Perspectives of vitamin C upregulating regulatory T cells in the era of thrombopoietin receptor agonists for immune thrombocytopenia
[Correction added on November 4, 2022 after first online publication: the Author Contributions, Acknowledgments, Funding, Conflicts of Interest, Data Availability Statement and Ethical approval has been updated].
Abstract
Background
Immune thrombocytopenia (ITP) is a rare disorder involving excessive peripheral destruction of platelets and inadequate bone marrow platelet production due to autoimmune reactions against megakaryocytes. Diagnosis is usually by the exclusion of primary causes; its rarity would only permit trial and error therapy experience.
Historically, for ITP, oral ascorbic acid (AA) gave inconsistent results. However, unlike other nutrients, AA is found to have an exceptionally tight intestinal absorption restriction mandating intravenous delivery for any therapeutic purposes. Recently, as there is more pre-clinical evidence of AA restoring regulatory T cells (Tregs) by robust demethylation, the historical oral route was likely to be the “bottle neck” restricting effective blood levels. During this era of thrombopoietin receptor agonists (TPO-RAs), planned tapering is often required upon discontinuation but unplanned discontinuations, for example, due to side effects, are more problematic. Moreover, combinations with TPO-RAs are currently being tried using steroids and rituximab except rituximab is inappropriate during pandemics. Notably, with the indirect (but prognostically relevant) action of TPO-RAs on Tregs, boosting Treg functions by adding other Treg active agents, for example, steroids or AA, seems appropriate, especially if without added toxicity upon combining.
Conclusions
With updated pharmacokinetic concepts, it is timely to repeat AA clinical trials for ITP but using intravenous administration. Initially, this may be on compassionate grounds for its probable role as an adjunct to TPO-RAs for unsuccessful tapering and, especially, for all unplanned discontinuations of TPO-RAs. Such off-label usage of AA is justified because of AA's robust pre-clinical evidence on demethylation and initial clinical experience (despite the inappropriate administrative route). Moreover, moderate intravenous AA dosages have one of the best safety profiles, let alone its eminent affordability. Admittedly, after the initial clinical experience, more systematic trials on AA are required, especially for the most appropriate dosage range and its efficacy as monotherapy.
1 BACKGROUND
Immune thrombocytopenia (ITP) is a rare autoimmune disorder involving an isolated very low platelet count with a normal bone marrow (BM) function and in the absence of other causes of thrombocytopenia. The acronym “ITP” now stands for “immune thrombocytopenia” as the term “purpura” is now avoided. The definition of “thrombocytopenia” may be taken to be platelets <100 × 109/L.1 Without specific markers, ITP is usually diagnosed by the exclusion of all other primary causes of thrombocytopenia. As details of ITP clinical management tactics (including specific recommendations during this coronavirus disease 2019 pandemic),2 are duly covered elsewhere, these are not included here. The main management difference during pandemics is that rituximab should be considered as contraindicated.2, 3
2 SALIENT POINTS ON CLINICAL MANAGEMENT OF ITP
Incidentally, the clinical experience in this era of cancer immunotherapy also tallied with published reports of a rare occurrence of secondary ITPs.4, 5 Although this article is on adult ITP, it should be noted that genetic defects may even manifest in older age groups. Fortunately, recent immunological testing helps considerably except the complexity of testing and the interpretation of results would usually demand adequate communications with laboratory immunologists. Moreover, there is a growing list of single-gene defects that may be associated with immune dysregulation and manifest as multiple haematological and immunological phenotypes, for example, autoimmune cytopenias (AICs).6 As AICs may be monogenic or polygenic, a preliminary serological and immunophenotyping assessment, including quantitative immunoglobulins,6 would help in picking up such cases. Because of the rarity of ITP, treatment choices may well be by “trial and error”, albeit evidence-based. Despite the current pandemic, most treatments, for example, intravenous immunoglobulin (IVIG), platelet transfusions, anti-RhD (immunoglobulin), splenectomy and thrombopoietin receptor agonists (TPO-RAs) are still valid,7 although steroid use is more cautious.2, 3, 8
As rituximab may now be contraindicated, it seems to be an appropriate time to re-consider vitamin C or ascorbic acid (AA) for ITP. Thirty-four years ago, Brox reported 2 g of AA was effective for ITP9 but subsequently, results were equivocal apart from anecdotal successes especially upon combining it with steroids.10 Retrospectively, this mysterious discrepancy, probably boils down to an inappropriate route of AA administration which warrants some discussion here.
3 SUB-CLINICAL AA DEFICIENCY
It turns out that, although most mammals can readily manufacture AA as required during unusual stress periods and disease states, all humans, guinea pigs, and some fruit bats fail to do so11 and are entirely dependent on external sources of AA. Thus, during normal life, one would have no problem with maintaining a reasonable blood level of AA by taking a healthy and balanced diet. However, during unusual stress periods and disease states, the AA blood level may well drop drastically. Thus, a sector of normal-looking people may well have subclinical AA deficiency12, 13 (see Section on “The Bare Truth of AA Deficiency”). As sub-clinical AA deficiencies are detected mainly by blood AA level determinations, these should actually be done when embarking on AA clinical trials, especially for those involving lower dosages of AA. For instance, Fowler et al. found AA blood levels documented before clinical trials to be as low as 17.9 ± 2.4 μM (μmol/L), well within the hypovitaminosis C range of <23 μM.14 Thus, failing to do so upon clinical trials involving lower dosages of AA may well jeopardize the trial results and the wrong interpretations may also ensue. Most probably, lower dosages of oral AA administered for trial purposes could have been consumed just for replenishing the pre-existing AA deficiencies and not much leftover AA is available for the specific therapeutic manoeuvres being tried.
4 THE CONTROVERSY OF HIGH-DOSE INTRAVENOUS VITAMIN C
The clinical use of high-dose intravenous vitamin C (HDIVC) has long been controversial15 especially when applied to cancer treatments, even though there are now more and more scientific data supporting the rationale of this usage.16, 17 Actually, various clinical trials of HDIVC for cancers are already ongoing or have recently been completed. Most importantly, unlike other nutrients, AA has very unique pharmacokinetics limiting oral absorption by imposing an exceptionally tight intestinal absorption limit so that plasma AA levels would hardly be > 0.2 mM (>200 μM). This tight intestinal control means that a very stable “physiological” AA blood level of up to 0.2 mM is always maintained regardless of how high an oral AA dose may be. Obviously, the only way to achieve pharmacologic blood levels of AA is to bypass this by the iv route of administration18 (see Table 1). With a better understanding of AA pharmacokinetic concepts, historical clinical trials using entirely oral AA administration, for example, those performed by Brox et al. in 1988, would thus be seriously challenged (see Section on “Sub-clinical AA Deficiency”). Now, even if there was never any sub-clinical AA deficiency in those trial patients, it is unreasonable to expect “physiological” AA blood levels to achieve any specific therapeutic purpose.19 Clearly, the iv route of administration would have been a much better route of administration employed for those historical AA trials.
| Route | Dose | Conc (mM) |
|---|---|---|
| Oral | 0 g | ≤0.011† |
| Oral | 1.25 g | 0.187 |
| Oral | 3.0 g | 0.206 |
| iv | 3.0 g | 1.76 |
| iv | 5.0 g | 2.87 |
| iv | 10.0 g | 5.58 |
| iv | 100 g | 15.38 |
- Data extracted from Reference 62 [Padayatti et al., 2004]. With the same dose of 3 g of vitamin C, the iv route of administration gives > 8.5 times the plasma concentration of vitamin C – a point highly relevant when designing vitamin C trials at such lower dosage ranges.
- † Scurvy level.
- Conc: concentration; iv: intravenous.
5 THE BARE TRUTH OF AA DEFICIENCY
Although the recommended dietary allowance (RDA) of AA is 75–90 mg/day, AA blood levels may readily decrease considerably upon stress, infections or accidental trauma, especially since humans cannot manufacture AA.11 Even marathon runners were found to require a week's supply of oral AA at 1.5 g/day (but not even 0.5 g/day) to attenuate plasma cytokine increases.20 Moreover, in healthy subjects, neutrophil movements were significantly enhanced through an antihistamine action as rendered by oral AA at 2 g/day for 2 weeks.21 Such enhanced neutrophil movements may be helpful for combating infections in general.22 Alarmingly, the reverse seems to be happening as reported in a New Zealand study on sepsis patients. It was found that 40% of those patients actually had AA at scurvy levels (≤11 μM) and most patients had hypovitaminosis C (<23 μM) blood levels despite adequate supplements.23 Naturally, the human inability to manufacture any AA would mandate a purposeful and adequate external AA supply, especially during exceptional stress periods. For documenting the AA requirement during such exceptional periods, perhaps neutrophil motility21, 22 would be a better marker.
6 THE 1988 ITP TRIAL
In 1988, 2 g doses of oral AA were administered for ITP. In retrospect, the high response rate of seven of 11 patients9 was actually unexpected, especially when viewed from the angle of current pharmacokinetic concepts. It follows that inconsistent results of a subsequent trial using the same 2 g oral AA dose were not surprising. It may also be expecting far too much for a merely “physiological” AA blood level to achieve specific pharmacologic end points. Moreover, common but subclinical AA deficiencies could have also affected the actual availability of the 2 g oral AA delivered (see Section on “Sub-clinical AA Deficiency”). Pragmatically, the AA delivered could have been consumed just to supplement pre-existing subclinical deficiencies. Clearly, the 2 g oral AA adopted by Brox et al.9 for ITP was too low judging from current pharmacokinetic concepts. Unfortunately, AA has since been forgotten. Nevertheless, HDIVC for ITP is worth pursuing especially as there is now good scientific evidence for being a potentially useful agent for ITP (see below).
7 SIDE EFFECTS OF HDIVC
HDIVC as being used for many decades by alternative medicine practitioners had remarkably few documented side effects.24 For instance, of 9328 patients on HDIVC averaging 28 g iv, there were 101 minor side effects such as lethargy, fatigue, change in mental status and injection site irritation. Another issue is oxalate metabolism and the potential formation of calcium oxalate crystals, especially in individuals predisposed to renal stone formation. Actually, any renal pathology and all renal failures would constitute contraindications to HDIVC, let alone only < 0.5% of AA is converted to oxalate.25 Moreover, a prospective study consisting of 157 cases having HDIVC of at least 15 g per infusion revealed no reported renal stones despite 8% of patients actually had a previous history of renal stones before HDIVC.26 Other exceedingly rare contraindications are primary hyperoxaluria (incidence rate of 1 per 120 000 population) and paroxysmal nocturnal hemoglobinuria (incidence rate of 1.5 per million population). Notably, glucose-6-phosphate dehydrogenase (G6PD) deficiency is known to be commoner in Asians and Africans and local routines often involve screening for this deficiency before any HDIVC (to avoid hemolysis). However, the author would concur with published literature27 that hemolysis usually does not occur with HDIVC doses of <7 g iv, regardless of G6PD deficiency. Lastly, false positive bed-side glucose meters are also well known upon HDIVC except laboratory-based tests are unaffected. Thus, pharmacologic AA concentrations, especially in dosages mostly in the range <7 g, would have an excellent safety profile.
8 ITP PATHOGENESIS
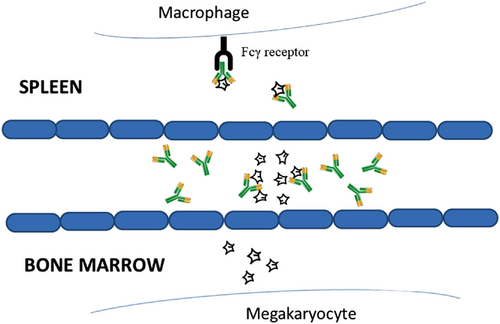
ITP involves excessive peripheral destruction of platelets in the body, especially in the spleen and the liver, along with inadequate BM platelet production due to an autoimmune reaction against megakaryocytes. Also documented are insufficient TPO levels. Platelet lysis is mostly mediated by complement-dependent cytotoxicity: the binding of antibodies to platelet glycoprotein (GP) forming the membrane attack complex (MAC).28, 29 Some platelets are being opsonized through complement activation in readiness for phagocytosis by macrophages in the spleen in an Fcγ receptor (FcγR)-dependent mechanism (Figure 1). In ITP patients, splenic macrophages are also the major antigen-presenting cells stimulating autoreactive CD4 T cells. Antiplatelet antibodies would trigger platelet desialylation. Desialylated platelets as recognized by hepatocytes are thus being removed from the liver. Importantly, this would also diminish the main trigger of the production of TPO, the major growth factor of megakaryocytes. In the marrow, an autoimmune response acts against megakaryocytes that express similar GPs as platelets so that they are also recognized by antiplatelet antibodies and similarly removed. Notably, CD8 cytotoxic T cells mediate cytotoxicity against platelets upon close contact, for example, in the spleen.28, 29 Thus, the autoimmune response is rendered by a loss of immune tolerance and supported by a deficiency of Tregs in the spleen, the blood and also the BM. Ultimately, the insufficient platelet production results from both the autoimmune response targeting megakaryocytes and insufficient levels of TPO.
9 DERANGED T CELL SUBSETS AND RELATED IMMUNE CHECKPOINTS
Evidently, in ITP patients, the ratio of Th1/Th2 in their peripheral blood (PB) was elevated.30, 31 Moreover, Ji, et al. also found the percentage of Tregs in the PB of ITP patients to be significantly lower than that of healthy controls (HCs). The percentage of Th17 cells also increased significantly at disease onset and the ratio of Treg/Th17 correlated with disease activity. Considering its relevance to the clinical diversity of ITP patients, the Treg/Th17 ratio may have prognostic significance in ITP patients.32 Moreover, the imbalance of Treg/Th17 may also be relevant in the pathogenesis of ITP.
Comparing the BM's frequency of T helper (Th) subsets, Tregs and their effector cytokines in a group of adult active ITP patients with those of the PB of HCs found higher frequencies of Th22, Th17, Th1, and follicular Th (Tfh) cells, but the Tregs were remarkably less numerous in the BM of the ITP patients than those of HCs. Evidently, in ITP, the numbers of BM Th22, Th17, Th1, Th2, and Tfh cells were significantly elevated than those in the PB; in contrast, their Treg numbers in the BM were considerably reduced than that in the PB. These findings may be relevant to the pathogenesis of ITP.33 In ITP, the BM's megakaryocytes were defective. As the significantly increased numbers of Th1, Th17, and Th22 cells were found to coincide with the significantly decreased numbers of Tregs in the BM of active ITP patients, dysregulated immune responses could have taken place in their BM. Interestingly, Th22 is a recently identified CD4+ Th subset being involved in human autoimmune diseases.33 In the PB of ITP patients, the increase of Th22 cells may indicate a higher probability of bleeding as Th22 cells act primarily in the skin and mucosal tissues.33 Moreover, Th17 cells having strong proinflammatory properties may play important roles in the pathogenesis of ITP.34 As in the ITP patients, there was a higher percentage of Th17 cells in the BM than in the PB coupled with a decreased percentage of Tregs in the BM than in the PB, their BM was deemed to have a more severe immune dysregulation.35 Thus, the higher numbers of Th22, Th17, Th1, and Tfh cells but a reduced percentage of Tregs in the BM may well be relevant in the pathophysiological process of ITP.
Moreover, another study found the percentages of PD-1+CD3+CD4+ T cells and PD-L1+HLA-DR+CD11c+ dendritic cells were higher in ITP patients than those of HCs. The levels of interferon-gamma (IFN-γ), interleukin-17(IL-17), and soluble PD-1 (sPD-1) in the serum of ITP patients were also found to be increased but transforming growth factor-β (TGF-β) levels were decreased. Notably, the level of sPD-1 was found to be negatively correlated with platelet counts. Moreover, IFN-γ and IL-17 levels of ITP patients were found to be decreased after sPD-L1 administration and the level of IFN-γ in ITP patients remained higher after the anti-PD-1 blockade.36
Theoretically, as the level of sPD-1 was found to be directly related to the severity of ITP, sPD-1 may be akin to a beneficial “braking effect” against excessive autoimmunity. Moreover, with more autoimmune actions, a proportionately heavier “braking effect” through the action of higher sPD-1 levels may be a “protective” mechanism. Notably, in ITP patients, adding an extra supply of sPD-L1 to activate PD-1/PD-L1 could help to restore the imbalance of Th1/Th2 and Treg/Th17 cell subtypes. Similarly, anti-PD-1 administration may exacerbate the disease by enhancing IFN-γ production. Moreover, as sPD-1 was negatively correlated with platelet counts, it could indicate the degree of severity of ITP.36 Now, the severance of such natural “protective” mechanisms (by administrating immune checkpoint inhibitors (ICIs)), may allow ITP to occur as the natural “protective” effect would then be overwhelmed by administering ICIs. Indeed, secondary ITP has never been reported following ICI administration for non-small cell lung cancer.4
10 REGULATORY T CELLS
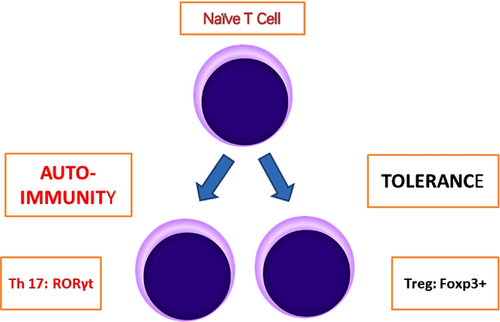
Basically, epigenetics may involve changes in gene expression not involving alterations in the DNA sequence, for example, DNA methylation, histone modifications, and inhibition by non-coding RNA (ncRNA). Upon methylation, gene expression is repressed. For demethylation, the active participation of the ten-eleven translocation (TET) enzymes is required.37 Characteristically, Tregs express the transcription factor forkhead box P3 (Foxp3). The subset of CD4+ Foxp3+ T cells is crucial for controlling autoimmunity, all immune responses and against environmental antigens. Tregs may develop naturally in the thymus (nTregs), or through the conversion of naïve CD4+ T cells in the periphery (pTregs).38 Central Tregs (cTregs) are found mostly in secondary lymphoid organs, while effector Tregs (eTregs) are found mostly in secondary lymphoid organs. Th17 are Tregs that express cytokine IL17, transcription factor RORγt (Figure 2) as well as other expressions and are found to be predominantly proinflammatory.34
Although Tregs may be found in various tissues of the body (e.g. the skin and the intestines), visceral adipose tissue (VAT) Tregs deserve more attention. They are nTregs and play a vital role in the regulation of glucose and insulin tolerance. Yet, obesity would lead to a diminished VAT Treg compartment. Incidentally, a high-fat diet may trigger increased production of pro-inflammatory cytokines, TNFα, IL-6, and macrophage infiltrations in the VAT. Metabolic derangements caused by VAT Treg cell dysfunctions may also mimic those that contribute to a higher incidence of type 2 diabetes mellitus and obesity.38
11 FOXP3 IS A “MASTER”
Tregs characteristically express Foxp3, a master regulator of Treg development and function. First identified in the Scurfy mice, the defective gene exhibiting hyperactive CD4+ T cells and proinflammatory cytokine overproduction, was subsequently also found to be responsible for IPEX (the human counterpart of the immune dysregulation named, immune dysregulation polyendocrinopathy enteropathy X-linked) – both having mutations of Foxp3. Eventually, it was found that CD25+CD4+ peripheral T cells and CD25+CD4+CD8- T cells in normal mice expressed Foxp3. After transduction by Foxp3, naïve T cells also upregulate the expression of CD25 and other Treg-associated cell-surface molecules, for example, cytotoxic T cell-associated antigen-4.39 Actually, apart from Foxp3, other genes, for example, Il2ra, Ctla4, Tnfrsf18, Ikzf2, and Ikzf4 are also active regarding Treg functions. These are responsible for various epigenetic changes, for example, DNA methylation – a repressive mark (decreased gene expression).37 Notably, the subpopulations of Foxp3+ T cells (nTregs and pTregs) are more effective and stable than others, for example, iTregs, central (cTregs) and effector (eTregs).
12 THE CONSERVED NON-CODING SEQUENCE OF FOXP3
The Foxp3 gene locus contains conserved non-coding sequence (CNS) elements for recruiting transcription factors to regulate gene expressions. Moreover, CNS2 has a Treg-specific demethylated region (TSDR), and this is largely demethylated in nTreg cells. Notably, AA may stabilize Foxp3 expression by demethylation of the CNS2 region in iTreg cells as mediated by the TET demethylase.40 In fact, Tregs are rather plastic. These specialized cells may not only retain the Foxp3 expression but also acquire the expression of transcription factors associated with effector T cell programs. Thus, plasticity is an important feature for Tregs as they have to exert a variety of specific suppressive activities toward various types of inflammation. Tregs (as often found in non-lymphoid organs, adipose tissues and the intestines) would display marked tissue-specific heterogeneity.
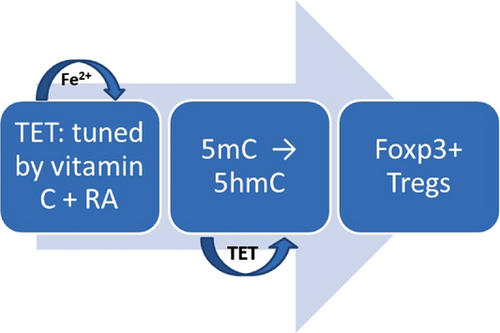
Although downstream regulatory elements are located in CNS1–3,41 the recently discovered CNS0 would diminish DNA demethylation in TSDRs. Its depletion may also affect the Foxp3 gene locus on several downstream enhancers (CNS0–3).37 Remarkably, although iTregs are unstable in vitro, AA potentiates CNS2 demethylation and the stability of Foxp3 expression in human iTreg cells.42 Demethylation in the CNS2 region of the Foxp3 gene is vital to ensure Treg stability and functionality (the major TSDR). Thus, AA has a remarkably profound effect on iTregs by acting as a cofactor for TET proteins (Figure 3). Importantly, studies on iTregs and AA found deep CNS2 demethylation in stimulated cells with high stability indicating the unique potential usefulness in therapeutic applications.37, 43
Taken together, although Tregs are very important for immune tolerance when subjected to various environmental stress, they may have altered stability, plasticity and tissue-specific heterogeneity, and their suppressive functions are thus affected. Various factors, for example, micronutrients, vitamins, and metabolites may affect Treg stability, plasticity, and tissue-specific heterogeneity.40 As Tregs lose their functions, peripheral tolerance is lost and autoimmune diseases would ensue. Yet, upon restoring tolerance by upregulating Tregs, it would have a natural advantage of restoring Tregs that are indispensable for the establishment and maintenance of immunological self-tolerance.44 Upon restoring malfunctioning Tregs, a durable state of affairs ensues even without undue adverse effects. This appears to be very fundamental, uniquely natural and highly appropriate.
13 TPO RECEPTOR AGONISTS
TPO-RAs recently approved work well for ITP. TPO-RAs activate TPO-Rs on megakaryocytes and stimulate platelet production. The response rate to TPO-RAs is 60%–80% despite chronic ITP refractory to one or more lines of therapy.45 Although the approval conditions appear too stringent as eligible patients are limited to those aged ≥1 year and with chronic ITP refractory to at least one other treatment, for example, steroids, IVIG, there may well be real concerns for the long-term safety of TPO-RAs. Moreover, “off-target” effects do occur, for example, eltrombopag would chelate extra- and intra-cellular calcium as well as iron and subsequently, shuttle iron out of cells.46 Remarkably, in the EXTEND study consisting of 302 patients, as many as 55% of study patients withdrew from the study because of adverse events (14%), patient decision (13%), lack of efficacy (11%) or other reasons. Moreover, in the long-term romiplostim study consisting of 292 patients, 31% discontinued due to patient decision (27%), adverse events (12%), alternative therapy (12%) or other reasons.47 Although 14% of patients on eltrombopag had stopped treatment because of adverse events, in general, TPO-RAs have an acceptable safety profile. For transaminitis that occurs in up to 10% (mostly due to eltrombopag), it was largely asymptomatic and reversible. Thrombosis occurred in only 6% and BM reticulin deposition was also rare (1.4%–6%).28 Other side effects, for example, the development of neutralizing antibodies, were mainly observed with romiplostim.
Pragmatically, romiplostim is given as a weekly subcutaneous injection. Although avatrombopag does not have any food intake restrictions, eltrombopag has to be taken 4 h after and 2 h before food that contains cations (iron, calcium, milk or other dairy products). For effectiveness, TPO-RAs were discontinued in approximately 1/3 of patients due to the lack of response.47
14 TPO-RAS: “REBOUND PHENOMENON”
Upon abrupt discontinuation of TPO-RAs, in up to 10% of ITP patients, platelet counts may drop temporarily to below pretreatment levels, and tapering is often adopted.47, 48 Nevertheless, 10%–30% of patients on TPO-RA, can discontinue their TPO-RA without further treatment. This is possible through the improvement of Treg function that can uniquely restore immune tolerance to platelets – an indirect effect of TPO-RAs that may ironically be crucial for maintaining a more prolonged response49 (see Section on “The CNS of Foxp3”). Actually, one of the ways for TPO-RAs to restore Treg function appears to be mediated through promoting the secretion of TGF-β1.49, 50 Of the three known isoforms of TGF-β, TGF-β1 is ubiquitously expressed.51 It can convert Foxp3- T cells to Foxp3+ Tregs. However, theoretically, such an indirect effect may be less consistent and this inconsistency might have affected the overall efficacy of TPO-RAs. Notably, steroids have been found to act on Tregs52 and clinical trials on the combination of TPO-RAs and steroids are also ongoing (see Table 2). Actually, other than steroids, rituximab has also been considered except for its inappropriateness during pandemic periods.
| Year | TPO-RA | F/R | Combination Agents | Endpoint | Evaluation Period | Country | NCT No. |
|---|---|---|---|---|---|---|---|
| 2020 | romiplostim | F | LD rituximab, HD dexamethasone | response | 6 mo | Mexico | NCT04588194 |
| 2020 | eltrombopag | R | Rituximab† | response, safety | 3 mo | China | NCT04518475 |
| 2020 | eltrombopag | F | HD dexamethasone | response, safety | 18 mo | Germany | NCT04346654 |
| 2018 | eltrombopag | F | dexamethasone, P, HD | response, safety | 6 mo | Hong Kong | NCT03830749 |
| 2015 | eltrombopag | F | LD rituximab, HD dexamethasone, | response | 6 mo | Mexico | NCT02834286 |
| 2012 | eltrombopag | F | HD dexamethasone | response | 6 mo | Mexico | NCT01652599 |
- † A single infusion of rituximab 375 mg/m2 per patient. F: fresh (treatment naïve); HD: high-dose: 40 mg orally; days 1–4); LD: low-dose (100 mg weekly for four weeks); mo: months; NCT: National Clinical Trial (US); P: pulsed; R: relapsed; TPO-RAs: thrombopoietin receptor agonists.
15 TPO-RA: A BONUS ACTION
Apart from the TPO action of TPO-RAs on megakaryocytes, actually, TGF-β1 secretion was also enhanced,49, 50 and Tregs were found to be upregulated. However, the polarizations of Th1 and Th17 were not rectified. In principle, with TPO-RA's additional action on Tregs, for those ITP patients not responding to or upon relapse after TPO-RA treatment, it is appropriate to boost the effect on Tregs by combining with other Treg enhancing agents, for example, AA or steroids.52 Currently, as clinical trials are already ongoing for combining with steroids (see Table 2), AA should now be considered seriously, especially for its effectiveness according to pre-clinical studies (see Section on “The CNS of Foxp3”) being coupled with a good safety profile and eminent affordability. As a pilot study, AA may be an appropriate adjunctive agent for unsuccessful tapering of TPO-RAs (to prevent the “rebound phenomenon”),47, 48 and especially in cases of unplanned discontinuation of TPO-RAs (e.g. mandated by the development of side effects). For all such problems related to the tapering of TPO-RAs, AA may well have significant contributions. Admittedly, some trial and error efforts on the dosage and scheduling are definitely required.
Incidentally, from unpublished personal anecdotal clinical experience, HDIVC in the range of 100–250 mg/kg was found to be a useful adjunct for managing oncology complications and related incidental treatment-related injuries.53 Although such an oncology experience may not be directly relevant to ITP, the dosage range appears to be close to that for preventing cytokine storms (see below). Even though this dose is below that of HDIVC for cancericidal purposes,54 it may well be a prudent adjunctive dosage of AA suitable for an initial pilot clinical study, for example, as an adjunct for failed tapering or unplanned discontinuation of TPO-RAs. After such pilot studies, more formal clinical trials of HDIVC for ITP may then be undertaken. In principle, AA is not a direct competitor of steroids. Rather, AA's role is more likely to be an alternative to rituximab which is inappropriate during periods of pandemics.
16 NOVEL AGENTS FOR ITP
Fostamatinib, a spleen tyrosine kinase inhibitor targeting macrophages, has been approved for ITP. It has an overall response rate of almost 50% with continual treatment and an 18% rate of stable responses in heavily pretreated ITP patients. However, its low stable response rate may render it unlikely to replace rituximab, TPO-RA or splenectomy. Despite this lower efficiency in multi-treated and refractory patients, fostamatinib would still be a viable option for refractory ITP.55 Other potentially useful agents may include rapamycin (that blocks the mTOR pathway) and low-dose decitabine which may have immunomodulating effects. However, as these have not yet been approved for ITP, better documentation of the long-term effects may be advisable.56 Moreover, side effects may be significant, for example, the oncology clinical experience on mTOR inhibitors – a potentially fatal interstitial pneumonitis.57
17 PERSPECTIVES IN THE ERA OF TPO-RA
As splenectomy is known to involve mortality of up to 1% and a sepsis risk of 2%, in the TPO-RA era when there is a reasonable chance of achieving spontaneous remission of ITP within the first year, splenectomy is now deferred until the patient is confirmed to have chronic ITP.7
Now, even in the TPO-RA era, the strategy of using a combination of agents is still worthwhile and probably more productive than TPO-RA monotherapy. For instance, initial attempts of adding steroids to TPO-RAs appear to overcome their “resistance”.58, 59 This is likely to be the mutual enhancement effect on Tregs as both have similar actions on Tregs, albeit one agent's action may be weaker than the other. Although a usual strategy is to combine agents having different mechanisms of action,56 combinations using AA may well be exceptional – as, with the good safety profile of AA, no significant toxicities are added to the combination.
Although the action of TPO-RAs is on enhanced platelet production, Liu et al. have recently found that there was also a mechanism for reducing the excessive phagocytic capacity of monocytes/macrophages – the restoration of monocyte FcγR balance toward inhibitory FcγRIIb. This would enable a correction of ITP's enhanced phagocytic capacity.60 Moreover, in ITP patients, opsonization of platelets resulted in enhanced platelet destruction through FcγR-mediated phagocytosis of macrophages. Thus, TPO-RAs can also increase platelets by decreasing platelet destruction. This is likely to be a bonus action that upregulates Tregs.45
Importantly, as with current pharmacokinetic concepts, iv AA can achieve very much higher pharmacologic blood levels than that by the oral route, HDIVC may now be tried as an adjunct agent for TPO-RA agents. Admittedly, pilot clinical experience on its effectiveness as monotherapy is also mandatory. Meanwhile, with its good safety profile, it might also be tried by combining with TPO-RAs on compassionate grounds, for example, in non-responding or relapsed cases after trying TPO-RAs. Such unresponsive cases may represent a less robust indirect action on Tregs by TPO-RAs.45, 50, 60
As there is a possible failure of the indirect effect of TPO-RAs on Tregs, it may be worthwhile to boost such Treg functions and eventually restore the desired self-tolerance effect.39 In case the failure is really due to an inadequate action of TPO-RA on Tregs, the addition of another agent to enhance the Treg function may work well by mutual enhancement. A rather extreme example of this is the very well-known risks of mixing a hypnotic with alcohol. Although not all mutual enhancements are as powerful, at least the side effects of HDIVC are less than combining TPO-RA with rituximab. Without adding significant toxicity, HDIVC combined with TPO-RAs may have the best of both worlds – not only a possible mutual enhancement effect on Tregs but also combining diverse drug mechanisms as TPO-RA acts mainly on megakaryocytes. Unfortunately, ITP is too uncommon to enable large and systematic clinical trials.
18 DISCUSSION
Despite kicking off a new era of managing ITPs, TPO-RAs may fall short of constituting a panacea for all pathologies of ITP. For instance, TPO-RAs cannot correct the polarizations of Th1 and Th1750 except upon combining with pulsed high-dose dexamethasone.61 This highlights the vital importance of adopting the combination strategy even in this era of TPO-RA.
In 1988, although a “child” was born when Brox et al. of Mc Gill University, Canada, reported AA to be active for ITP in seven of 11 patients,9 this “child” had apparently “failed to thrive”. As subsequent series had merely equivocal results, AA for ITP has been gradually forgotten. Fortunately, this “child” has actually grown when one sheds light on the very peculiar pharmacokinetic concepts of AA more than 20 years ago.18, 62-64 Unlike other nutrients, AA has an exceptionally tight intestinal absorption restriction permitting only a narrow range of blood levels. Most surprisingly, the historical AA oral dose of 2 g has not changed throughout 3 decades, despite new pharmacokinetic concepts. Even a few years ago, the same dose of AA was still mentioned.65 Actually, with the current pharmacokinetic concept, HDIVC should now be used for any therapeutic purposes. Moreover, it is also well known that the blood level of AA may drastically be reduced especially with chronic illness. Theoretically, the historical 2 g oral AA dose could have been consumed for replenishing pre-existing but sub-clinical AA deficits without much leftover to act on ITP. In ITP patients, Tregs are much fewer, malfunctioning and immune tolerance is also lost. Restoring such abnormalities not only benefits the autoimmune disorder but also the immunological self-tolerance.39, 44 A durable state of affairs can thus be achieved but without undue adverse effects: a fundamental, natural and highly appropriate remedy.
Intriguingly, similar to the Treg malfunctioning of ITP, Tregs are also abnormal in cancer – being too active.66 The Treg pathology has been described as at the “intersection” between autoimmune diseases and cancer.67 Moreover, the demethylation mechanisms also appear to benefit both autoimmunity and cancer and they also involve closely similar mechanisms, for example, both involve TET enzymes.68 With such a co-benefit, a similar experience of using AA for demethylating purposes for cancer may help; to guide the decision on an appropriate dosage for demethylation in ITP. For instance, AA is known to cause genome-wide demethylation in cancers through demethylation as enhanced by TET enzymes.68 Moreover, for renal cell carcinoma cells, although 5 mM of AA for 4 h was required for adequate cytotoxicity, 1 mM of AA for 2 h was already adequate for demethylation purposes.69 As oral AA would never achieve a blood level close to 1 mM (see Table 1), obviously, the equivocal experience on oral AA for ITP has to be renewed.
Although alternative medicine practitioners may give HDIVC averaging 28 g iv for various indications,54 the personal compassionate usage of HDIVC in oncology involved lower dosages of 100–250 mg/kg. Moreover, such a lower HDIVC dosage was also found to be sufficient for neutralizing the harmful effects of cytokines. For instance, for ultra-marathon runners, AA 1 g iv may decrease potentially harmful blood cytokines.20 Moreover, IL-6 may well be specifically responsible for the highly lethal “cytokine storm” of severe sepsis that occasionally occurs in cancer patients. Thus, the use of HDIVC to reduce such significant cytokine effects has also been considered for reducing mortality rates. According to a limited meta-analysis, effective levels of HDIVC appeared to be iv 25–50 mg/kg.70 Fowler et al. had also found that either 50 or 200 mg/kg of HDIVC is useful for controlling excessive inflammation in sepsis.14 Thus, the adoption of a dose of 50 mg/kg of AA given iv once to twice a week may well be appropriate for pilot studies for ITP. Moreover, the blood levels of AA should be documented in all clinical trials before HDIVC, otherwise, the usefulness of results may be jeopardized. Admittedly, although drug testing for other common diseases is very much more systematic, the rarity of ITP precludes such an approach.
Despite high AA intakes, a fixed plateau blood level (70–80 μM) ensues,18, 71 and tissue distributions diversify, for example, from 0.05 mM (erythrocytes) to 0.8(brain) to 2 mM (adrenals). Intriguingly, for stress and infections, neutrophils’ content can abruptly increase, from 1.4 to 12 mM.72 Importantly, considerable stress effectively exhausting AA reserves may risk reduced immunity and even frank exposure to harmful cytokines purposely induced.14, 20, 70 Moreover, although an antioxidant at physiological concentrations, AA becomes a pro-oxidant at higher concentrations (e.g. at >15 mM) exhibiting cancericidal,54 bacteriostatic,73 or antiproliferative74 effects. Normally, HDIVC induces a rapid and quantitative renal clearance, rather than the usual saturable active transport64; boasting urinary concentrations of 1500:1. Thus, normal plasma concentrations soon resume in ∼16 h.64 With a short plasma half-life (2 h), peak plasma AA concentrations of even 1.5–37.8 mM (infusing 5–60 g HDIVC) had post-infusion plasma levels declining very rapidly.75 Thus, AA's pharmacokinetics may demand daily administrations for actions effective only at top AA concentrations.54, 73, 74 However, for actions requiring much lower dosages (100–250 mg/kg), personal anecdotal experience (mainly for managing oncology complications) suggested that twice weekly HDIVC was already active despite its very short half-life.
Intriguingly, besides enhancing demethylation, AA may also have opposite effects, for example, IL-17 induction under Th1 polarization (by Song's group)76 and Th1 enhancement (upon in vivo parasitic infestation).77, 78 Song's group purposely induced naïve CD4+ T cells to produce IL-1776; AA's concentration was similar to human physiological levels (10 μg/ml [57 μM]), but other antioxidants studied lacked such effects and no Th1 changes were reported. The purposeful IL-17 induction was akin to an incidental clinical event. During infestations, and especially infections, neutrophils promptly acquire AA too much higher levels79; antiviral cytokines may also be induced.80 With its known dual role (pathogenic and protective),81 IL-17 is unlikely the major player (difficult to balance related prevailing factors between two diverse actions). Despite the pre-clinical effects on demethylation (by either IL-17 or Th1), clinically, IL-17 induction, say upon infection, is often exceptional and short-lived. Moreover, Th1 enhancement for parasites77, 78 is even more exceptional in developed countries. Effectively, demethylation interference is probably minor clinically. Importantly, Song's group also reported that a higher AA concentration (100 μg/ml [570 μM]) possessed demethylation effects.82 Others also reported AA concentrations of ∼1000 μM69 had highly stable and deep demethylation effects37: most probably more resistant to Th1 or IL-17 interference. Moreover, steroids may correct the polarizations of Th1 and Th17 of ITP,61, 73 despite the failure of TPO-RAs.50 Clinically, significant infections often attract steroid administrations83: likely masking most other effects (see above). Eventually, clinical trials may determine the most desirable dosage. Nevertheless, both of the aforementioned pre-clinical studies may help to throw light on AA's inconsistent historical results.9, 84
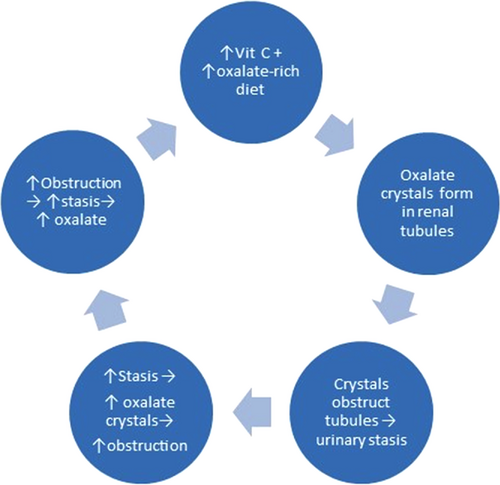
Although AA is metabolized to oxalate, three factors may reduce this process. First, as AA tends to bind with calcium ions85; calcium oxalate crystallization is actually reduced by AA's presence. Secondly, with a normal renal function having negligible urinary stasis, only 0.5% of AA converts to oxalate.25, 86 Lastly, even HDIVC had no notable renal stone formation, despite 8% of those patients having a history of renal stones.26 Importantly, the amount of AA converting to oxalate is crucially proportional to the renal time span.86 With increasing urinary obstruction and stasis, oxalate crystallization from AA would increase proportionately. The incidental personal experience also suggested a “taboo combination” of prolonged gram-dose AA with excessive oxalate-rich food: oxalate nephropathy (ON) might ensue.87 ON has been reviewed in detail elsewhere.88 Briefly, it is renal failure due to calcium oxalate crystal obstruction. Chocolates, spinach, carrots, celery, rhubarb, nuts, and starfruit88, 89 are oxalate-rich. Especially, when starfruit is coupled with dehydration, ON may rapidly ensue.89 Thus, apart from ON's other causes (primary and enteric hyperoxaluria90 and fat malabsorption),88 for ingestion hyperoxaluria, AA is hardly the main culprit of the “taboo combination”. Clearly, ONs have occurred even without any AA supplementation).89.91 Moreover, even HDIVC lacked notable oxalate stone formation problems,25, 86 let alone oral intakes. Moreover, dehydration/hydration is closely associated with oxalate crystallization.91, 92 Although initially scanty oxalate crystals would hardly obstruct renal tubules, as crystals accumulate progressively, especially when precipitated by dehydration, progressive obstruction due to rapidly accumulating oxalate crystals may cause significant urinary stasis. More and more crystals so formed with increasing urinary stasis would initiate a vicious cycle (Figure 4). Ultimately, ON ensues. Not only oxalate-rich food but also AA supplementation must discontinue promptly as the oxalate crystals had already caused a “secondary” renal dysfunction contraindicating gram dosages of AA.
19 CONCLUSIONS
Tor ITP, although the historical oral dose of 2 g of AA gave variable results, there is ample pre-clinical evidence that AA actually has a robust demethylating action.37, 43 As Tregs so restored play a critical role in the maintenance of peripheral tolerance,49 AA may thus have a good potential role for treating ITP. Notably, the modern pharmacokinetic concept on an exceptionally tight intestinal AA absorption restriction would demand iv administration for employing AA for therapy purposes. Moreover, as Sakaguchi has indicated,93 for deranged Tregs, it is most prudent to restore Tregs so as to achieve a stable and lasting self-tolerance.
It is now very appropriate to repeat the clinical trials for ITP but to use iv AA administration to bypass the tight intestinal control on oral AA. In this era of TPO-RA, both unsuccessful tapering of TPO-RA and especially the unplanned discontinuations (e.g. due to side effects) would offer opportunities for trials on adjunctive HDIVC. Actually, both steroids and rituximab have been found to be potentially useful adjuncts to TPO-RAs and clinical trials, mostly on combining with steroids, are already ongoing (see Table 2). However, during pandemics, rituximab would be contraindicated and recruitment to trials is thus affected. Appropriately, HDIVC may now be explored initially for its role as an adjunctive agent. Now, the recently discovered bonus effect of TPO-RAs on Tregs may or may not always be consistent and would thus be another opportunity for trials on HDIVC. Moreover, as both steroids and AA act on Tregs, a mutual enhancement effect may exist. If so, this would be another advantage to combine them, especially as no toxicity is added. Lastly, despite the failure of TPO-RAs to correct the polarization of Th-1 and Th17,50 steroids commonly given clinically may act on Tregs.52 Thus, the trials on combining with steroids (see Table 2) may be very appropriate.
After some initial experience on HDIVC for ITP, more systematic trials may then be considered. Conceptually, AA is not a competitor of steroids which is expected to work well with TPO-RAs. However, with a good safety profile and eminently affordability, AA would appear to be a close rival to rituximab, especially during pandemic periods (see Table 3). Nevertheless, how well would HDIVC compare with rituximab remains to be determined. By all means, before testing possible combinations of HDIVC for ITP, one needs adequate clinical trial data for the most appropriate dosage range, scheduling and clinical effectiveness, especially as monotherapy.
| Rituximab | Vitamin C (HDIVC) | |
|---|---|---|
| Main action | Anti-CD20 antibody | Treg restoration |
| Regulatory status | Off-label use, 2nd line | Compassionate use§ |
| Recommended dosage | 375 mg/m2 | 50 mg/kg |
| Administrative mode | Infusion | Infusion |
| Administrative frequency | Weekly | Weekly† |
| Overall response rate | 60%94 | 0%–60%ǂ |
| Adverse effects | Significant | Insignificant |
| Contraindications | During pandemics2 | G6PD def; RF, RS |
| Healthcare costs | High | Low |
| Biosimilar availability | Yes95 | NA |
| Preparation availability | Off the shelf (in stock) | Import license§ |
- † : proposed.
- ǂ : inappropriate route of administration employed.
- § : required, for example, for “compassionate use” (used on compassionate grounds upon exhausting all other drugs) for the “preservative-free” preparation for doses higher than 1 g; approved preparations, for example, of 0.5 g may contain preservatives (doses >1-2 g not recommended).
- Def: deficiency; HDIVC: high-dose intravenous vitamin C; NA: not applicable; RF: renal failure; RS: renal stone.
AUTHOR CONTRIBUTIONS
Dr. Shiu Ying Tsao contributed to the preparation and collection of original literatures and figures and the writing and editing of manuscript. Dr. Shiu Ying Tsao was responsible for the structural designs, scientific quality and writing.
ACKNOWLEDGEMENTS
Not applicable.
FUNDING INFORMATION
Not applicable.
CONFLICT OF INTEREST
The author declares no conflict of interest. The paper was handled by editors and has undergone a rigorous peer-review process. Dr. Shiu Ying Tsao was not involved in the journal's review of/or decisions related to this manuscript.
ETHICAL APPROVAL
Not applicable.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.




