Effect of Proteolytic Modification on Texture and Mastication of Heat-Treated Egg White Gels
Abstract
Raw egg white undergoes sol–gel transition by heat treatment, which changes it to an elastic gel. Here, protease treatment to render a new texture to heated egg white gel was applied. Protease-treated gels exhibited ductile flow without obvious rupture points. Transmission electron microscopy analysis showed that in protease-treated gels, protein aggregates were distributed more homogeneously compared with that observed in the untreated control, probably because ovalbumin was digested into small peptides as revealed by SDS-PAGE. The properties of the gel were evaluated by sensory tests and by measuring the movement of the masseter muscle, using surface electromyography. Results showed that maximum bite force and mastication duration were decreased for the protease-treated gels, which were evaluated as being softer, smoother, less elastic and better textured. Overall, our results indicate that protease-treated egg white gel has superior qualities and is easier to swallow than the untreated gel.
Practical Applications
In the food industry, the use of egg white is limited compared with that of egg yolk and whole eggs. In this study, we performed protease treatment to generate a new food material with smoother and softer texture compared with heat treated egg white. Our findings may expand the consumption of egg white, which can be consumed by people with mastication and swallowing disorders, and reduce the waste of egg white as a surplus product.
Introduction
Diet composition and food texture are critical for people who have mastication and swallowing disorders, i.e., dysphagia, associated with many medical conditions. As mastication and swallowing are the first steps of the digestion and nutrient absorption processes, problems with initial food processing in the oral cavity negatively affect energy and nutrient intake.
During mastication, solid foods are mechanically broken and then mixed with saliva to form a bolus of appropriate hardness and size that is easier to swallow. Bolus formation is affected by the physical properties of food such as hardness, moisture and fat content and structure. Therefore, to maintain oral food intake and improve the nutritional status for dysphagic patients, food products with a modified texture are often necessary. Currently, many foods with improved physical properties have been developed and characterized.
In this study, we attempted to generate a food product with high nutritional value that is easy to swallow. Egg white was selected for this purpose because it is rich in high-quality protein and is easy to obtain as a surplus product during the manufacture of several food products such as mayonnaise. Furthermore, egg white generally subjected to heat-treatment before consumption for hygienic reasons, and it turns into a gel with heat-treatment. Some people with mastication and swallowing disorders have difficulties eating heat-treated egg white gel because of the physical changes that occur with heat-treatment. Heat-treated egg white gel is elastic and breaks when eaten, and when the heat-treated egg white gel is reduced to very small sized pieces in the process of preparation or mastication, it results in discrete pieces that are unable to hold together well.
The aim of this study was to produce egg white-based gels with a modified texture by using proteases. We have already developed innovative food materials with altered physical properties by using Molsin, an Aspergillus saitoi protease preparation, which also contains β-glucosidase (Yoshida 1956; Ichishima and Yoshida 1965). Thus, Molsin has been applied to improve the texture of soy curd and convert isoflavone into aglycon via β-glucosidase activity; the resulting gel had a smooth texture and had increased nutritional value because of higher availability of aglycon (Nishinoaki et al. 2008). These data indicate that enzymatic treatment can be successfully applied to improving the texture and nutritional value of foods and developing new healthy food products for people with dysphagia.
In the present study, we subjected heat-treated egg white to protease digestion and the resultant product was analyzed for hardness by using a creepmeter and for structure by electron microscopy. In addition, we evaluated product properties by performing sensory tests and measuring mastication parameters by surface electromyography (sEMG) to assess changes in muscular nerve potential during jaw masseter muscle contraction and extension. Mastication energy, jaw movement, and chewing force have been widely used to analyze the physical properties of foods (Karkazis and Kossioni 1997; Mioche et al. 2003; Lee et al. 2009; Kohyama et al. 2010; Ishihara et al. 2011; Shimada et al. 2012). Our results indicate that protease treatment of heated egg white gel improved its texture and facilitated mastication.
Materials and Methods
Materials
Hen eggs were purchased from a local market. Protease used in the experiments (Protease N Amano G; Amano Enzyme Inc., Aichi, Japan) had the activity of 3.51 × 105 U/g determined by the method of Anson–Hagiwara (Hagiwara 1956) using casein as a substrate.
Preparation of Egg White Gels and Enzymatic Treatment
Egg white (235 g) was poured into a stainless-steel mold (8 × 12 × 5 cm) and heated at 76.5C for 30 min, then quickly cooled to room temperature by immersing in ice-cold water for 20 min and refrigerated at 4C for 1 h. The resulting gels were cut into 2 × 2 × 2 cm cubes which were covered with 0.5 mm thick absorbent cotton and soaked in 1.5 mL of protease solution (0.003, 0.05 and 0.2%, pH 5.2) containing 5% NaCl to prevent microbiological spoilage, for 96 h at 4C. A control sample was treated under the same conditions without protease.
Mechanical Texture Measurements
Mechanical texture measurements were performed using a creepmeter (RE2-33005S; Yamaden Co., Ltd., Tokyo, Japan). Each sample was pressed with a plunger (3 mm in diameter) at the sample holder speed of 1 mm/s.
Scanning Electron Microscopy (SEM)
Egg white gels were sliced from the inside with a razor (FH-10; Feather Safety Razor Co., Ltd., Osaka, Japan) into pieces of about 5 × 5 × 5 mm. Samples were fixed in 3% glutaraldehyde in 88 mM phosphate buffer (pH 7.4) at 4C for 3 days, washed in 100 mM phosphate buffer (pH 7.4) and dehydrated by passing through graded ethyl alcohol solutions (50–100%). Then, samples were treated with t-butanol (50–100%), dried in a centrifugal concentrator (VC-360; Taitec Co., Ltd., Saitama, Japan), mounted on aluminum stubs (OP-51436; Keyence Corporation, Osaka, Japan) with carbon double-sided tape (Nisshin EM Corporation, Tokyo, Japan), and coated with gold using Quick Coater (SC-701; Sanyu Electron Co., Ltd., Tokyo, Japan). The samples were observed under an SE microscope (VE-7800; Keyence Corporation, Osaka, Japan) at 20 kV.
Transmission Electron Microscopy (TEM)
Small gel pieces (2 × 2 × 2 mm) cut from the inside of the gel samples were fixed with 2.5% glutaraldehyde in 90 mM cacodylate buffer (pH 7.4) for 12 h at room temperature, washed with 100 mM cacodylate buffer (pH 7.4), and post-fixed with 2% osmium tetraoxide for 1.5 h at room temperature. After washing with 100 mM cacodylate buffer (pH 7.4), samples were dehydrated using a graded series of ethanol solutions (50–100%), transferred to propylene oxide, and incubated in propylene oxide/epoxy resin (2:1, 1:1 and 1:2, respectively). The samples were then embedded in 100% epoxy resin (EPON 812; TAAB Laboratories Equipment Ltd, Berkshire, UK) and polymerized at 35C for 12 h, 45C for 12 h and 60C for 48 h. Sections (60-nm thick) were cut using an ultramicrotome (UCT; Leica Microsystems GmbH, Wetzlar, Germany), stained with uranyl acetate followed by lead citrate, and observed under an electron microscope (JEM-1011; JEOL Ltd., Tokyo, Japan).
SDS-Polyacrylamide Gel Electrophoresis (SDS-PAGE)
Protein samples (9 µg) were prepared in 62.5 mM Tris–HCl, pH 6.8, containing 2% SDS, 15% glycerol and 5% β-mercaptoethanol, and loaded into 10–20% premade gels (Multigel II mini 10/20; Cosmo Bio Co., Ltd., Tokyo, Japan). SDS-PAGE was performed at a constant current of 20 mA in 49.5 mM Tris–glycine, pH 8.3, containing 0.1% SDS. Gels were stained with Coomassie Brilliant Blue R250 and destained in methanol/acetic acid/water (75:21:204 v/v/v, respectively). Molecular weight markers Daiichi III (Daiichi Kagaku Yakuhin, Tokyo, Japan) were used to assess protein size.
Sensory Evaluation
Ten students of the Fukuoka Women's University (20–24 years old) were recruited for sensory evaluation. They were asked to assess product texture of control gels and 0.2% protease-treated gels served as 2 × 2 × 2 cm cubes. For training purposes, the participants were asked to perform the procedure multiple times before actual experiment. Five texture parameters were evaluated: (1) softness, (2) smoothness, (3) elasticity, (4) residue feeling in mouth and (5) overall texture appreciation. Comparative evaluation was conducted using a bipolar scale (hard–soft; not smooth–smooth, strong–weak, many–few, bad–good) and scores from 1 to 7 (1, very much; 2, rather; 3, slightly; 4, usually; 5, slightly; 6, rather; 7, very much). Samples were placed on alumite plates coded by random three-digit numbers and served at 13.8 ± 1.6C. The room was kept at 23C.
Evaluation of Egg White Gels by sEMG
Participants, men (n = 2) and women (n = 3) aged 20–22 years, were recruited among people who did not have current or previous abnormalities in jaw function or the oral cavity. Data were collected from 20 sEMG measurements using a surface electromyography system equipped with the IEMG software (Oisaka Electronic Equipment Ltd., Hiroshima, Japan). Wet sensors and a Blue sensor M (M-00-S; Ambu A/S, Ballerup, Denmark) were taped to the masseter muscle where movements could be clearly detected. To ensure that the sensor was accurately attached, the skin was wiped with cotton dampened in 70% ethanol to remove moisture and oil. The attachment points along the same muscle fiber were searched in the process of chewing and bipolar electrodes were taped in tandem at 1 cm intervals. As a reference, a sensor was attached to the left elbow bone to avoid influence of muscle and fat tissues (all subjects were right-handed). The room temperature was maintained at 23C, and the samples were kept at 4C. Food samples were placed on a spoon and eaten ad libitum.
The start of mastication and the end of swallowing were indicated by the participants who were instructed to concentrate on eating and avoid movement during mastication to minimize disturbance of the electric potential.
Data Acquisition from sEMG
From the start to the end of chewing, the change in the muscle potential was captured by a computer. Total muscle work, peak-to-peak voltage (Vp-p), and mastication duration were measured (Fig. 1A). Total muscle work is the intensity of the total EMG activity for the muscle during the entire masticatory sequence calculated by the IEMG software and divided by the mastication time (Fig. 1B), and Vp-p is the maximum amplitude (Fig. 1A). Mastication duration was determined from the start of masseter muscle response to the end of mastication after swallowing the bolus.
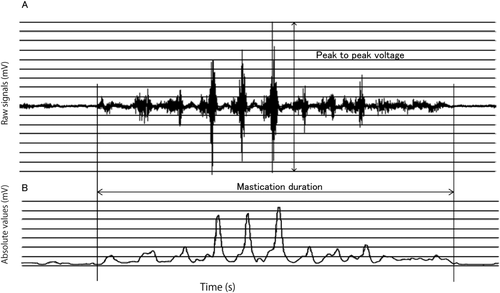
Representative Mastication Recordings Using Electromyography
(A) Signals from the masseter muscle. (B) Power Frequency analysis of the signals by the IEMG software. All signals were rectified.
Data acquisition was performed under the following conditions: sampling speed was 3 kHz, the low-pass filter (LPD) was automatically determined as 44.23 kHz, and the high-pass filter (HPF) was 2 Hz. The signals were amplified 10,000 fold.
Statistical Analysis
To assess the differences between the control and protease-treated gels, each dataset (total muscle work, Vp-p, mastication duration) was analyzed by paired t-test. Sensory evaluation data were analyzed by ANOVA using F-test and then Welch t-test. The analyses were performed using the JMP 9.02 statistical software (SAS Institute Inc., Cary, NC).
Results
Physical Properties
Figure 2 shows stress–strain curves of the control gels (A) or with 0.003, 0.05 and 0.2% protease preparation (B−D). Stress–strain curves of the 0.2% protease-treated gels were significantly different from those of the control gels in waveform morphology. A typical waveform for the control gel was characterized by a linear stress increase during the first 20% of strain, which indicates elasticity, while rupture was clearly detected at the elastic deformation point. In contrast, the waveform for the 0.2% protease-treated gels did not show any obvious rupture point, and the gels exhibited ductile flow. The stress–strain curves of all protease-treated gels were lower than those of the control gel, and the effect was more pronounced at higher protease concentrations, indicating that gel stress was decreased in a protease concentration-dependent manner. Therefore, the 0.2% protease-treated gel was used to compare protease treatment with control in the following experiments.
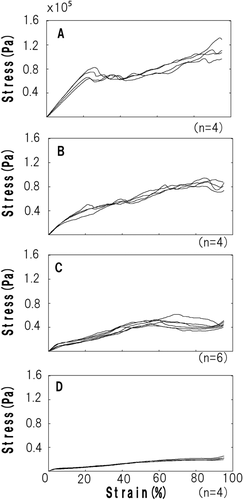
Stress–Strain Curves of the Egg White Gels
(A) Control gel. (B-D) Gels treated with different protease concentrations: (B) 0.003%, (C) 0.05% and (D) 0.2%.
Gel Appearance
Figure 3 shows the appearance of the egg white gels. Visual comparison indicated that there was no significant difference in the appearance between the control gel (A) and the 0.2% protease-treated gel (B). The protease-treated gel did not lose shape, but its corners were slightly rounded.
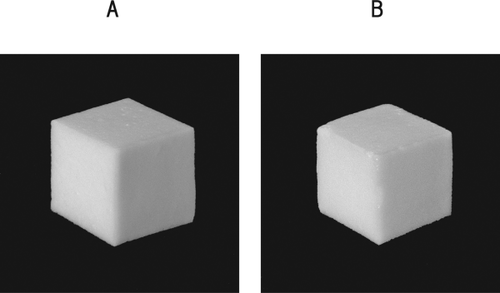
Appearance of the Control Gel (A) and 0.2% Protease-Treated Gel (B)
Gel Structure
The SEM analysis revealed minor differences between the control and 0.2% protease-treated gels (Fig. 4).
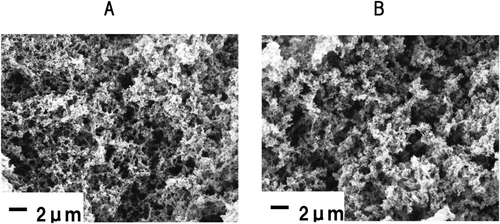
Scanning Electron Micrographs of the Control Gel (A) and 0.2% Protease-Treated Gel (B)
According to TEM images, protein aggregates observed as black entities were more homogeneous in the 0.2% protease-treated gel than in the control gel (Fig. 5).
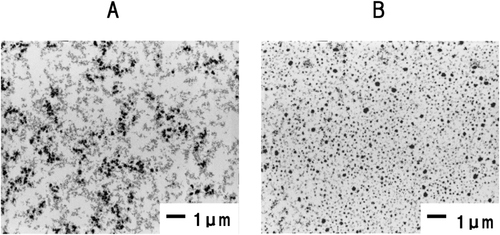
Transmission Electron Micrographs of the Control Gel (A) and 0.2% Protease-Treated Gel (B)
Protein Hydrolysis in Egg White Gels
SDS-PAGE analysis of protein composition revealed obvious degradation of ovalbumin and an increase in the proportion of low-molecular-weight proteins/peptides in the 0.2% protease-treated gel compared with the control gel (Fig. 6). In addition, some degradation of ovotransferrin was observed, while lysozyme seemed intact.
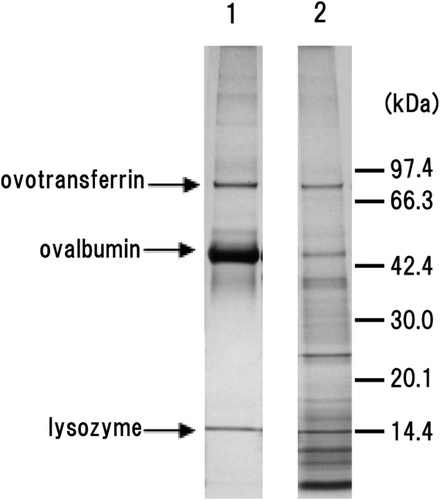
Representative Images of SDS-Page Separation of Egg White Proteins. Lane 1, Control Gel; Lane 2, 0.2% Protease-Treated Gel. Molecular Weight Markers are Shown on the Right
Sensory Evaluation
There were statistically significant differences between the 0.2% protease-treated gel and the control gel in softness, smoothness, elasticity, residue feeling in mouth and overall texture appreciation (P < 0.001; Table 1). The 0.2% protease-treated gel was evaluated as being softer, smoother, and less elastic and having less of a residue feeling in mouth than that in the control gel. In addition, the 0.2% protease-treated gel was highly preferred over the control gel.
| Control | Protease-treated gel | ||||
|---|---|---|---|---|---|
| AV | SD | AV | SD | t-test (Welch) P-value (Prob>|t|) | |
| (1) Softness | 1.6 | 0.70 | 7.0 | 0.0 | <0.0001a |
| (2) Smoothness | 1.8 | 0.92 | 7.0 | 0.0 | <0.0001a |
| (3) Elasticity | 1.8 | 0.92 | 7.0 | 0.0 | <0.0001a |
| (4) Residue feeling in mouth | 1.7 | 0.95 | 6.9 | 0.32 | <0.0001a |
| (5) Overall texture appreciation | 2.7 | 1.64 | 5.9 | 1.85 | 0.0007a |
- p < 0.001.
- AV, average; SD, standard deviation.
Mastication
The data were screened using the outlier analysis of jackknife distance; statistical analysis is presented in Table 2. No significant difference between the 0.2% protease-treated and control gel groups was observed in total muscle work (P = 0.53). However, Vp-p was significantly higher (P = 0.013) and the time between the first bite and completion of swallowing was significantly longer (P < 0.0001) for the control gel group compared with the protease-treated gel group. The Pearson's correlation coefficient showing the correlation between the parameters (total muscle work, Vp-p and mastication duration) of two samples for each subject was 0.54, 0.42 and 0.71, respectively, indicating medium correlativity in total muscle work and Vp-p, and high correlativity in mastication duration. Participants with long mastication of protease-treated gels tended to have extended mastication of control gels as well.
| Total muscle work (mV/s) | Vp-p (mV) | Mastication duration (s) | |
|---|---|---|---|
| Sample size | 16 | 16 | 16 |
| Mean of the 0.2% protease-treated gel | 100.3 | 0.016 | 8.09 |
| Mean of the control gel | 106.1 | 0.021 | 13.31 |
| Mean of a difference | −5.8 | −0.005 | −5.22 |
| Standard error | 9.0 | 0.002 | 0.78 |
| 95% of CI upper limit | 13.4 | −0.001 | −3.59 |
| 95% of CI lower limit | −25.0 | −0.009 | −6.84 |
| Pearson's correlation coefficient | 0.54 | 0.42 | 0.71 |
| t-value | −0.65 | −2.84 | −6.85 |
| P-value by a two-sided test | 0.53 | 0.013 | <0.0001 |
Discussion
In the present study, we succeeded in producing egg white gels with a new texture by sequentially applying protease treatment. The measurements of gel physical properties by using a creepmeter indicate that the protease-treated gels exhibited less overall stress than did the control gels, as evidenced by the absence of clear breaking points. In addition, stress–strain curves reveal ductile flow of the protease-treated gel, indicating that it was soft and extendable, probably because ovalbumin was readily degraded into smaller fragments by the protease. It is, therefore, likely that ovalbumin degradation accounts for the observed changes in the physical properties of the protease-treated gel. Nevertheless, the appearance of the control gel did not significantly differ from that of the protease-treated gel. It has been reported that in egg white gels, heating causes protein agglutination and changes in the protein-formed network (Clark 1998), which is further destroyed by protease treatment. However, even after protease digestion, the structure of the protein network did not immediately collapse and the gel retained its shape. Thus, unless the protease-treated gel is subjected to external force, its shape does not undergo deformation. After external force such as mastication is applied, soft and smooth texture of the deformed gel can be sensed.
According to sEMG, no difference was observed in total muscle work required for the mastication of the control and protease-treated gels; in contrast, Vp-p and mastication duration were significantly different. Total muscle work is indicated by the energy spent per second of mastication, Vp-p is the maximum force required to break the sample, and mastication duration is an indicator of how easily the food can be processed in the oral cavity. Overall, our results demonstrate that protease treatment of egg white gel shortens the time required to form the bolus to be swallowed but does not affect the energy spent for mastication.
Previous studies have shown that chewing time tends to be longer for hard food than for soft food (Karkazis and Kossioni 1997; Kohyama et al. 2005; Pereira et al. 2006). The hardness of the bolus affects food digestion and absorption rate in the elderly. Mioche et al. (2004) have reported a decline in the mastication ability of elderly people owing to the hardness of the bolus despite a long chewing period. According to electromyography, compared with younger people, elderly people have an inferior ability to masticate cooked meat, suggesting that even if food is well chewed, it may remain difficult to swallow. In the present study, we successfully applied protease treatment for the degradation of protein structure in the heat-treated egg white gel, thus transforming it into a softer material that is easier to swallow and requires less time for processing in the oral cavity. Thus, the protease-treated white egg gel can be used by elderly people with weakened chewing abilities and can be applied to develop novel food products with improved texture.
Conclusion
In this study, we produced a novel egg white gel by protease treatment. Ovalbumin, the major protein in egg white, was digested into small peptides; as a result, the gel exhibited ductile flow without any obvious rupture points and was easy to swallow as indicated by the electromyography analysis of the masseter muscle movements. Thus, our approach was successful in the production of the egg white gel with soft and smooth texture without compromising its original shape.
Acknowledgments
We thank Hayaka Mogami and Ikumi Matsuo for their technical assistance. This work was partly supported by JSPS KAKENHI grants (number 24500951 to J.F., 25242012 to T.A., and 23500951 to M.M), the fund from the Research Project on the Development of Agricultural Products and Foods with Health-promoting benefits (NARO), and by the User Science Institute of Kyushu University established by the Ministry of Education, Culture, Sports, Science and Technology as one of its “Strategic Research Center Developing Programs” funded by Special Coordination Funds for Promoting Science and Technology.




