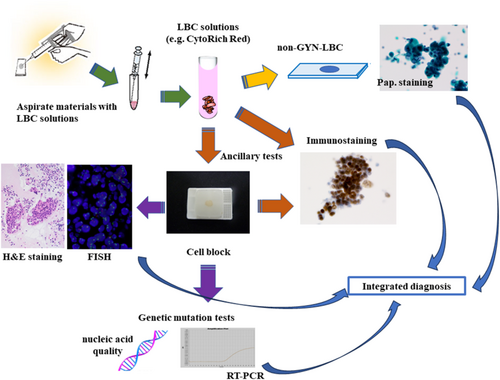
Advances in diagnostic liquid-based cytology
Hideyuki Abe
Department of Diagnostic Pathology, Kurume University Hospital, Kurume, Japan
Search for more papers by this authorCorresponding Author
Akihiko Kawahara
Department of Diagnostic Pathology, Kurume University Hospital, Kurume, Japan
Correspondence
Akihiko Kawahara, Department of Diagnostic Pathology, Kurume University Hospital, 67 Asahi-machi, Kurume, Fukuoka 830-0011, Japan.
Email: [email protected]
Search for more papers by this authorJun Akiba
Department of Diagnostic Pathology, Kurume University Hospital, Kurume, Japan
Search for more papers by this authorRin Yamaguchi
Department of Diagnostic Pathology, Nagasaki University Hospital, Nagasaki, Japan
Search for more papers by this authorHideyuki Abe
Department of Diagnostic Pathology, Kurume University Hospital, Kurume, Japan
Search for more papers by this authorCorresponding Author
Akihiko Kawahara
Department of Diagnostic Pathology, Kurume University Hospital, Kurume, Japan
Correspondence
Akihiko Kawahara, Department of Diagnostic Pathology, Kurume University Hospital, 67 Asahi-machi, Kurume, Fukuoka 830-0011, Japan.
Email: [email protected]
Search for more papers by this authorJun Akiba
Department of Diagnostic Pathology, Kurume University Hospital, Kurume, Japan
Search for more papers by this authorRin Yamaguchi
Department of Diagnostic Pathology, Nagasaki University Hospital, Nagasaki, Japan
Search for more papers by this authorAbstract
Liquid-based cytology (LBC) has changed the landscape of gynaecological cytology. A growing demand exists for LBC in diagnostic cytology, particularly for ancillary testing, such as immunocytochemistry and molecular testing. Ancillary testing solely based on conventional preparation (CP) methods remains challenging. Recently, the increased demand for specialist testing and minimally invasive techniques, such as endoscopic ultrasonography fine-needle aspiration, to obtain cellular samples has led to an increasing demand for ancillary testing on cytology LBC supernatant, slides and cell block (CB). This facilitates the diagnosis and prognosis in cytology samples enabling personalized treatment. An understanding of the history and future prospects of LBC is crucial for its application in routine diagnostics by cytopathologists and cytotechnologists. In this review, we initiated an internet search using the keyword ‘liquid-based cytology’, and we conducted a literature review to discuss the usefulness of combined diagnosis of LBC and CP, immunocytochemistry and molecular testing and assessed the quality of nucleic acids in diagnostic LBC. High-quality and cell-rich diagnostic LBC surpassed the CP method alone in terms of reliability and versatility of ancillary testing in cytological diagnosis. Conclusively, diagnostic LBC lends itself to various new technologies and is expected to continue evolving with innovations in the future.
Graphical Abstract
Cancer is a disease with highly diverse forms and characteristics. Diagnostic LBC is an essential tool for understanding this diversity of cancer.
This study aims to review the literature on diagnostic liquid-based cytology. Additionally, it provides an overview of the cytological applications of non-gynaecological liquid-based cytology.
CONFLICT OF INTEREST STATEMENT
The authors made no disclosures.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1Hajdu SI. The first cytopathologist. J Am Soc Cytopathol. 2014; 3: 5-6.
- 2Naylor B, Tasca L, Bartziota E, Schneider V. In Romania it's the Méthode Babeş-Papanicolaou. Acta Cytol. 2002; 46: 1-12.
- 3Papanicolaou GN, Traut HF. The diagnostic value of vaginal smears in carcinoma of the uterus. Am J Obstet Gynecol. 1941; 42: 139-206.
- 4Cancer of the cervix: Death by incompetence (editorial). Lancet. 1985; 2: 363-364.
- 5Howell LP, Wilton M, Bishop J, Afify A. Living with uncertainty: equivocal pap test results and the evolution of ASC terminology. Diagn Cytopathol. 2010; 38: 221-232.
- 6Papillo JL, Zarka MA, St John TL. Evaluation of the ThinPrep pap test in clinical practice. A seven-month, 16,314-case experience in northern Vermont. Acta Cytol. 1998; 42: 203-208.
- 7Linder J. Recent advances in thin-layer cytology. Diagn Cytopathol. 1998; 18: 24-32.
10.1002/(SICI)1097-0339(199801)18:1<24::AID-DC5>3.0.CO;2-U CAS PubMed Web of Science® Google Scholar
- 8Rezende MT, Bianchi AGC, Carneiro CM. Cervical cancer: automation of pap test screening. Diagn Cytopathol. 2021; 49: 559-574.
- 9Ferenczy A, Braun L, Shah KV. Human papillomavirus (HPV) in condylomatous lesions of cervix. Am J Surg Pathol. 1981; 5: 661-670.
- 10Pinto AP, Carvalho MC, Kolb S, Tirone FA, Maia LR, Escobar CS. Value of cytology in papillary condylomatous lesions of the cervix. Acta Cytol. 2007; 51: 51-60.
- 11Richart RM. Screening. The next century. Cancer. 1995; 76: 1919-1927.
10.1002/1097-0142(19951115)76:10+<1919::AID-CNCR2820761308>3.0.CO;2-9 CAS PubMed Web of Science® Google Scholar
- 12Nayar R, Wilbur DC. The Bethesda system for reporting cervical cytology: a historical perspective. Acta Cytol. 2017; 61: 359-372.
- 13Pangarkar MA. The Bethesda system for reporting cervical cytology. Cytojournal. 2022; 30(19): 28.
10.25259/CMAS_03_07_2021 Google Scholar
- 14Gibb RK, Martens MG. The impact of liquid-based cytology in decreasing the incidence of cervical cancer. Rev Obstet Gynecol. 2011; 4: S2-S11.
- 15Dasgupta S. The efficiency of cervical pap and comparison of conventional pap smear and liquid-based cytology: a review. Cureus. 2023; 15:e48343.
- 16Puliti D, Ciatto S, Confortini M, Sani C, Zappa M. Comparison of the conventional cervical smear and liquid-based cytology: results of a controlled, prospective study in the Abruzzo region of Italy. Acta Cytol. 2008; 52: 568-574.
- 17Fremont-Smith M, Marino J, Griffin B, Spencer L, Bolick D. Comparison of the SurePath liquid-based Papanicolaou smear with the conventional Papanicolaou smear in a multisite direct-to-vial study. Cancer. 2004; 102: 269-279.
- 18McQueen F, Duvall E. Using a quality control approach to define an ‘adequately cellular’ liquid-based cervical cytology specimen. Cytopathology. 2006; 17: 168-174.
- 19Arbyn M, Herbert A, Schenck U, et al. European guidelines for quality assurance in cervical cancer screening: recommendations for collecting samples for conventional and liquid-based cytology. Cytopathology. 2007; 18: 133-139.
- 20Norimatsu Y, Shigematsu Y, Sakamoto S, et al. Nuclear features in endometrial cytology: comparison of endometrial glandular and stromal breakdown and endometrioid adenocarcinoma grade 1. Diagn Cytopathol. 2012; 40: 1077-1082.
- 21Sams SB, Currens HS, Raab SS. Liquid-based Papanicolaou tests in endometrial carcinoma diagnosis. Performance, error root cause analysis, and quality improvement. Am J Clin Pathol. 2012; 137: 248-254.
- 22Norimatsu Y, Yanoh K, Kobayashi TK. The role of liquid-based preparation in the evaluation of endometrial cytology. Acta Cytol. 2013; 57: 423-435.
- 23Yanoh K, Norimatsu Y, Munakata S, et al. Evaluation of endometrial cytology prepared with the Becton Dickinson SurePath™ method: a pilot study by the Osaki study group. Acta Cytol. 2014; 58: 153-161.
- 24Fulciniti F, Yanoh K, Karakitsos P, et al. The Yokohama system for reporting directly sampled endometrial cytology: the quest to develop a standardized terminology. Diagn Cytopathol. 2018; 46: 400-412.
- 25Norimatsu Y, Maeda Y, Malara N, Fulciniti F, Kobayashi TK. A review of the directly sampled endometrial cytology on LBC samples: classification, microscopic criteria and beyond. Cytopathology. 2024; 35: 350-361.
- 26Kenyon S, Sweeney BJ, Happel J, Marchilli GE, Weinstein B, Schneider D. Comparison of BD Surepath and ThinPrep pap systems in the processing of mucus-rich specimens. Cancer Cytopathol. 2010; 118: 244-249.
- 27Son SM, Koo JH, Choi SY, et al. Evaluation of urine cytology in urothelial carcinoma patients: a comparison of CellprepPlus® liquid-based cytology and conventional smear. Korean J Pathol. 2012; 46: 68-74.
- 28Norimatsu Y, Kawanishi N, Shigematsu Y, Kawabe T, Ohsaki H, Kobayashi TK. Use of liquid-based preparations in urine cytology: an evaluation of Liqui-PREP and BD SurePath. Diagn Cytopathol. 2010; 38: 702-704.
- 29Chong Y, Baek KH, Kim JY, Kim TJ, Lee EJ, Kang CS. Comparison of EASYPREP® and SurePath® in thyroid fine-needle aspiration. Diagn Cytopathol. 2016; 44: 283-290.
- 30Lee YM, Hwang JY, Son SM, et al. Comparison of diagnostic accuracy between CellprepPlus® and ThinPrep® liquid-based preparations in effusion cytology. Diagn Cytopathol. 2014; 42: 384-390.
- 31Zhao J, Yao X, Song C, Wang C. A comparative study of two liquid-based preparation methods: membrane-based and sedimentation in fine needle aspiration cytology diagnosis in thyroid nodules. World J Surg Oncol. 2020; 18: 13.
- 32Belsley NA, Tambouret RH, Misdraji J, Muzikansky A, Russell DK, Wilbur DC. Cytologic features of endocervical glandular lesions: comparison of SurePath, ThinPrep, and conventional smear specimen preparations. Diagn Cytopathol. 2008; 36: 232-237.
- 33Morii E, Hatanaka Y, Motoi N, et al. Guidelines for handling of cytological specimens in cancer genomic medicine. Pathobiology. 2023; 90: 289-311.
- 34Kawahara A, Taira T, Abe H, et al. Fixation effect of SurePath preservative fluids using epidermal growth factor receptor mutation-specific antibodies for immunocytochemistry. Cancer Cytopathol. 2014; 122: 145-152.
- 35Risse EK, Ouwerkerk-Noordam E, Boon ME. Endometrial cells in liquid-based cervical cytology: a diagnostic pitfall solved by preparing cytohistology from the residual thin layer sample. Acta Cytol. 2011; 55: 327-333.
- 36Tyler KL, Selvaggi SM. Morphologic features of prostatic adenocarcinoma on ThinPrep® urinary cytology. Diagn Cytopathol. 2011; 39: 101-104.
- 37Makde MM, Sathawane P. Liquid-based cytology: technical aspects. Cytojournal. 2022; 19: 41.
- 38Tisserand P, Fouquet C, Marck V, et al. ThinPrep-processed fine-needle samples of breast are effective material for RNA- and DNA-based molecular diagnosis: application to p53 mutation analysis. Cancer. 2003; 99: 223-232.
- 39Lee JK, Choi ER, Jang TH, et al. A prospective comparison of liquid-based cytology and traditional smear cytology in pancreatic endoscopic ultrasound-guided fine needle aspiration. Acta Cytol. 2011; 55: 401-407.
- 40Shukla S, Einstein A, Shukla A, Mishra D. Comparison of specimen adequacy and smear quality in oral smears prepared by manual liquid-based cytology and conventional methods. J Oral Maxillofac Pathol. 2015; 19: 315-318.
- 41Norimatsu Y, Ohsaki H, Masuno H, Kagawa A, Teramoto N, Kobayashi TK. Efficacy of CytoLyt® hemolytic action on ThinPrep® LBC using cultured osteosarcoma cell line LM8. Acta Cytol. 2014; 58: 76-82.
- 42Thompson CH, Rose BR. Deleterious effects of formalin/acetic acid/alcohol (FAA) fixation on the detection of HPV DNA by in situ hybridization and the polymerase chain reaction. Pathology. 1991; 23: 327-330.
- 43Kotikalapudi R, Patel RK, Katragadda S. Standardization of DNA extraction from methanol acetic acid fixed cytogenetic cells of cattle and buffalo. Pak J Biol Sci. 2013; 16: 1823-1825.
- 44McMenamin M, McKenna M. Stability of human papillomavirus (HPV) in cervical ThinPrep specimens previously lysed with glacial acetic acid: effect on cobas 4800 HPV test performance. Cancer Cytopathol. 2014; 122: 250-256.
- 45Hoda RS. Non-gynecologic cytology on liquid-based preparations: a morphologic review of facts and artifacts. Diagn Cytopathol. 2007; 35: 621-634.
- 46Bürger M, Heidrich A, Petersen I, Stallmach A, Schmidt C. Increased accuracy of FNA-based cytological diagnosis of pancreatic lesions by use of an ethanol-based fixative system: a STROBE compliant study. Medicine (Baltimore). 2022; 101:e30449.
- 47Rossi ED, Morassi F, Santeusanio G, Zannoni GF, Fadda G. Thyroid fine needle aspiration cytology processed by ThinPrep: an additional slide decreased the number of inadequate results. Cytopathology. 2010; 21: 97-102.
- 48Sharma V, Gupta V, Parmar P, Jain P, Thakran D, Sen R. Comparative analysis of liquid based and conventional cytology smears in fine needle aspirates from breast lesions. J Cytol. 2019; 36: 89-93.
- 49Martini M, Capodimonti S, Cenci T, et al. To obtain more with less: cytologic samples with ancillary molecular techniques-the useful role of liquid-based cytology. Arch Pathol Lab Med. 2018; 142: 299-307.
- 50Rossi D, Belotti A, di Tonno C, et al. Changes in thyroid fine needle aspiration practice during the COVID-19 pandemic. Cytopathology. 2021; 32: 732-737.
- 51Cox JT. Liquid-based cytology: evaluation of effectiveness, cost-effectiveness, and application to present practice. J Natl Compr Canc Netw. 2004; 2: 597-611.
- 52Armstrong SF, Guest JF. Cost-effectiveness and cost-benefit of cervical cancer screening with liquid based cytology compared with conventional cytology in Germany. Clinicoecon Outcomes Res. 2020; 12: 153-166.
- 53Zendehrokh N, Olejnicka B, Westman A, Dejmek A. Liquid-based cytology using CytoRich red/Tripath is diagnostically equivalent to conventional smears for bronchial washings and brushings and reduces the cost. Diagn Cytopathol. 2013; 41: 876-884.
- 54Feoli F, Ameye L, Van Eeckhout P, Paesmans M, Marra V, Arisio R. Liquid-based cytology of the breast: pitfalls unrecognized before specific liquid-based cytology training - proposal for a modification of the diagnostic criteria. Acta Cytol. 2013; 57: 369-376.
- 55Rossi E, Bizzarro T, Martini M, et al. The role of liquid based cytology and ancillary techniques in the peritoneal washing analysis: our institutional experience. PLoS One. 2017; 12:e0168625.
- 56Archondakis S, Roma M, Kaladelfou E. Two-year experience of the implementation of the Milan for reporting salivary gland cytopathology at a private medical laboratory. Head Neck Pathol. 2021; 15: 780-786.
- 57Parfitt JR, McLachlin CM, Weir MM. Comparison of ThinPrep and conventional smears in salivary gland fine-needle aspiration biopsies. Cancer. 2007; 111: 123-129.
- 58Sharma S, Agarwal S, Jain M, Singh GB, Andley M. Cytomorphological differences between liquid-based cytology and conventional smears in fine-needle aspirates of thyroid lesions. J Cytol. 2018; 35: 208-211.
- 59Gerhard R, Schmitt FC. Liquid-based cytology in fine-needle aspiration of breast lesions: a review. Acta Cytol. 2014; 58: 533-542.
- 60Budhwar A, Kataria SP, Kumar S, Singh G, Kaushik N, Sen R. Fine needle aspiration cytology of cervical lymph nodes: comparison of liquid based cytology (SurePath) and conventional preparation. Diagn Cytopathol. 2021; 49: 18-24.
- 61Doxtader EE, Cheng YW, Zhang Y. Molecular testing of non-small cell lung carcinoma diagnosed by endobronchial ultrasound-guided transbronchial fine-needle aspiration: the cleveland clinic experience. Arch Pathol Lab Med. 2019; 143: 670-676.
- 62Sato A, Matsuda K, Motoyama T, et al. 53BP1 expression as a biomarker to differentiate thyroid follicular tumors. Endocr Connect. 2021; 10: 309-315.
- 63Osugi M, Kinoshita K, Sugita A, Kito K, Maeda T. Expression of p63 immunostaining in liquid-based cytology (BD SurePath) of breast fine-needle aspiration. Diagn Cytopathol. 2018; 46: 845-852.
- 64Tripathy K, Mishra A, Singh AK, Panda PL, Mahapatra A, Lenka A. Immunocytochemistry using liquid-based cytology: a tool in hormone receptor analysis of breast cancer. J Cytol. 2018; 35: 260-264.
- 65Fan YB, Wu X, Fu ZM, Wu GP. Clinical application of the SurePath liquid-based pap test in cytological screening of bronchial brushing for the diagnosis of lung cancer. Cytotechnology. 2010; 62: 53-59.
- 66Ryu HS, Park IA, Park SY, Jung YY, Park SH, Shin HC. A pilot study evaluating liquid-based fine needle aspiration cytology of breast lesions: a cytomorphological comparison of SurePath® liquid-based preparations and conventional smears. Acta Cytol. 2013; 57: 391-399.
- 67Kim SY, Kim EK, Moon HJ, et al. Combined use of conventional smear and liquid-based preparation versus conventional smear for thyroid fine-needle aspiration. Endocrine. 2016; 53: 157-165.
- 68Pao W, Miller V, Zakowski M, et al. EGF receptor gene mutations are common in lung cancers from ‘never smokers’ and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci U S A. 2004; 101: 13306-13311.
- 69Soda M, Takada S, Takeuchi K, et al. A mouse model for EML4-ALKpositive lung cancer. Proc Natl Acad Sci U S A. 2008; 105: 19893-19897.
- 70Kawahara A, Yamamoto C, Nakashima K, et al. Molecular diagnosis of activating EGFR mutations in non-small cell lung cancer using mutation-specific antibodies for immunohistochemical analysis. Clin Cancer Res. 2010; 16: 3163-3170.
- 71Abe H, Kawahara A, Azuma K, et al. Heterogeneity of anaplastic lymphoma kinase gene rearrangement in non-small-cell lung carcinomas: a comparative study between small biopsy and excision samples. J Thorac Oncol. 2015; 10: 800-805.
- 72Schmitt F, Cochand-Priollet B, Toetsch M, Davidson B, Bondi A, Vielh P. Immunocytochemistry in Europe: results of the European Federation of Cytology Societies (EFCS) inquiry. Cytopathology. 2011; 22: 238-242.
- 73Srebotnik Kirbiš I, Rodrigues Roque R, Bongiovanni M, Strojan Fležar M, Cochand-Priollet B. Immunocytochemistry practices in European cytopathology laboratories-review of European Federation of Cytology Societies (EFCS) online survey results with best practice recommendations. Cancer Cytopathol. 2020; 128: 757-766.
- 74Aikawa E, Kawahara A, Hattori S, et al. Comparison of the expression levels of napsin a, thyroid transcription factor-1, and p63 in nonsmall cell lung cancer using cytocentrifuged bronchial brushings. Cancer Cytopathol. 2011; 119: 335-345.
- 75Dalin MG, Katabi N, Persson M, et al. Multi-dimensional genomic analysis of myoepithelial carcinoma identifies prevalent oncogenic gene fusions. Nat Commun. 2017; 8: 1197.
- 76Haller F, Skálová A, Ihrler S, et al. Nuclear NR4A3 immunostaining is a specific and sensitive novel marker for acinic cell carcinoma of the salivary glands. Am J Surg Pathol. 2019; 43: 1264-1272.
- 77Skaugen JM, Seethala RR, Chiosea SI, Landau MS. Evaluation of NR4A3 immunohistochemistry (IHC) and fluorescence in situ hybridization and comparison with DOG1 IHC for FNA diagnosis of acinic cell carcinoma. Cancer Cytopathol. 2021; 129: 104-113.
- 78Zhao LL, Sun Y, Wang C, et al. Clinical study of brush liquid-based cytology combined with automatic immunohistochemistry in the classification and diagnosis of lung cancer. Zhonghua Zhong Liu Za Zhi. 2019; 41: 326-330.
- 79Wise O, Howard MR. Thyroid cytology: a review of current international reporting systems and emerging developments. Cytopathology. 2016; 27: 161-167.
- 80Chong Y, Ji SJ, Kang CS, Lee EJ. Can liquid-based preparation substitute for conventional smear in thyroid fine-needle aspiration? A systematic review based on meta-analysis. Endocr Connect. 2017; 6: 817-829.
- 81Baum JE, Soong L, Scognamiglio T, Margolskee EM, Hoda RS, Rao R. Cytological diagnosis of papillary thyroid carcinoma with tall cells on ThinPrep liquid-based cytology. Diagn Cytopathol. 2019; 47: 541-546.
- 82Luu MH, Fischer AH, Pisharodi L, Owens CL. Improved preoperative definitive diagnosis of papillary thyroid carcinoma in FNAs prepared with both ThinPrep and conventional smears compared with FNAs prepared with ThinPrep alone. Cancer Cytopathol. 2011; 119: 68-73.
- 83Maurya MK, Yadav R, Kumar M, Singh HP, Mishra A, Goel MM. A comparative analysis of liquid-based cytology and conventional smears in fine-needle aspirates of thyroid lesions. Cureus. 2023; 15:e45353.
- 84Suzuki A, Hirokawa M, Higuchi M, et al. Cytological characteristics of papillary thyroid carcinoma on LBC specimens, compared with conventional specimens. Diagn Cytopathol. 2015; 43: 108-113.
- 85Mahajan S, Rajwanshi A, Srinivasan R, Radotra BD, Panda N. Should liquid based cytology (LBC) be applied to thyroid fine needle aspiration cytology samples?: comparative analysis of conventional and LBC smears. J Cytol. 2021; 38: 198-202.
- 86Fischer AH, Clayton AC, Bentz JS, et al. Performance differences between conventional smears and liquid-based preparations of thyroid fine-needle aspiration samples: analysis of 47,076 responses in the college of american pathologists interlaboratory comparison program in non-gynecologic cytology. Arch Pathol Lab Med. 2013; 137: 26-31.
- 87Nagarajan N, Schneider EB, Ali SZ, Zeiger MA, Olson MT. How do liquid-based preparations of thyroid fine-needle aspiration compare with conventional smears? An analysis of 5475 specimens. Thyroid. 2015; 25: 308-313.
- 88Robinson I. A diagnostic head and neck fine needle aspiration service can be provided using liquid-based cytology only. Cytopathology. 2017; 28: 24-30.
- 89Sadullahoğlu C, Yıldırım HT, Nergiz D, et al. The risk of malignancy according to Milan reporting system of salivary gland fine-needle aspiration with Becton Dickinson SurePath liquid-based processing. Diagn Cytopathol. 2019; 47: 863-868.
- 90Kord S, Mokhtari M, Tahmasebi S. Comparison of liquid-based and conventional cytology in diagnosis of breast mass. J Cytol. 2019; 36: 22-27.
- 91Scarpa Carniello JV, Pareja F, Santos-Zabala ML, Edelweiss M. Diagnostic dilemmas and pitfalls in ThinPrep® cytology of breast fine needle aspiration biopsy: report of six cases with histological correlates. Diagn Cytopathol. 2017; 45: 655-661.
- 92Schiettecatte A, Bourgain C, Breucq C, Buls N, de Wilde V, de Mey J. Initial axillary staging of breast cancer using ultrasound-guided fine needle aspiration: a liquid-based cytology study. Cytopathology. 2011; 22: 30-35.
- 93Nishimura R, Aogi K, Yamamoto T, et al. Usefulness of liquid-based cytology in hormone receptor analysis of breast cancer specimens. Virchows Arch. 2011; 458: 153-158.
- 94Campion MB, Kipp BR, Humphrey SK, Zhang J, Clayton AC, Henry MR. Improving cellularity and quality of liquid-based cytology slides processed from pancreatobiliary tract brushings. Diagn Cytopathol. 2010; 38: 627-632.
- 95Govil H, Reddy V, Kluskens L, et al. Brush cytology of the biliary tract: retrospective study of 278 cases with histopathologic correlation. Diagn Cytopathol. 2002; 26: 273-277.
- 96Taira T, Kawahara A, Yamaguchi T, et al. Morphometric image analysis of pancreatic disease by ThinPrep liquid-based cytology. Diagn Cytopathol. 2012; 40: 970-975.
- 97Cermak TS, Wang B, DeBrito P, Carroll J, Haddad N, Sidawy MK. Optimizing visualization of the sampled material by omitting the preparation of direct smears and rinsing the entire sample in liquid-based media demonstrated a trend toward improving the diagnostic rate. Cancer Cytopathol. 2012; 120: 319-325.
- 98Kitano M, Yoshida T, Itonaga M, Tamura T, Hatamaru K, Yamashita Y. Impact of endoscopic ultrasonography on diagnosis of pancreatic cancer. J Gastroenterol. 2019; 54: 19-32.
- 99Ko SH, Pyo JS, Son BK, Lee HY, Oh IW, Chung KH. Comparison between conventional smear and liquid-based preparation in endoscopic ultrasonography-fine needle aspiration cytology of pancreatic lesions. Diagnostics (Basel). 2020; 10: 293.
- 100Chandan S, Mohan BP, Khan SR, et al. Comparison of EUS-guided conventional smear and liquid-based cytology in pancreatic lesions: a systematic review and meta-analysis. Endosc Int Open. 2020; 8: E1611-E1622.
- 101Yan X, Zhou G, Ji J, et al. Evaluation of the diagnostic efficacy of liquid-based cytology obtained via percutaneous ultrasound-guided fine-needle aspiration for pancreatic masses: a large tertiary center's 8-year experience. J Cancer Res Clin Oncol. 2023; 149: 17189-17197.
- 102Zhou W, Gao L, Wang SM, et al. Comparison of smear cytology and liquid-based cytology in EUS-guided FNA of pancreatic lesions: experience from a large tertiary center. Gastrointest Endosc. 2020; 91: 932-942.
- 103Yeon MH, Jeong HS, Lee HS, et al. Comparison of liquid-based cytology (CellPrepPlus) and conventional smears in pancreaticobiliary disease. Korean J Intern Med. 2018; 33: 883-892.
- 104Li Z, Tabbara SO, Nwosu A, et al. Pancreaticobiliary cytology practice in 2021: results of a college of American pathologists survey. Arch Pathol Lab Med. 2024; 148: 677-685. doi:10.5858/arpa.2023-0167-CP
- 105Miyamoto K, Matsumoto K, Kato H, et al. The efficacy of pancreatic juice cytology with liquid-based cytology for evaluating malignancy in patients with intraductal papillary mucinous neoplasm. BMC Gastroenterol. 2020; 20: 319.
- 106Kitagawa K, Mitoro A, Tomooka F, et al. Diagnostic yield of liquid-based cytology in serial pancreatic juice aspiration cytological examination. DEN Open. 2022; 3:e177.
10.1002/deo2.177 Google Scholar
- 107Naito Y, Kawahara A, Okabe Y, et al. SurePath® LBC improves the diagnostic accuracy of intrahepatic and hilar cholangiocarcinoma. Cytopathology. 2018; 29: 349-354.
- 108Lee MW, Paik WH, Lee SH, et al. Usefulness of liquid-based cytology in diagnosing biliary tract cancer compared to conventional smear and forceps biopsy. Dig Dis Sci. 2023; 68: 274-283.
- 109Volmar KE, Vollmer RT, Routbort MJ, Creager AJ. Pancreatic and bile duct brushing cytology in 1000 cases: review of findings and comparison of preparation methods. Cancer. 2006; 108: 231-238.
- 110Sung S, Sireci AN, Remotti HE, et al. Plasma-thrombin cell blocks: potential source of DNA contamination. Cancer Cytopathol. 2019; 127: 771-777.
- 111Nambirajan A, Jain D. Cell blocks in cytopathology: an update. Cytopathology. 2018; 29: 505-524.
- 112Noda Y, Fujita N, Kobayashi G, et al. Diagnostic efficacy of the cell block method in comparison with smear cytology of tissue samples obtained by endoscopic ultrasound-guided fine-needle aspiration. J Gastroenterol. 2010; 45: 868-875.
- 113Torous VF, Chen Y, VanderLaan PA. Comparison of plasma-thrombin, HistoGel, and CellGel cell block preparation methods with paired ThinPrep slides in the setting of mediastinal granulomatous disease. J Am Soc Cytopathol. 2019; 8: 52-60.
- 114Wilson BL, Russell D, Evans SK, Agrawal T. Cell blocks in urine cytopathology: do they add value to the diagnosis? A pilot study. J Am Soc Cytopathol. 2021; 10: 47-55.
- 115Tanaka R, Ohtsuka K, Ogura W, et al. Subtyping and EGFR mutation testing from blocks of cytological materials, based on liquid-based cytology for lung cancer at bronchoscopic examinations. Diagn Cytopathol. 2020; 48: 516-523.
- 116He QL, Zhu YZ, Zheng GJ, Shi LC, Hu SW, Li CT. A new convenient technique for making cell blocks. Cell Tissue Res. 2012; 350: 395-400.
- 117Wilgenbusch H, Molm C, Aslan D, Berg B. It is all in the bag: collodion bag versus HistoGel cell block method. J Am Soc Cytopathol. 2020; 9: 20-25.
- 118Noda Y, Fujita N, Kobayashi G, et al. Prospective randomized controlled study comparing cell block method and conventional smear method for bile cytology. Dig Endosc. 2013; 25: 444-452.
- 119Mohapatra DS, Gupta P, Gupta N, et al. Evaluation of the utility of liquid-based cytology, cell-blocks, and flow cytometric immunophenotyping on endobronchial ultrasound-guided transbronchial needle aspiration samples in the diagnosis of sarcoidosis. J Bronchology Interv Pulmonol. 2022; 29: 260-268.
- 120Zhang XH, Ma SY, Liu N, et al. Comparison of smear cytology with liquid-based cytology in pancreatic lesions: a systematic review and meta-analysis. World J Clin Cases. 2021; 9: 3308-3319.
- 121Erdogan-Durmus S, Erdem ZB, Yulek O. Diagnostic value of preparing additional liquid-based cytology slides and cell blocks from residue material in thyroid fine needle aspiration. J Cytol. 2023; 40: 95-98.
- 122Abe H, Kawahara A, Azuma K, et al. Copy number gain in recurrent anaplastic lymphoma kinase (ALK) rearrangement-lung adenocarcinoma in the pleural effusion. Diagn Cytopathol. 2018; 46: 744-747.
- 123Chua TH, Chuah KL. Concordance of cytological specimens with histological tissue for detection of epidermal growth factor receptor mutation in non-small cell lung cancer: a systematic review. Acta Cytol. 2022; 66: 61-71.
- 124Sekita-Hatakeyama Y, Nishikawa T, Takeuchi M, et al. K-ras mutation analysis of residual liquid-based cytology specimens from endoscopic ultrasound-guided fine needle aspiration improves cell block diagnosis of pancreatic ductal adenocarcinoma. PLoS One. 2018; 13:e0193692.
- 125Abe H, Takase Y, Sadashima E, et al. Insulinoma-associated protein 1 is a novel diagnostic marker of small cell lung cancer in bronchial brushing and cell block cytology from pleural effusions: validity and reliability with cutoff value. Cancer Cytopathol. 2019; 127: 598-605.
- 126van Hemel BM, Suurmeijer AJ. Effective application of the methanol-based PreservCyt™ fixative and the Cellient™ automated cell block processor to diagnostic cytopathology, immunocytochemistry, and molecular biology. Diagn Cytopathol. 2013; 41: 734-741.
- 127Choi SJ, Choi YI, Kim L, et al. Preparation of compact agarose cell blocks from the residues of liquid-based cytology samples. Korean J Pathol. 2014; 48: 351-360.
- 128Qin SY, Zhou Y, Li P, Jiang HX. Diagnostic efficacy of cell block immunohistochemistry, smear cytology, and liquid-based cytology in endoscopic ultrasound-guided fine-needle aspiration of pancreatic lesions: a single-institution experience. PLoS One. 2014; 9:e108762.
- 129Prendeville S, Brosnan T, Browne TJ, McCarthy J. Automated Cellient™ cytoblocks: better, stronger, faster? Cytopathology. 2014; 25: 372-380.
- 130Chen YA, Lai YC, Lin SJ, Yang CS. Utility of cell block as an adjunct to liquid-based cytology for diagnosing papillary thyroid carcinoma. Indian J Pathol Microbiol. 2020; 63: 581-586.
- 131Collins GR, Thomas J, Joshi N, Zhang S. The diagnostic value of cell block as an adjunct to liquid-based cytology of bronchial washing specimens in the diagnosis and subclassification of pulmonary neoplasms. Cancer Cytopathol. 2012; 120: 134-141.
- 132Woo CG, Son SM, Han HS, et al. Diagnostic benefits of the combined use of liquid-based cytology, cell block, and carcinoembryonic antigen immunocytochemistry in malignant pleural effusion. J Thorac Dis. 2018; 10: 4931-4939.
- 133Ren S, Solomides C, Draganova-Tacheva R, Bibbo M. Overview of nongynecological samples prepared with liquid-based cytology medium. Acta Cytol. 2014; 58: 522-532.
- 134Watanabe M, Hashida S, Yamamoto H, et al. Estimation of age-related DNA degradation from formalin-fixed and paraffin-embedded tissue according to the extraction methods. Exp Ther Med. 2017; 14: 2683-2688.
- 135Abe H, Kawahara A, Sadashima E, et al. Nucleic acid quality in sodium alginate cell blocks made from liquid-based cytology specimens. J Jpn Soc Clin Cytol. 2021; 60: 102-109. (in Japanese with English summary).
10.5795/jjscc.60.102 Google Scholar
- 136Groelz D, Viertler C, Pabst D, Dettmann N, Zatloukal K. Impact of storage conditions on the quality of nucleic acids in paraffin embedded tissues. PLoS One. 2018; 13:e0203608.
- 137Satoh Y, Matsuo Y, Kuba T, et al. EGFR mutation genotyping and ALK status determination in liquid-based cytology samples of non-small cell lung cancer. Virchows Arch. 2020; 476: 753-762.
- 138Spathis A, Georgoulakis J, Foukas P, et al. KRAS and BRAF mutation analysis from liquid-based cytology brushings of colorectal carcinoma in comparison with formalin-fixed, paraffin-embedded tissue. Anticancer Res. 2010; 30: 1969-1975.
- 139Malapelle U, de Rosa N, Rocco D, et al. EGFR and KRAS mutations detection on lung cancer liquid-based cytology: a pilot study. J Clin Pathol. 2012; 65: 87-91.
- 140Lv M, Fan YB, Zhao YJ, Wang TY, Wu GP. Expression and clinical significance of glucose transporter 1 mRNA in bronchial brushing liquid-based cytology specimens from patients with and without lung cancer. Cytopathology. 2012; 23: 108-113.
- 141Itonaga M, Ashida R, Murata SI, et al. Kras gene analysis using liquid-based cytology specimens predicts therapeutic responses and prognosis in patients with pancreatic cancer. Cancers (Basel). 2022; 14: 551.
- 142Reynolds JP, Tubbs RR, Minca EC, et al. EGFR mutational genotyping of liquid based cytology samples obtained via fine needle aspiration (FNA) at endobronchial ultrasound of non-small cell lung cancer (NSCLC). Lung Cancer. 2014; 86: 158-163.
- 143Nishikawa T, Fujii T, Tatsumi S, et al. Molecular analysis of liquid-based cytological specimen using virtually positive sputum with adenocarcinoma cells. Diagnostics (Basel). 2020; 10: 84.
- 144Jikuzono T, Horikawa A, Ishikawa T, et al. Proteinase K treatment improves RNA recovery from thyroid cells fixed with liquid-based cytology solution. BMC Res Notes. 2018; 11: 822.
- 145Kim WY, Oh SY, Kim H, Hwang TS. DNA degradation in liquid-based cytology and its comparison with conventional smear. Diagn Cytopathol. 2016; 44: 450-458.
- 146Fujii T, Asano A, Shimada K, Tatsumi Y, Obayashi C, Konishi N. Evaluation of RNA and DNA extraction from liquid-based cytology specimens. Diagn Cytopathol. 2016; 44: 833-840.
- 147Matsuo Y, Yoshida T, Yamashita K, Satoh Y. Reducing DNA damage by formaldehyde in liquid-based cytology preservation solutions to enable the molecular testing of lung cancer specimens. Cancer Cytopathol. 2018; 126: 1011-1021.
- 148Dejmek A, Zendehrokh N, Tomaszewska M, Edsjö A. Preparation of DNA from cytological material: effects of fixation, staining, and mounting medium on DNA yield and quality. Cancer Cytopathol. 2013; 121: 344-353.
- 149Amemiya K, Hirotsu Y, Nagakubo Y, et al. Actionable driver DNA variants and fusion genes can be detected in archived cytological specimens with the Oncomine dx target test multi-CDx system in lung cancer. Cancer Cytopathol. 2021; 129: 729-738.
- 150Kawahara A, Azuma K, Sumi A, et al. Identification of non-small-cell lung cancer with activating EGFR mutations in malignant effusion and cerebrospinal fluid: rapid and sensitive detection of exon 19 deletion E746-A750 and exon 21 L858R mutation by immunocytochemistry. Lung Cancer. 2011; 74: 35-40.
- 151Kimura H, Fujiwara Y, Sone T, et al. EGFR mutation status in tumour-derived DNA from pleural effusion fluid is a practical basis for predicting the response to gefitinib. Br J Cancer. 2006; 95: 1390-1395.
- 152Jian G, Songwen Z, Ling Z, et al. Prediction of epidermal growth factor receptor mutations in the plasma/pleural effusion to efficacy of gefitinib treatment in advanced non-small cell lung cancer. J Cancer Res Clin Oncol. 2010; 136: 1341-1347.
- 153Kawahara A, Fukumitsu C, Azuma K, et al. Combined test using both cell sediment and supernatant cell-free DNA in pleural effusion shows increased sensitivity in detecting activating EGFR mutation in lung cancer patients. Cytopathology. 2018; 29: 150-155.
- 154Kawahara A, Abe H, Murata K, et al. Screening system for EGFR mutation detection in cytology cell-free DNA of cerebrospinal fluid based on assured sample quality. Cytopathology. 2019; 30: 144-149.
- 155Chiang CL, Shen CI, Huang HC, Chang HJ, Huang YT, Chiu CH. Cytology-based specimen triage for epidermal growth factor receptor mutation testing of malignant pleural effusions in non-small cell lung cancer. Front Oncol. 2022; 12:810124.
- 156Kawahara A, Fukumitsu C, Taira T, et al. Epidermal growth factor receptor mutation status in cell-free DNA supernatant of bronchial washings and brushings. Cancer Cytopathol. 2015; 123: 620-628.
- 157Takase Y, Naito Y, Kawahara A, et al. KRAS mutation analysis using cell-free DNA of pancreatic cancer. Anticancer Res. 2023; 43: 2683-2690.
- 158Wang JH, Gouda-Vossos A, Dzamko N, Halliday G, Huang Y. DNA extraction from fresh-frozen and formalin-fixed, paraffin-embedded human brain tissue. Neurosci Bull. 2013; 29: 649-654.
- 159Kofanova O, Bellora C, Garcia Frasquilho S, et al. Standardization of the preanalytical phase of DNA extraction from fixed tissue for next-generation sequencing analyses. N Biotechnol. 2020; 54: 52-61.
- 160Mathieson W, Guljar N, Sanchez I, Sroya M, Thomas GA. Extracting DNA from FFPE tissue biospecimens using user-friendly automated technology: is there an impact on yield or quality? Biopreserv Biobank. 2018; 16: 191-199.
- 161Matsuo Y, Yamashita K, Yoshida T, Satoh Y. Method for preservation of DNA stability of liquid-based cytology specimens from a lung adenocarcinoma cell line. Virchows Arch. 2021; 478: 507-516.
- 162Sauer T, Ebeltoft K, Pedersen MK, Kåresen R. Liquid based material from fine needle aspirates from breast carcinomas offers the possibility of long-time storage without significant loss of immunoreactivity of estrogen and progesterone receptors. Cytojournal. 2010; 7: 24.
- 163Kim Y, Choi KR, Chae MJ, et al. Stability of DNA, RNA, cytomorphology, and immunoantigenicity in residual ThinPrep specimens. APMIS. 2013; 121: 1064-1072.
- 164Field AS, Raymond WA, Rickard M, Schmitt F. Breast fine needle aspiration biopsy cytology: the potential impact of the international academy of cytology Yokohama system for reporting breast fine needle aspiration biopsy cytopathology and the use of rapid on-site evaluation. J Am Soc Cytopathol. 2020; 9: 103-111.
- 165Li HX, Wang MR, Zhao H, Cao J, Li CL, Pan QJ. Comparison of fluorescence in situ hybridization, NMP22 bladderchek, and urinary liquid-based cytology in the detection of bladder urothelial carcinoma. Diagn Cytopathol. 2013; 41: 852-857.
- 166Oya K, Kondo Y, Fukuda Y, Kishino M, Toyosawa S. TUBB3 immunostaining improves the diagnostic accuracy of oral liquid-based cytology in squamous cell carcinoma. Cytopathology. 2022; 33: 374-379.
- 167Minca EC, Lanigan CP, Reynolds JP, et al. ALK status testing in non-small-cell lung carcinoma by FISH on ThinPrep slides with cytology material. J Thorac Oncol. 2014; 9: 464-468.
- 168Bellevicine C, Malapelle U, Vigliar E, de Luca C, Troncone G. Epidermal growth factor receptor test performed on liquid-based cytology lung samples: experience of an academic referral center. Acta Cytol. 2014; 58: 589-594.
- 169Osorio-Osorno YA, Arboleda Toro D, Arango JC, Parada-Sanchez MT. Optimized liquid-based cytology for the cellular and molecular analysis of oral keratinocytes: a promising diagnostic tool. Diagn Cytopathol. 2021; 49: 96-104.
- 170Araki Y, Arihiro K, Yamaguchi K, et al. Analysis of microRNA expression in liquid-based cytology samples may be useful for primary lung cancer diagnosis. Am J Clin Pathol. 2021; 156: 644-652.
- 171Ali SZ, Baloch ZW, Cochand-Priollet B, Schmitt FC, Vielh P, VanderLaan PA. The 2023 Bethesda system for reporting thyroid cytopathology. Thyroid. 2023; 33: 1039-1044.
- 172VandenBussche CJ. A review of the Paris system for reporting urinary cytology. Cytopathology. 2016; 27: 153-156.
- 173Vlajnic T, Gut A, Savic S, Bubendorf L. The Paris system for reporting urinary cytology in daily practice with emphasis on ancillary testing by multiprobe FISH. J Clin Pathol. 2020; 73: 90-95.
- 174Rossi ED, Baloch Z, Barkan G, et al. Second edition of the Milan system for reporting salivary gland cytopathology: refining the role of salivary gland FNA. Cancer Cytopathol. 2024; 132: 10-21. doi:10.1002/cncy.22753
- 175Dubucs C, Basset C, D'Aure D, Courtade-Saïdi M, Evrard SM. A 4-year retrospective analysis of salivary gland cytopathology using the Milan system for reporting salivary gland cytology and ancillary studies. Cancers (Basel). 2019; 11:1912.
- 176Evrard SM, Meilleroux J, Daniel G, et al. Use of fluorescent in-situ hybridisation in salivary gland cytology: a powerful diagnostic tool. Cytopathology. 2017; 28: 312-320.
- 177Makino R, Kawahara A, Abe H, et al. A case of mucoepidermoid carcinoma of the parotid gland diagnosed by fine-needle aspiration cytology using SurePath® liquid-based sampling. J Jpn Soc Clin Cytol. 2023; 62: 252-257. (in Japanese with English summary).
10.5795/jjscc.62.252 Google Scholar
- 178Chang H, Lee H, Yoon SO, Kim H, Kim A, Kim BH. BRAF(V600E) mutation analysis of liquid-based preparation-processed fine needle aspiration sample improves the diagnostic rate of papillary thyroid carcinoma. Hum Pathol. 2012; 43: 89-95.
- 179Rossi ED, Martini M, Capodimonti S, et al. Diagnostic and prognostic value of immunocytochemistry and BRAF mutation analysis on liquid-based biopsies of thyroid neoplasms suspicious for carcinoma. Eur J Endocrinol. 2013; 168: 853-859.
- 180Agrawal T, Xi L, Navarro W, et al. An effective approach for BRAF V600E mutation analysis of routine thyroid fine needle aspirates. Cytopathology. 2022; 33: 344-349.
- 181Krane JF, Cibas ES, Alexander EK, Paschke R, Eszlinger M. Molecular analysis of residual ThinPrep material from thyroid FNAs increases diagnostic sensitivity. Cancer Cytopathol. 2015; 123: 356-361.
- 182Mian C, Lodde M, Comploj E, et al. Liquid-based cytology as a tool for the performance of uCyt+ and Urovysion multicolour-FISH in the detection of urothelial carcinoma. Cytopathology. 2003; 14: 338-342.
- 183Comploj E, Mian C, Ambrosini-Spaltro A, et al. uCyt+/ImmunoCyt and cytology in the detection of urothelial carcinoma: an update on 7422 analyses. Cancer Cytopathol. 2013; 121: 392-397.
- 184Iwaya H, Tanimoto A, Toyodome K, et al. Next-generation sequencing analysis of pancreatic cancer using residual liquid cytology specimens from endoscopic ultrasound-guided fine-needle biopsy: a prospective comparative study with tissue specimens. Diagnostics (Basel). 2023; 13: 1078.
- 185Sekita-Hatakeyama Y, Fujii T, Nishikawa T, et al. Evaluation and diagnostic value of next-generation sequencing analysis of residual liquid-based cytology specimens of pancreatic masses. Cancer Cytopathol. 2022; 130: 202-214.
- 186Malapelle U, de Rosa N, Bellevicine C, et al. EGFR mutations detection on liquid-based cytology: is microscopy still necessary? J Clin Pathol. 2012; 65: 561-564.
- 187Yamaguchi T, Akahane T, Harada O, et al. Next-generation sequencing in residual liquid-based cytology specimens for cancer genome analysis. Diagn Cytopathol. 2020; 48: 965-971.
- 188Reynolds JP, Zhou Y, Jakubowski MA, et al. Next-generation sequencing of liquid-based cytology non-small cell lung cancer samples. Cancer Cytopathol. 2017; 125: 178-187.
- 189Akahane T, Yamaguchi T, Kato Y, et al. Comprehensive validation of liquid-based cytology specimens for next-generation sequencing in cancer genome analysis. PLoS One. 2019; 14:e0217724.
- 190Pisapia P, Pepe F, Sgariglia R, et al. Next generation sequencing in cytology. Cytopathology. 2021; 32: 588-595.
- 191Tanaka R, Fujiwara M, Nakazato Y, et al. Optimal preservations of cytological materials using liquid-based cytology fixatives for next-generation sequencing analysis. Acta Cytol. 2022; 66: 457-465.
- 192Akahane T, Isochi-Yamaguchi T, Hashiba-Ohnuki N, et al. Cancer gene analysis of liquid-based cytology specimens using next-generation sequencing: a technical report of bimodal DNA- and RNA-based panel application. Diagn Cytopathol. 2023; 51: 493-500.
- 193Tafoya M, Judd A, Chiotti K, et al. Performance of a 50-gene next generation sequencing panel with post-centrifuge supernatant cytology fluid in non-small-cell lung cancer. Diagn Cytopathol. 2021; 49: 1173-1178.
- 194Wheeldon L, Jones M, Probyn B, Shetty D, Garvican J. Use of the biocartis Idylla™ platform for the detection of epidermal growth factor receptor, BRAF and KRAS proto-oncogene mutations in liquid-based cytology specimens from patients with non-small cell lung carcinoma and pancreatic adenocarcinoma. J Mol Pathol. 2022; 3: 104-114.
- 195Ikeda K, Sakabe N, Maruyama S, et al. Relationship between a deep learning model and liquid-based cytological processing techniques. Cytopathology. 2023; 34: 308-317.