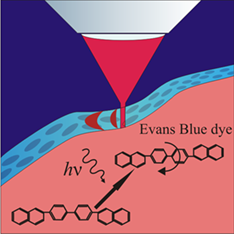
Intravital molecular tagging velocimetry of cerebral blood flow using Evans Blue
Corresponding Author
Anton A. Namykin
Saratov State University (National Research University), Saratov, Russia
Correspondence
Anton A. Namykin, Saratov State University (National Research University), 83 Astrakhanskaya str., 410012 Saratov, Russia.
Email: [email protected]
Search for more papers by this authorNatalia A. Shushunova
Saratov State University (National Research University), Saratov, Russia
Search for more papers by this authorMaria V. Ulanova
Saratov State University (National Research University), Saratov, Russia
Search for more papers by this authorOxana V. Semyachkina-Glushkovskaya
Saratov State University (National Research University), Saratov, Russia
Search for more papers by this authorValery V. Tuchin
Saratov State University (National Research University), Saratov, Russia
Tomsk State University (National Research University), Tomsk, Russia
Institute of Precision Mechanics and Control, Russian Academy of Sciences, Saratov, Russia
Search for more papers by this authorIvan V. Fedosov
Saratov State University (National Research University), Saratov, Russia
Search for more papers by this authorCorresponding Author
Anton A. Namykin
Saratov State University (National Research University), Saratov, Russia
Correspondence
Anton A. Namykin, Saratov State University (National Research University), 83 Astrakhanskaya str., 410012 Saratov, Russia.
Email: [email protected]
Search for more papers by this authorNatalia A. Shushunova
Saratov State University (National Research University), Saratov, Russia
Search for more papers by this authorMaria V. Ulanova
Saratov State University (National Research University), Saratov, Russia
Search for more papers by this authorOxana V. Semyachkina-Glushkovskaya
Saratov State University (National Research University), Saratov, Russia
Search for more papers by this authorValery V. Tuchin
Saratov State University (National Research University), Saratov, Russia
Tomsk State University (National Research University), Tomsk, Russia
Institute of Precision Mechanics and Control, Russian Academy of Sciences, Saratov, Russia
Search for more papers by this authorIvan V. Fedosov
Saratov State University (National Research University), Saratov, Russia
Search for more papers by this authorAbstract
The effects of light-driven enhancement of Evans Blue dye complexes with blood plasma proteins were observed for the first time, both in vitro and in vivo. The possible background of the effect concerns the photochemical cis-trans isomerization of the azo dye molecules. The effect was induced in the solution with a red laser with a wavelength of 638 nm, which corresponds to the peak of the dye absorption. The lifetime of the enhanced fluorescence is approximately 1 second and enables its use as an optically tagged molecular flow tracer for blood flow velocity measurements. Utilizing the effect, we performed for the first time the intravital molecular tagging velocimetry of the blood velocity in blood vessels in a living animal. The results of the measurements of the blood flow velocities in the cerebral veins of a group of healthy mice are presented.
REFERENCES
- 1C. P. Gendrich, M. M. Koochesfahani, D. G. Nocera, in Handbook of experimental fluid dynamics (Eds: J. Foss, C. Tropea, A. Yarin), Springer-Verlag, Berlin, Heidelberg 2007.
- 2R. Leigh, L. Knutsson, J. Zhou, P. C. van Zijl, J. Cereb. Blood Flow Metab. 2017, 1.
- 3A. Chien, F. Viñuela, Interv. Neuroradiol. 2017, 23, 427.
- 4L. Ramm, B. Schwab, R. Stodtmeister, M. Hammer, L. Sauer, E. Spörl, L. E. Pilluna, N. Terai, Curr. Eye Res. 2017, 42, 1313.
- 5B. Djurić, S. Suzić, B. Stojadinović, Z. Nestorović, M. Ivanović, J. Suzić-Lazić, D. Žikić, Biomed. Microdevices 2017, 19(3), 48.
- 6B. H. H. Lang, C. K. Wong, H. T. Hung, K. P. Wong, K. L. Mak, K. B. Au, Surgery 2017, 161(1), 87.
- 7S. C. Gnyawali, K. Blum, D. Pal, S. Ghatak, S. Khanna, S. Roy, C. K. Sen, Sci. Rep. 2017, 7, 41048.
- 8V. Rajan, B. Varghese, v. T. G. Leeuwen, W. Steenbergen, Lasers Med. Sci. 2009, 24(2), 269.
- 9C. Poelma, A. Kloosterman, B. P. Hierck, J. Westerweel, PloS One 2012, 7(9), e45247.
- 10C. E. Riva, Ocular Blood Flow, Springer, Berlin, Heidelberg 2012.
10.1007/978-3-540-69469-4_7 Google Scholar
- 11T. Kyoden, S. Naruki, S. Akiguchi, H. Ishida, T. Andoh, Y. Takada, T. Hachiga, J. Appl. Phys. 2016, 120(8), 084701.
- 12W. Trasischker, R. M. Werkmeister, S. Zotter, B. Baumann, T. Torzicky, M. Pircher, C. K. Hitzenberger, J. Biomed. Opt. 2013, 18(11), 116010.
- 13I. V. Fedosov, V. V. Tuchin, Handbook of Coherent-Domain Optical Methods, Springer Science+Business Media, New York 2013, p. 487.
10.1007/978-1-4614-5176-1_13 Google Scholar
- 14P. Vennemann, K. T. Kiger, R. Lindken, B. C. W. Groenendijk, S. Stekelenburg-de Vos, T. L. M. ten Hagen, N. T. C. Ursem, R. E. Poelmann, J. Westerweel, B. P. Hierck, J. Biomech. 2006, 39(7), 1191.
- 15F. C. Delori, C. K. Dorey, G. Staurenghi, O. Arend, D. G. Goger, J. J. Weiter, Invest. Ophthalmol. Vis. Sci. 1995, 36(3), 718.
- 16J. G. Gibson 2nd., W. A. Evans Jr., J. Clin. Investig. 1937, 16(3), 301.
- 17Y. Shapira, D. Setton, A. A. Artru, E. Shohami, Anesth. Analg. 1993, 77(1), 141.
- 18D. A. Berk, M. A. Swartz, A. J. Leu, R. K. Jain, Am. J. Physiol. 1996, 270(1), H330.
- 19S. Pant, H. B. Tripathi, D. D. Pant, J. Photochem. Photobiol. A Chem. 1994, 81(1), 7.
- 20F. B. Freedman, J. A. Johnson, Am. J. Physiol. 1969, 216(3), 675.
- 21 V. V. Tuchin Ed., Handbook of Optical Biomedical Diagnostics: Methods, Vol. 1, SPIE Press, Bellingham 2016.
- 22G. S. Hartley, Nature 1937, 140(3537), 281.
- 23G. S. Hartley, J. Chem. Soc. 1938, 633.
- 24P. S. Zacharias, S. Korupoju, J. Chem. Soc., Perkin Trans. 1998, 2(9), 2055.
10.1039/a706775e Google Scholar
- 25M. Han, M. Hara, J. Am. Chem. Soc. 2005, 127(31), 10951.
- 26P. Smitha, S. K. Asha, J. Phys. Chem. B 2007, 111(23), 6364.
- 27O. Haruta, Y. Matsuo, K. Ijiro, Colloids Surf. A Physicochem. Eng. Asp. 2008, 313, 595.
- 28F. Ma, N. Zhou, J. Zhu, W. Zhang, L. Fan, X. Zhu, Eur. Polym. J. 2009, 45(7), 2131.
- 29X. Ran, H. Wang, L. Shi, J. Lou, B. Liu, M. Li, L. Guo, J. Mater. Chem. C 2014, 2, 9866.
- 30T. L. Lee, C. T. Lo, J Polym Sci B 2017, 55(10), 793.
- 31J. Griffiths, Chem. Soc. Rev. 1972, 1(4), 481.
- 32H. Puchtler, F. Sweat, S. Gropp, J. Microsc. 1967, 87(3–4), 309.
- 33H. Rau, Angew. Chem. Int. Ed. 1973, 12(3), 224.
- 34H. El-Sayed, S. R. Goodall, R. Hainsworth, Clin. Lab. Haematol. 1995, 17(2), 189.
- 35P. W. Hamer, J. M. McGeachie, M. J. Davies, M. D. Grounds, J. Anat. 2002, 200(1), 69.
- 36A. Michalicova, J. Galba, M. Novak, A. Kovac, J. Liq. Chromatogr. Relat. Technol. 2017, 40, 442.
- 37S. J. Isak, E. M. Eyring, J. D. Spikes, P. A. Meekins, J. Photochem. Photobiol. A Chem. 2000, 134(1), 77.
- 38A. Saria, J. M. Lundberg, J. Neurosci. Methods 1983, 8(1), 41.
- 39K. J. Bertram, M. T. Shipley, M. Ennis, P. R. Sanberg, A. B. Norman, Exp. Neurol. 1994, 127(2), 245.
- 40D. Magde, R. Wong, P. G. Seybold, Photochem. Photobiol. 2002, 75(4), 327.