and MT. MT is reported to enhance phenylalanine ammonia-lyase (PAL) activity, leading to cell wall strengthening by deposition of phenolic compounds and thus maintaining higher membrane integrity (Aghdam and Fard, 2017). This provides an extra barrier against gasses and water vapor exchange.
The current study results further showed that the CH and CH+MT treatments were significantly more effective (p < 0.001) in minimizing respiration of aril-sacs compared with the control treatment (Figure 1b). However, the changes in aril-sacs respiration were mainly influenced by the interaction between coating treatments and the storage period (p < 0.001).
Aril-sacs respiration rate initially decreased until day 12 of storage which thereafter increased, irrespective of the treatment. The explanation for this pattern is still unclear; however, markedly, the coated sacs respired lower than the control aril-sacs. In support of our results, Fawole and Opara (2013b) observed a decrease in the respiratory rate of “Ruby” and “Bhagwa” pomegranates fruits during the first 4 weeks of storage, followed by an indistinct respiratory pattern as the storage period progressed, and this is hypothesized to be due to senescence.
Notably, on day 15 of storage, a sharp peak in the respiration rate of uncoated aril-sacs was observed. The coated aril-sacs later exhibited a rise in respiration rate on day 18 of storage, indicating that coating treatments effectively delayed respiration rate peak, an effect associated with quality maintenance of fresh produce. On day 21 of storage, the highest peak respiration rate was observed in control aril-sacs (40.06 ml CO2/kg/h), which was insignificantly different from the CH (36.65 ml CO2/kg/h), CH+0.1 mM MT (28.98 ml CO2/kg/h), and CH+0.4 mM MT (33.24 ml CO2/kg/h) treatments. Combination treatments of CH and MT at 0.8 and 1 mM, respectively, showed about 64.81% and 84.87% lower respiration rates than the control. In support of our results, Gao et al. (2016) reported a significantly lower respiratory rate of peach fruit treated with 0.1 mM MT stored at ambient temperature for 7 days.
The high respiration rate of minimally processed products results from a large surface area exposed, which results in high O2 intake into the cells and an increased rate of metabolic processes, including respiration (Aindongo et al., 2014b; Zagory, 1999). Thus, the lower respiration of CH and CH+MT-treated aril-sacs results from CH function as a barrier to gaseous exchange, leading to increased CO2/O2 concentration ratio and, thus, lower respiration rate (Sayyari et al., 2016). The incorporation of MT extends this effect by enhancing the activity of antioxidant compounds and enzymes, thus alleviating the oxidative stress resulting from the processing. Such an effect is associated with reduced respiration rates and delayed senescence of fresh produce.
3.2 Browning index
Browning was evaluated as a key parameter that affects the marketability of minimally processed products (Antunes et al., 2012). This parameter is also a direct indication of fruit decay and senescence. The results indicated a significant effect (p < 0.001) of interaction between treatment and storage duration on the browning index. Browning of minimally processed pomegranate aril-sacs increased progressively (p < 0.001) across all treatments during storage (Figure 2). However, the coating treatments significantly inhibited the increase in browning compared with the control treatment, which showed a higher browning index throughout the storage period. Aril-sacs treated with CH alone showed a higher browning index among the CH and CH-MT treatments. In contrast, aril-sacs treated with MT 0.8 and 1 mM, respectively, in combination with CH, showed a lower browning index throughout storage.
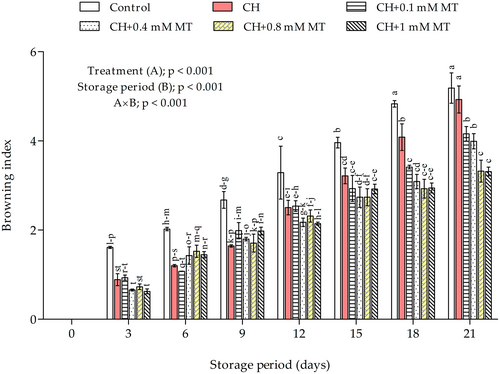
Browning is understood to occur through the oxidation of phenols by the enzymatic action of polyphenol oxidase (PPO) using oxygen as a co-substrate, which ultimately produces the browning appearance (Wu et al., 2021). Furthermore, factors such as rapid moisture loss have also been reported to enhance fruit browning (Kumari et al., 2015). Thus, the efficacy of CH+0.8–1 mM MT treatments to inhibit browning is attributed to the reported role of MT in reducing PPO activity (Li et al., 2021; Sun et al., 2020; Zhang et al., 2018). Further, the desiccation of the peel causes the leakage of phenolic substrates and, thus, browning due to contact with PPO. Therefore, the synergistic effect of MT in combination with CH in reducing weight loss could also explain the reduced browning in CH+MT coated aril-sacs.
3.3 Aril texture
Aril texture is also one of the key parameters for consumer acceptance (Szychowski et al., 2015). The aril texture declined progressively (p < 0.001) with the advancement of storage regardless of the treatment (Table 1). The changes in aril texture during storage were mainly driven by the interaction effect (p < 0.001) between coating treatments and the storage period. On the 15th day of storage, aril-sacs treated with CH+MT at 0.8 and 1 mM, respectively, exhibited the highest retention of aril texture while it was minimum in control aril-sacs.
| Treatments | Storage period (days) | |||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 3 | 6 | 9 | 12 | 15 | 18 | 21 | |
| Texture (N) | 13.52 ± 0.35a | |||||||
| Control | 11.61 ± 0.30b–k | 11.44 ± 0.67c–k | 11.58 ± 0.89c–k | 11.51 ± 0.26c–k | 9.13 ± 0.39m | 12.66 ± 0.33a–d | 11.52 ± 0.41a–h | |
| CH 0.5% | 12.28 ± 0.41a–g | 11.75 ± 0.36b–j | 10.36 ± 0.78h–m | 12.39 ± 0.75a–e | 9.84 ± 0.46k–m | 9.57 ± 0.40lm | 10.93 ± 0.29d–m | |
| CH+0.1 mM MT | 13.44 ± 0.74a–b | 11.11 ± 0.70c–l | 10.48 ± 0.45f–m | 11.82 ± 0.50a–j | 10.91 ± 0.55d–m | 10.43 ± 0.77g–m | 10.17 ± 0.78i–m | |
| CH+0.4 mM MT | 11.46 ± 0.65c–k | 12.95 ± 0.55a–c | 12.34 ± 0.68a–f | 10.93 ± 0.53d–m | 9.98 ± 0.38i–m | 10.21 ± 0.48h–m | 10.72 ± 0.46e–m | |
| CH+0.8 mM MT | 12.35 ± 0.55a–f | 11.97 ± 0.41a–i | 12.25 ± 0.67a–g | 11.64 ± 1.18b–k | 11.03 ± 0.66d–l | 11.71 ± 0.59b–k | 11.40 ± 0.43c–l | |
| CH+1.0 mM MT | 12.40 ± 0.62a–e | 12.09 ± 0.45a–h | 11.02 ± 0.56d–i | 10.17 ± 0.29i–m | 11.70 ± 0.35b–k | 10.65 ± 0.39e–m | 11.86 ± 0.41a–j | |
| Significance level (p) | ||||||||
| Treatment (A) | Storage period (B) | AxB | ||||||
| 0.204 | <0.001 | <0.001 | ||||||
- Note: Data points are presented as mean ± standard error of means (SEM). Characters on the tables show significant differences (p < 0.05) between data points.
- Abbreviations: CH, chitosan; MT, melatonin.
Markedly, there was a sharp increase in aril texture of control aril-sacs on day 18 of storage. This peak in aril-sacs texture was later observed on day 21 of storage in CH-treated aril-sacs. The aril texture in the study was measured by the maximum force required to release all the juice from the aril completely. This is primarily affected by the arils' membrane integrity and the turgor pressure. Thus, as senescence approaches, the arils lose cell membrane integrity and turgor pressure. Consequently, it becomes easier to ooze the juice from the aril membrane; however, the probe compresses the kernel inside the aril, increasing the aril hardness. Therefore, the sharp increase in aril texture on day 18 of storage for control treatment and day 21 for coated aril-sacs indicated aril senescence.
While the treatments did not significantly affect aril texture, it was observed that the CH+MT treatments delayed the loss of aril's textural properties. In support of our results, Gao et al. (2016) and Liu et al. (2018) reported higher texture of peach and strawberry fruits, respectively, treated with MT during postharvest storage. Delayed textural loss of the coated arils could result from the lower cell wall degradation enzyme activity and enhanced catalase (CTA) antioxidant enzyme activity by MT, which prevents membrane damage (Zhang et al., 2018).
3.4 Color attributes
The changes in color parameters; a*, C*, and h° as a function of storage period and coating treatments are shown in Table 2. An indistinct pattern of arils redness was noted with the advancement of storage. Statistical analysis revealed that aril redness was predominantly affected by the interaction between treatments and storage period. Individual factor analysis demonstrated that this was due to the effect of the storage period (p < 0.001) because no significance (p < 0.061) was observed between the coating treatments. In support of our results, Fawole et al. (2020) observed no significant differences in aril redness of “Wonderful” pomegranate treated with putrescine treatments during storage at 4°C for 4 months.
| Treatments | Storage period (days) | |||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 3 | 6 | 9 | 12 | 15 | 18 | 21 | |
| a* | ||||||||
| Control | 17.52 ± 0.39a–f | 18.32 ± 1.03a–d | 16.52 ± 1.30a–g | 14.05 ± 1.05c–g | 16.70 ± 2.58a–g | 18.35 ± 2.01a–d | 15.34 ± 1.31a–g | 14.72 ± 1.53a–g |
| CH 0.5% | 17.52 ± 0.39a–f | 15.74 ± 1.37a–g | 11.97 ± 1.54g | 13.39 ± 0.98d–g | 19.48 ± 2.07ab | 14.17 ± 1.08b–g | 18.98 ± 2.04a–c | 18.85 ± 1.61a–c |
| CH+0.1 mM MT | 17.52 ± 0.39a–f | 12.31 ± 0.92fg | 13.96 ± 1.89b–g | 13.59 ± 0.76c–g | 18.04 ± 2.35a–e | 11.65 ± 1.42g | 14.81 ± 1.88a–g | 13.34 ± 1.37d–g |
| CH+0.4 mM MT | 17.52 ± 0.39a–f | 14.27 ± 1.47b–g | 15.00 ± 0.56a–g | 12.71 ± 0.70e–g | 18.19 ± 2.39a–d | 14.74 ± 0.71a–g | 12.78 ± 1.02e–g | 15.91 ± 1.83a–g |
| CH+0.8 mM MT | 17.52 ± 0.39a–f | 11.87 ± 1.05g | 15.76 ± 2.36a–g | 16.16 ± 0.72a–g | 13.63 ± 1.73c–g | 18.37 ± 2.37a–d | 15.82 ± 2.27a–g | 15.95 ± 1.75a–g |
| CH+1.0 mM MT | 17.52 ± 0.39a–f | 13.25 ± 0.86d–g | 12.26 ± 1.47fg | 13.67 ± 1.60c–g | 13.67 ± 1.56c–g | 19.66 ± 1.61a | 14.85 ± 1.93a–g | 15.41 ± 1.43a–g |
| C* | ||||||||
| Control | 23.08 ± 0.57b–f | 20.88 ± 1.05c–j | 19.04 ± 1.59e–m | 15.40 ± 1.11k–o | 19.10 ± 3.02e–m | 19.85 ± 2.27c–l | 17.05 ± 1.44g–o | 16.44 ± 1.60h–o |
| CH 0.5% | 23.08 ± 0.57b–f | 17.00 ± 1.20g–o | 13.23 ± 1.65no | 14.66 ± 0.96l–o | 21.82 ± 2.36c–h | 14.97 ± 1.13l–o | 21.05 ± 2.40c–i | 20.78 ± 1.73c–k |
| CH+0.1 mM MT | 23.08 ± 0.57b–f | 13.34 ± 0.88no | 15.09 ± 2.01i–o | 14.78 ± 0.77l–o | 19.74 ± 2.51d–l | 12.14 ± 1.52° | 16.21 ± 2.06i–o | 15.20 ± 1.56l–o |
| CH+0.4 mM MT | 23.08 ± 0.57b–f | 16.78 ± 1.67g–o | 15.84 ± 0.51i–o | 14.63 ± 0.59l–o | 19.79 ± 2.57d–l | 15.36 ± 0.82k–o | 13.94 ± 1.17l–o | 17.77 ± 2.00f–n |
| CH+0.8 mM MT | 23.08 ± 0.57b–f | 22.04 ± 0.85b–f | 28.61 ± 1.17a | 24.79 ± 1.45a–d | 22.61 ± 1.24b–f | 19.55 ± 3.46d–l | 23.18 ± 1.98b–f | 25.35 ± 0.16a–c |
| CH+1.0 mM MT | 23.08 ± 0.57b–f | 22.50 ± 1.60b–f | 28.79 ± 1.46a | 27.30 ± 1.71ab | 23.41 ± 1.21b–e | 15.51 ± 1.68j–o | 22.46 ± 0.94b–f | 24.43 ± 1.98a–e |
| h° | ||||||||
| Control | 32.16 ± 0.70a | 28.65 ± 1.30a–f | 29.36 ± 1.93a–d | 24.19 ± 1.34d–i | 27.77 ± 2.98a–g | 22.04 ± 1.04g–j | 25.85 ± 1.07d–g | 26.63 ± 0.91a–g |
| CH 0.5% | 32.16 ± 0.70a | 22.09 ± 2.96g–j | 25.22 ± 1.67d–h | 23.79 ± 2.24d–i | 26.35 ± 2.12c–g | 18.76 ± 1.02i–k | 25.14 ± 1.08d–h | 24.90 ± 0.88d–h |
| CH+0.1 mM MT | 32.16 ± 0.70a | 22.20 ± 2.92g–j | 22.34 ± 1.81g–j | 23.21 ± 0.52f–j | 23.94 ± 1.12d–i | 15.88 ± 1.23k | 24.01 ± 0.40d–i | 28.53 ± 1.61a–f |
| CH+0.4 mM MT | 32.16 ± 0.70a | 31.66 ± 1.55a–c | 17.87 ± 2.88jk | 29.08 ± 3.09a–e | 23.28 ± 0.57f–j | 15.53 ± 1.98k | 23.27 ± 0.96f–j | 26.50 ± 0.77c–g |
| CH+0.8 mM MT | 32.16 ± 0.70a | 22.04 ± 0.85g–j | 28.61 ± 1.17a–f | 24.79 ± 1.45d–h | 22.61 ± 1.24g–j | 19.55 ± 3.46h–k | 23.18 ± 1.98f–j | 25.35 ± 0.16d–g |
| CH+1.0 mM MT | 32.16 ± 0.70a | 22.50 ± 1.60g–j | 28.79 ± 1.46a–f | 27.30 ± 1.71a–g | 23.41 ± 1.21e–j | 15.51 ± 1.68k | 22.46 ± 0.94g–j | 24.43 ± 1.98d–i |
| Significance level (p) | ||||||||
| Treatment (A) | Storage period (B) | AxB | ||||||
| a* | 0.061 | <0.001 | 0.006 | |||||
| C* | 0.020 | 0.051 | 0.005 | |||||
| h° | 0.007 | <0.001 | <0.001 | |||||
- Note: Data points are presented as mean ± standard error of means (SEM). Characters on the tables show significant differences (p < 0.05) between data points.
- Abbreviations: CH, chitosan; MT, melatonin; a*, redness; C*, chroma; h°, hue angle.
In contrast, coating treatments mainly influenced (p = 0.020) the arils chroma and no significant differences were observed with storage (p = 0.051) (Table 2) with. Similarly, Fawole and Opara (2013b) found no significant differences in arils chroma with the advancement of storage. The authors highlighted that pomegranate arils' color intensity presented by chroma is relatively stable during storage. The analysis of variance in our study revealed that the CH+MT at 0.8 and 1 mM coatings, respectively, maintained the highest chroma of arils throughout storage. Further, these treatments retained chroma by 43% and 39%, respectively, on the final day of storage, compared with the control.
The aril hue angle initially declined till day 15 of storage, which increased thereafter. Noticeably, slight significant differences were observed on 21 day of storage among coating treatments and control.
3.5 Total soluble solids and titratable acidity
Changes in total soluble solids (TSS), titratable acidity (TA), and TSS/TA of control and treated aril-sacs are shown in Table 3. The TSS content was mainly affected (p < 0.001) by the interaction between coating treatments and the storage period. Overall, TSS increased from days 0 to 21 days of storage, with control aril-sacs having higher values than coated aril-sacs. On day 21 of storage, the highest TSS content (18.46°Brix) was measured in control aril-sacs, while the lowest (17.7°Brix) was measured in aril-sacs treated with CH+1 mM MT.
| Treatments | Storage period (days) | |||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 3 | 6 | 9 | 12 | 15 | 18 | 21 | |
| TSS (°Brix) | ||||||||
| Control | 17.4 ± 0.23n–p | 17.47 ± 0.12m–p | 18.00 ± 0.06e–l | 17.63 ± 0.07l–p | 18.03 ± 0.12e–l | 18.20 ± 0.06c–i | 18.73 ± 0.15a | 18.47 ± 0.13a–e |
| CH 0.5% | 17.4 ± 0.23n–p | 17.77 ± 0.09h–o | 18.13 ± 0.03e–k | 17.73 ± 0.03i–o | 17.47 ± 0.26m–p | 18.60 ± 0.10a–d | 18.40 ± 0.06a–f | 17.97 ± 0.13f–l |
| CH+0.1 mM MT | 17.4 ± 0.23n–p | 17.23 ± 0.15p | 18.03 ± 0.07e–l | 17.80 ± 0.10h–n | 18.63 ± 0.09a–c | 18.70 ± 0.12a | 18.37 ± 0.07a–f | 18.03 ± 0.07e–l |
| CH+0.4 mM MT | 17.4 ± 0.23n–p | 17.97 ± 0.17f–l | 18.03 ± 0.07e–l | 17.83 ± 0.12g–n | 17.93 ± 0.03f–m | 18.30 ± 0.00a–g | 18.23 ± 0.15b–h | 17.87 ± 0.12g–n |
| CH+0.8 mM MT | 17.4 ± 0.23n–p | 17.30 ± 0.26op | 18.10 ± 0.36e–l | 17.67 ± 0.03k–p | 18.07 ± 0.12e–l | 18.03 ± 0.03e–l | 17.93 ± 0.03f–m | 18.07 ± 0.15e–l |
| CH+1.0 mM MT | 17.4 ± 0.23n–p | 17.60 ± 0.06e–l | 18.13 ± 0.13d–k | 17.73 ± 0.18i–o | 18.23 ± 0.03b–h | 18.17 ± 0.03d–j | 18.67 ± 0.15ab | 17.70 ± 0.10j–p |
| TA (% citric acid) | ||||||||
| Control | 1.32 ± 0.04ab | 1.10 ± 0.11d–h | 1.17 ± 0.04b–f | 1.23 ± 0.08a–f | 1.21 ± 0.12a–f | 1.24 ± 0.06a–f | 1.24 ± 0.01a–f | 1.06 ± 0.01e–h |
| CH 0.5% | 1.32 ± 0.04ab | 0.94 ± 0.10gh | 1.23 ± 0.07a–f | 1.12 ± 0.06c–g | 1.09 ± 0.02d–h | 1.22 ± 0.02a–f | 1.26 ± 0.06a–e | 1.12 ± 0.02b–g |
| CH+0.1 mM MT | 1.32 ± 0.04ab | 1.21 ± 0.06a–f | 1.29 ± 0.03a–d | 1.12 ± 0.06b–g | 1.22 ± 0.04a–f | 1.13 ± 0.05b–g | 1.13 ± 0.05b–f | 1.14 ± 0.05b–f |
| CH+0.4 mM MT | 1.32 ± 0.04ab | 1.30 ± 0.04a–c | 1.06 ± 0.04f–h | 1.24 ± 0.07a–f | 1.22 ± 0.02a–f | 1.26 ± 0.14a–e | 1.21 ± 0.07a–f | 1.23 ± 0.05a–f |
| CH+0.8 mM MT | 1.32 ± 0.04ab | 1.10 ± 0.12d–h | 1.17 ± 0.03b–f | 1.16 ± 0.06b–f | 1.22 ± 0.03a–f | 0.93 ± 0.03h | 1.30 ± 0.03a–c | 1.25 ± 0.04a–f |
| CH+1.0 mM MT | 1.32 ± 0.04ab | 1.26 ± 0.03a–d | 1.18 ± 0.08b–f | 1.16 ± 0.01b–f | 1.39 ± 0.04a | 1.11 ± 0.01c–h | 1.28 ± 0.00a–f | 1.25 ± 0.01a–f |
| TSS/TA | ||||||||
| Control | 15.07 ± 0.32a–f | 16.26 ± 1.75a–e | 15.41 ± 0.57a–f | 14.40 ± 0.93c–f | 15.20 ± 1.51a–f | 14.68 ± 0.64a–f | 15.11 ± 0.24a–f | 16.99 ± 0.30ab |
| CH 0.5% | 15.07 ± 0.32a–f | 16.97 ± 1.93ab | 14.87 ± 0.79a–f | 15.94 ± 0.91a–e | 16.06 ± 0.12a–e | 15.22 ± 0.29a–f | 14.66 ± 0.68a–f | 15.99 ± 0.38a–e |
| CH+0.1 mM MT | 15.07 ± 0.32a–f | 14.35 ± 0.71c–f | 14.05 ± 0.36d–f | 15.98 ± 0.99a–e | 15.27 ± 0.58a–f | 16.69 ± 0.80a–c | 16.31 ± 0.77a–e | 16.21 ± 0.75a–e |
| CH+0.4 mM MT | 15.07 ± 0.32a–f | 13.84 ± 0.29ef | 17.13 ± 0.72a | 14.46 ± 0.75b–f | 14.67 ± 0.25a–f | 14.95 ± 1.77a–f | 15.20 ± 0.91a–f | 14.53 ± 0.63b–f |
| CH+0.8 mM MT | 15.07 ± 0.32a–f | 16.15 ± 1.66a–e | 15.50 ± 0.39a–f | 15.37 ± 0.77a–f | 14.80 ± 0.44a–f | 15.08 ± 0.56a–f | 13.79 ± 0.32ef | 14.45 ± 0.39b–f |
| CH+1.0 mM MT | 15.07 ± 0.32a–f | 14.28 ± 0.35c–f | 15.48 ± 0.88a–f | 15.36 ± 0.20a–f | 13.17 ± 0.39f | 16.44 ± 0.20a–d | 14.89 ± 0.28a–f | 14.11 ± 0.13d–f |
| Significance level (p) | ||||||||
| Treatment (A) | Storage period (B) | AxB | ||||||
| TSS | <0.05 | <0.001 | <0.001 | |||||
| TA | <0.05 | 0.001 | <0.001 | |||||
| TSS/TA | 0.201 | 0.758 | 0.023 | |||||
- Note: Data points are presented as mean ± standard error of means (SEM). Characters on the tables show significant differences (p < 0.05) between data points.
- Abbreviations: CH, chitosan; MT, melatonin; TSS, total soluble solids; TA, titratable acidity.
Studies reporting postharvest changes in TSS contents differ depending on cultivar/variety, storage condition, and processing. This study observed an increase in TSS from days 0 to 21 of storage, with control aril-sacs having higher values than coated aril-sacs. In contrast, Fawole and Opara (2013b) reported a decrease in TSS content of pomegranate fruit (cv. Ruby) with prolonged storage at different storage conditions for 12 weeks. Similarly, Mwelase and Fawole (2022) reported a significant decrease in TSS contents of ‘Wonderful’ pomegranates treated with chitosan combined with epibrassinolide during storage for 12 weeks at 5°C. However, Ghafir et al. (2010) reported an increase in TSS of “Shlefy” pomegranate fruit during 16 weeks of storage at 5 and 7°C, respectively. Similarly, Kawhena et al. (2020) observed a significant increase in TSS of minimally processed arils during storage at 5°C for 16 days. TSS are substrates used as energy sources for fruit respiration; thus, the content is expected to decline with prolonged storage due to senescence. However, the increasing content may be attributed to high moisture loss, highly influenced by fruit processing, leading to an increased concentration of sugars within the arils.
Table 3 also shows the changes in TA of control and coated minimally processed pomegranate aril-sacs fruit during storage. Overall, the interaction between coating treatments and the storage period affected the juice TA content. The coating treatments were the main contributing factor (p < 0.05), with the coated aril-sacs showing better retention of TA than control sacs. On the final day of storage, aril-sacs treated with CH+1 mM MT showed the highest levels (1.25% citric acid) of TA, followed by CH+MT 0.8 and 0.4 mM, respectively. In contrast, TA's lowest level (1.06% citric acid) was measured in control aril-sacs. TA is interrelated with the levels of organic acids, which are primary respiratory substrates (Sayyari et al., 2011). Therefore, the improved efficiency of the CH+MT coatings in inhibiting the rise in respiration rate may explain the high levels of TA of the coated aril-sacs.
An increase in TSS/TA was observed with prolonged storage; however, the CH+MT coatings inhibited this rise (Table 3). Moreover, the main differences in TSS/TA were due to the interaction effect (p = 0.023) between coating treatments and the storage period.
3.6 Ascorbic acid content
The ascorbic acid content was mainly influenced (p < 0.001) by the interaction effect between coating treatments and the storage period (Figure 3). The ascorbic acid content declined considerably (p < 0.001) with storage, irrespective of the treatment. However, the levels were consistently high in treated aril-sacs and lower in control aril-sacs. On day 21 of storage, the lowest ascorbic acid content (28.36 mg AAE/100 ml PJ) was measured in control aril-sacs, while the highest retention was measured in CH+1 mM MT coated aril-sacs. However, no significant differences in ascorbic acid content were observed between CH and CH+MT treatments.
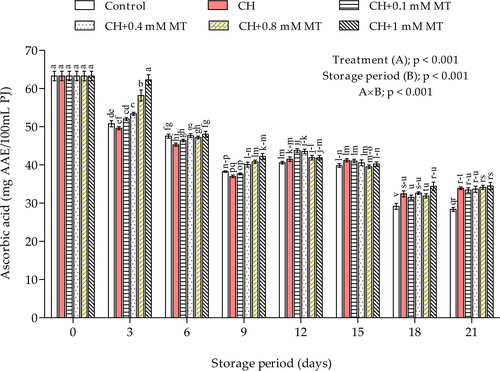
Ascorbic acid (vitamin C) is an important water-soluble antioxidant integrated into plant defense mechanisms against oxidation stress (Mditshwa et al., 2017). A decline in ascorbic acid content is due to the ascorbate oxidase enzyme activity with O2 as a co-substrate which causes a breakdown in ascorbic acid to secondary products (Mditshwa et al., 2017). Therefore, the excellent barrier properties of CH resulting in lower O2 within the fruit prevents the degradation of ascorbic acid by inhibiting ascorbate oxidase activity (Cazón et al., 2017). In support of our observations, Petriccione et al. (2014) reported higher ascorbic acid content in cherry fruits treated with CH. Further, Gao et al. (2016) also observed higher ascorbic acid content of peach fruit treated with 0.1 mM MT and stored at ambient conditions for 7 days.
3.7 Total anthocyanin content
The anthocyanin content increased significantly (p < 0.001) during storage, irrespective of the treatment (Figure 4). However, the changes in anthocyanin content were mainly affected by the interaction effect (p < 0.001) between coating treatments and the storage period. As a result, the increase was substantially higher in treated aril-sacs than in control aril-sacs. A sharp rise in anthocyanin content was observed on day 12 and 15 of storage, respectively, which thereafter decreased; however, the CH+MT treatments at 0.8 and 1 mM, respectively, maintained higher anthocyanin content throughout storage. The remarkable increase in anthocyanin content on days 12 and 15 could be attributed to the aforementioned high moisture loss, resulting in more concentrated juice pigment (Fawole and Opara, 2013b).
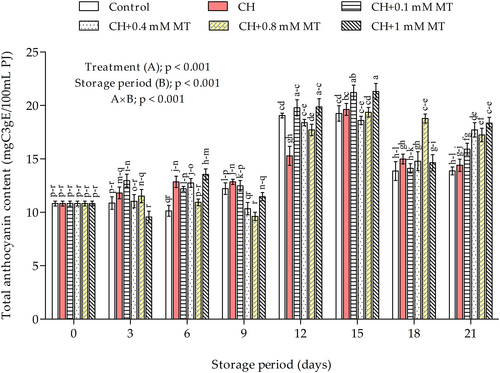
On day 21 of storage, highly significant differences were found between control, CH, and CH+MT-treated aril-sacs. The highest anthocyanin content (18.35 mgC3gE/100 ml PJ) was measured in CH+1 mM MT-treated aril-sacs and non-significantly followed by CH+0.8 mM MT. In comparison, the lowest anthocyanin content (13.88 mgC3gE/100 ml PJ) was measured in control aril-sacs with sacs treated with CH alone slightly above that level (14.40 mgC3gE/100 ml PJ).
In support of our results, Aghdam and Fard (2017) reported higher anthocyanin accumulation of litchi fruit treated with 0.4 mM MT during storage at 4°C for 12 days. Similarly, Liu et al. (2018) observed high anthocyanins accumulation in strawberries treated with MT at 0.1 mM during storage at 25°C for 8 days. The authors further noted that the higher anthocyanin content in fruit treated with melatonin resulted from higher PAL antioxidant activity, consequently leading to enhanced ROS scavenging capacity of the produce. Further, anthocyanins are known as antioxidant compounds in plants, indicating that melatonin-induced accumulation in anthocyanins is part of the systems used by the compound to preserve quality and delay senescence of pomegranate aril-sacs.
3.8 DPPH radical-scavenging activity and ferric reducing antioxidant power (FRAP)
The effects of coating treatments and storage period on DPPH radical-scavenging activity (RSA) and FRAP are shown in Table 4. The RSA of minimally processed pomegranate aril-sacs declined significantly (p < 0.05) with extension of storage, regardless of the treatment. While the coating treatments and storage period significantly affected RSA, the RSA changes were mainly driven by the interaction effect between the two factors. Rapid decline RSA was measured in control aril-sacs, whereas enhanced retention of RSA was measured in CH+1 mM MT coated aril-sacs. On the final day of storage, CH+1 mM MT coated aril-sacs had about 25% higher RSA than control aril-sacs.
| Treatments | Storage period (days) | |||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 3 | 6 | 9 | 12 | 15 | 18 | 21 | |
| DPPH (mM AAE/100 ml PJ) | ||||||||
| Control | 2600.59 ± 31.59a | 2379.30 ± 41.65c–f | 2484.09 ± 36.89a–d | 2459.10 ± 52.71a–e | 2095.55 ± 52.85h–j | 1601.25 ± 95.44°–q | 1587.73 ± 136.96p–r | 1451.34 ± 65.96r |
| CH 0.5% | 2600.59 ± 31.59a | 2444.59 ± 31.18a–f | 2278.54 ± 27.43e–g | 2356.73 ± 34.79c–f | 1989.14 ± 46.82i–k | 1832.76 ± 68.67k–n | 1685.20 ± 64.04n–q | 1526.31 ± 59.58qr |
| CH+0.1 mM MT | 2600.59 ± 31.59a | 2455.07 ± 36.32a–e | 2255.97 ± 35.78f–h | 2432.50 ± 54.33a–f | 2137.47 ± 108.97g–i | 1889.99 ± 80.44k–m | 1771.50 ± 79.48l–p | 1734.42 ± 53.01m–p |
| CH+0.4 mM MT | 2600.59 ± 31.59a | 2326.10 ± 25.74c–f | 2505.05 ± 113.76a–c | 2304.33 ± 33.18d–g | 1989.14 ± 46.19i–k | 1869.04 ± 74.35k–n | 1736.83 ± 57.30m–p | 1681.21 ± 70.70n–q |
| CH+0.8 mM MT | 2600.59 ± 31.59a | 2376.88 ± 42.23c–f | 2309.17 ± 42.68d–g | 2419.60 ± 55.23a–f | 1952.06 ± 32.34j–l | 1694.11 ± 82.23n–q | 1924.66 ± 94.68j–l | 1773.11 ± 69.56l–p |
| CH+1.0 mM MT | 2600.59 ± 31.59a | 2465.55 ± 31.27a–e | 2358.34 ± 33.51c–f | 2330.13 ± 17.80c–f | 2002.04 ± 62.26i–k | 1723.13 ± 71.47m–q | 1823.09 ± 32.84k–o | 1869.84 ± 71.46k–n |
| FRAP (mM TE/100 ml PJ) | ||||||||
| Control | 1501.58 ± 30.32a | 1385.65 ± 60.33bc | 1024.95 ± 34.07p | 1167.51 ± 47.74k–m | 1165.05 ± 12.27k–m | 1209.91 ± 28.75h–l | 1188.40 ± 35.34i–l | 1000.07 ± 36.59op |
| CH 0.5% | 1501.58 ± 30.32a | 1342.33 ± 47.96b–d | 1241.40 ± 44.81d–l | 1168.28 ± 30.41k–m | 1182.72 ± 43.26i–l | 1266.59 ± 37.83d–k | 1160.44 ± 36.14k–n | 1053.06 ± 12.84n–p |
| CH+0.1 mM MT | 1501.58 ± 30.32a | 1415.14 ± 43.27b | 1174.42 ± 14.15i–m | 1208.68 ± 33.50h–l | 1301.62 ± 32.16b–i | 1215.74 ± 31.27f–l | 1257.07 ± 18.19d–l | 1063.81 ± 10.18m–p |
| CH+0.4 mM MT | 1501.58 ± 30.32a | 1355.69 ± 69.32b–d | 1262.75 ± 45.71d–l | 1144.31 ± 59.14l–o | 1182.72 ± 43.26i–m | 1221.58 ± 14.47e–l | 1337.10 ± 35.55b–e | 1208.68 ± 23.85h–l |
| CH+0.8 mM MT | 1501.58 ± 30.32a | 1291.78 ± 28.41c–j | 1270.28 ± 28.61c–k | 1009.43 ± 37.55p | 1294.55 ± 32.16c–i | 1290.40 ± 11.43c–j | 1331.73 ± 38.47b–g | 1212.82 ± 19.88g–l |
| CH+1.0 mM MT | 1501.58 ± 30.32a | 1334.49 ± 97.97b–f | 1329.88 ± 98.66b–g | 1167.81 ± 29.05k–m | 1301.62 ± 90.01b–i | 1156.14 ± 39.43k–n | 1311.45 ± 32.03b–h | 1348.01 ± 68.53b–d |
| Significance level (p) | ||||||||
| Treatment (A) | Storage period (B) | AxB | ||||||
| DPPH | <0.05 | <0.001 | <0.001 | |||||
| FRAP | <0.001 | <0.001 | <0.001 | |||||
- Note: Data points are presented as mean ± standard error of means (SEM). Characters on the tables show significant differences (p < 0.05) between data points.
- Abbreviations: CH, chitosan; MT, melatonin; DPPH, 2,2-diphenyl-1-picrylhydrazyl; FRAP, ferric reducing antioxidant power.
Despite the noticeable changes in RSA with the advancement of the storage period, slight significant differences were observed in the antioxidant power of the aril-sacs with storage (Table 4). However, the coating treatments largely influenced the aril-sacs antioxidant power (p < 0.001). The control aril-sacs showed a more rapid decline in antioxidant power compared with the other treatments. Control aril-sacs showed a reduction in antioxidant capacity of approximately 38% within 7 days of storage. In contrast, aril-sacs treated with CH+1 mM MT retained about 88% of the initial antioxidant power after 6 days of storage. On the last day of storage, the lowest antioxidant power (1000.07 mM TE/100 ml PJ) was measured in control aril-sacs and the highest (1348.01 mM TE/100 ml PJ) was observed on CH+1 mM MT-treated aril-sacs.
Several bioactive compounds, including phenols, ascorbic acid, flavonoids, and anthocyanins, contribute to the antioxidant power and free radical scavenging activity (Kumari et al., 2015). Also, in the current study, the coated aril-sacs with higher antioxidant power (FRAP) and RSA had higher ascorbic acid and anthocyanins contents. Therefore, we hypothesize that the higher RSA and antioxidant power are attributed to the bioactive compounds' enhanced retention. In support of our results, Kumari et al. (2015) reported higher RSA of litchi fruit treated with chitosan combined with salicylic acid, which was positively and significantly correlated with the content of bioactive compounds. Similarly, Zhao et al. (2020) reported about two folds higher free radical scavenging ability of CH+MT 50 and 100 mg/L treatments, respectively, compared with the control treatment. Further, superoxide dismutase (SOD) and CAT are the most important antioxidant enzymes responsible for fresh produce's enhanced free radical scavenging ability. SOD dismutase O2− to H2O2 that is rapidly broken down into water and oxygen by CTA. Zhang et al. (2018) reported higher SOD and CAT enzyme activities in litchi's treated with 0.4 mM MT during storage at 25°C for 8 days. Also, Aghdam and Fard (2017) reported higher SOD and CAT enzyme activities in strawberry fruits treated with 100 μM MT during storage at 4°C for 12 days.
3.9 Multivariate data analysis
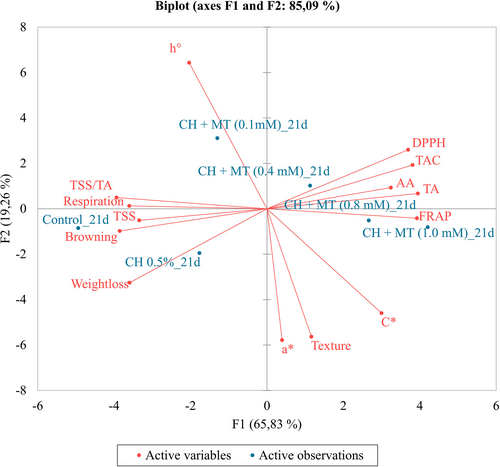
Principal component analysis (PCA) biplot was generated to demonstrate clarity between the coating treatments based on the assessed parameters on the last day of storage (Figure 5). The first principal component (F1) accounted for the maximum variation of the data (65.83%), while the second principal component (F2) accounted for 19.26% of the total variance. CH+0.8 mM MT and CH+1 mM MT were found to be significantly and positively correlated. However, these treatments were negatively correlated to control and CH 0.5% treatments. The control and CH 0.5% treatments were characterized by higher weight loss, browning, respiration, and TSS, which indicate faster senescence and decay (Figure 2, Figures S1 and S2). In contrast, the CH+0.8 mM MT and CH+1 mM MT were characterized by higher FRAP, TA, ascorbic acid, anthocyanins, and free radical scavenging ability. This supports the literature reports, as aforementioned, that these parameters are integrated into MT mechanism to maintain the quality of fresh produce. A significant and positive correlation was observed between DPPH, TAC, AA, TA, and FRAP. The parameters were significantly and negatively correlated with weight loss, browning, respiration, TSS, and TSS/TA (Figure 5). From these results, we can presume that higher respiration results in increased weight loss and browning of minimally processed aril-sacs. a*, C*, and texture were closely correlated and negatively correlated with h° (Figure 5). However, these parameters were not significantly correlated with the other evaluated parameters.
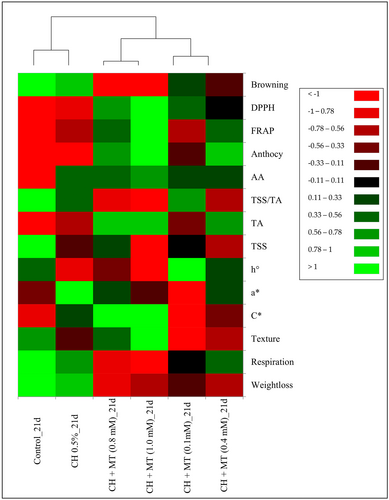
To further investigate the relationships between these treatments and quality attributes, heat map cluster analysis was carried out to discriminate the similarities or propinquity of data (Figure 6). Three groups were clustered in the heatmap. CH+0.1 mM MT, CH+0.4 mM MT, CH+0.8 mM MT and CH+1 mM MT merged first; however, these group was further separated to two clusters of CH+0.1 mM MT and CH+0.4 mM MT, and CH+0.8 mM MT and CH+1 mM MT, respectively. The control and CH 0.5% were the separate cluster from the beginning. The heat map analysis revealed that CH+MT 0.8–1 mM treatments are the best-performing treatments. These treatments significantly reduced aril-sacs weight loss, browning, and respiration rate. This was attributed to higher retentions of ascorbic acid, anthocyanins, and antioxidant capacity.
4 CONCLUSION
Our results showed that incorporating melatonin with chitosan greatly improved the functional properties of chitosan coating, which effectively inhibited surface browning of arils-sacs and retained chroma of arils during cold storage. The combination of CH with MT, particularly 0.8–1 mM MT, enhanced the antioxidant capacity; DPPH and FRAP attributed to the higher ascorbic acid and total anthocyanins content, which contribute to quality maintenance and delay senescence of minimally pomegranate aril-sacs. Thus, it can be concluded that CH+0.8–1 mM MT treatments could be potentially adopted as a safe preservation strategy to maintain the quality of minimally pomegranate aril-sacs during cold storage. However, further research is still essential to understand the mode of action before commercialization.
AUTHOR CONTRIBUTIONS
Conceptualization, O.A.F.; methodology, S.M.; software, S.M. and O.A.F.; validation, S.M., and O.A.F.; formal analysis, S.M.; investigation, S.M.; resources, O.A.F.; writing—original draft preparation, S.M.; writing—review and editing, S.M., U.L.O., and O.A.F.; visualization, U.L.O. and O.A.F.; supervision, O.A.F.; project administration, O.A.F.; funding acquisition, O.A.F., and U.L.O. All authors have read and agreed to the published version of the manuscript.
ACKNOWLEDGMENTS
This work was supported by the National Research Foundation of South Africa (NRF) (grant numbers: 129295 and 64813) and University of Johannesburg Research Committee.
Open Research
DATA AVAILABILITY STATEMENT
Data available on request from the authors.




