All-Dry Fabrication of Poly(methacrylic acid)-Based Membranes with Controlled Dissolution Behavior
Scott Seidel
Mork Family Department of Chemical Engineering and Materials Science, University of Southern California, 925 Bloom Walk, Los Angeles, California 90089, USA
Search for more papers by this authorChristopher Chu Cheong
Mork Family Department of Chemical Engineering and Materials Science, University of Southern California, 925 Bloom Walk, Los Angeles, California 90089, USA
Search for more papers by this authorPhilip Kwong
Mork Family Department of Chemical Engineering and Materials Science, University of Southern California, 925 Bloom Walk, Los Angeles, California 90089, USA
Search for more papers by this authorCorresponding Author
Malancha Gupta
Mork Family Department of Chemical Engineering and Materials Science, University of Southern California, 925 Bloom Walk, Los Angeles, California 90089, USA
Search for more papers by this authorScott Seidel
Mork Family Department of Chemical Engineering and Materials Science, University of Southern California, 925 Bloom Walk, Los Angeles, California 90089, USA
Search for more papers by this authorChristopher Chu Cheong
Mork Family Department of Chemical Engineering and Materials Science, University of Southern California, 925 Bloom Walk, Los Angeles, California 90089, USA
Search for more papers by this authorPhilip Kwong
Mork Family Department of Chemical Engineering and Materials Science, University of Southern California, 925 Bloom Walk, Los Angeles, California 90089, USA
Search for more papers by this authorCorresponding Author
Malancha Gupta
Mork Family Department of Chemical Engineering and Materials Science, University of Southern California, 925 Bloom Walk, Los Angeles, California 90089, USA
Search for more papers by this authorAbstract
The all-dry fabrication of porous poly(methacrylic acid)-based membranes displaying tunable dissolution behaviors in aqueous media is presented. Poly(methacrylic acid) (PMAA) membranes were fabricated using a low temperature, solventless technique with gaseous initiator and monomer precursors. The PMAA was then converted to poly(methacrylic acid-co-methacrylic anhydride) by thermal annealing. By controlling the annealing time, the methacrylic anhydride (MAN) content was varied, which allowed for the dissolution behavior to be tuned. The incorporation of MAN moieties in the membranes also allowed for crosslinking via a vapor phase reaction with 1,3-diaminopropane. The membranes can be deposited on a variety of substrates, including gauze.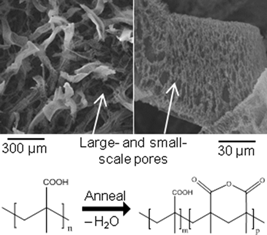
References
- 1 F. Galeotti, F. Trespidi, G. Timo, M. Pasini, ACS Appl. Mater. Interfaces 2014, 6, 5827.
- 2 D. Schmaljohann, Adv. Drug Deliv. Rev. 2006, 58, 1655.
- 3 J.-F. Jhong, A. Venault, L. Liu, J. Zheng, S.-H. Chen, A. Higuchi, J. Huang, Y. Chang, ACS Appl. Mater. Interfaces 2014, 6, 9858.
- 4 S. C. Hess, A. X. Kohll, R. A. Raso, C. M. Schumacher, R. N. Grass, W. J. Stark, ACS Appl. Mater. Interfaces 2015, 7, 611.
- 5 M. Ulbricht, Polymer 2006, 47, 2217.
- 6 X. Meng, F. Tian, J. Yang, C.-N. He, N. Xing, F. Li, J. Mater. Sci. Mater. Med. 2010, 21, 1751.
- 7 F.-L. Mi, Y.-B. Wu, S.-S. Shyu, J.-Y. Schoung, Y.-B. Huang, Y.-H. Tsai, J. Y. Hao, J. Biomed. Mater. Res. 2002, 59, 438.
- 8 S. Kovacic, F. Preishuber-Pflugl, C. Slugovc, Macromol. Mater. Eng. 2014, 299, 843.
- 9 R. Yin, K. Wang, Y. Lu, J. Nie, Macromol. Mater. Eng. 2015, 300, 291.
- 10 R. Sakai, B. John, M. Okamoto, J. V. Seppala, J. Vaithilingam, H. Hussein, R. Goodridge, Macromol. Mater. Eng. 2013, 298, 45.
- 11 Y. Zhang, L. Ionov, ACS Appl. Mater. Interfaces 2014, 6, 10072.
- 12 E. S.-Q. Lee, G. P. Rangaiah, Ind. Eng. Chem. Res. 2009, 48, 7662.
- 13 S. Sharma, Y. C. Chua, G. P. Rangaiah, Mater. Manuf. Process. 2011, 26, 431.
- 14 A. Figoli, T. Marino, S. Simone, E. Di Nicolo, X.-M. Li, T. He, S. Tornaghi, E. Drioli, Green Chem. 2014, 16, 4034.
- 15 S. Mehrabani, P. Kwong, M. Gupta, A. M. Armani, Appl. Phys. Lett. 2013, 102, 241101.
- 16 K. K. S. Lau, J. Bico, K. B. K. Teo, M. Chhowalla, G. A. J. Amaratunga, W. I. Milne, G. H. McKinley, K. K. Gleason, Nano Lett. 2003, 3, 1701.
- 17 B. Chen, S. Seidel, H. Hori, M. Gupta, ACS Appl. Mater. Interfaces 2011, 3, 4201.
- 18 P. Kwong, C. A. Flowers, M. Gupta, Langmuir 2011, 27, 10634.
- 19 B. Chen, P. Kwong, M. Gupta, ACS Appl. Mater. Interfaces 2013, 5, 12701.
- 20 R. Tao, M. Anthamatten, Macromol. Rapid Commun. 2013, 34, 1755.
- 21 S. Seidel, P. Kwong, M. Gupta, Macromolecules 2013, 46, 2976.
- 22 S. Seidel, M. Gupta, J. Vac. Sci. Technol. A 2014, 32, 041514.
- 23 P. Kwong, S. Seidel, M. Gupta, ACS Appl. Mater. Interfaces 2013, 5, 9714.
- 24 R. D. Porasso, J. C. Benegas, M. A. G. T. van den Hoop, J. Phys. Chem. B 1999, 103, 2361.
- 25 S. Zhou, B. Chu, J. Phys. Chem. B 1998, 102, 1364.
- 26 D. H. Grant, N. Grassie, Polymer 1960, 1, 125.
- 27 Y. Mao, K. K. Gleason, Langmuir 2006, 22, 1795.
- 28 B.-C. Ho, Y.-D. Lee, W.-K. Chin, J. Polym. Sci. Polym. Chem. 1992, 30, 2389.
- 29 T. G. Fox, P. J. Flory, J. Appl. Phys. 1950, 21, 581.
- 30Sigma-Aldrich. Thermal Transitions of Homopolymers: Glass Transition & Melting Point. https://www.sigmaaldrich.com/content/dam/sigma-aldrich/docs/Aldrich/General_Information/thermal_transitions_of_homopolymers.pdf, accessed: November, 2014.
- 31 K. E. Uhrich, S. M. Cannizzaro, R. S. Langer, K. M. Shakesheff, Chem. Rev. 1999, 99, 3181.
- 32 C. T. Hess, Wound Care, 5th edition, Lippincott Williams & Wilkins, Philadelphia, PA, USA 2005.
- 33 J. S. Boateng, K. H. Matthews, H. N. E. Stevens, G. M. Eccleston, J. Pharm. Sci. 2008, 97, 2892.
- 34 P. H. Corkhill, C. J. Hamilton, B. J. Tighe, Biomaterials 1989, 10, 3.




