Multifunctional inorganic nanomaterials for cancer photoimmunotherapy
Abstract
Phototherapy and immunotherapy in combination is regarded as the ideal therapeutic modality to treat both primary and metastatic tumors. Immunotherapy uses different immunological approaches to stimulate the immune system to identify tumor cells for targeted elimination. Phototherapy destroys the primary tumors by light irradiation, which induces a series of immune responses through triggering immunogenic cancer cell death. Therefore, when integrating immunotherapy with phototherapy, a novel anti-cancer strategy called photoimmunotherapy (PIT) is emerging. This synergistic treatment modality can not only enhance the effectiveness of both therapies but also overcome their inherent limitations, opening a new era for the current anti-cancer therapy. Recently, the advancement of nanomaterials affords a platform for PIT. From all these nanomaterials, inorganic nanomaterials stand out as ideal mediators in PIT due to their unique physiochemical properties. Inorganic nanomaterials can not only serve as carriers to transport immunomodulatory agents in immunotherapy owing to their excellent drug-loading capacity but also function as photothermal agents or photosensitizers in phototherapy because of their great optical characteristics. In this review, the recent advances of multifunctional inorganic nanomaterial-mediated drug delivery and their contributions to cancer PIT will be highlighted.
Abbreviations
-
- APC
-
- antigen-presenting cell
-
- AuNPs
-
- Gold nanoparticles
-
- BP
-
- Black phosphorus
-
- BPNS
-
- Black phosphorus nanosheet
-
- BPQD
-
- Black phosphorus quantum dot
-
- CAR
-
- chimeric antigen receptor
-
- Ce6
-
- chlorin e6
-
- CNT
-
- carbon nanotube
-
- CTL
-
- cytotoxic T lymphocyte
-
- CuS nanoparticles
-
- copper sulfide nanoparticles
-
- DC
-
- dendritic cell
-
- DOX
-
- doxorubicin
-
- GC
-
- glycated chitosan
-
- GO
-
- graphene oxide
-
- GSH
-
- glutathione
-
- HA
-
- hyaluronic acid
-
- HMS
-
- hollow mesoporous silica
-
- ICD
-
- immunogenic cell death
-
- ICG
-
- indocyanine green
-
- IONPs
-
- Iron oxide nanoparticles
-
- LY
-
- LY364947
-
- MSNs
-
- mesoporous silica nanoparticles
-
- MWNT
-
- multi-walled nanotube
-
- NE
-
- neutrophil
-
- NIR
-
- near-infrared
-
- PDT
-
- photodynamic therapy
-
- PEI
-
- polyethyleneimine
-
- PET
-
- positron emission tomography
-
- PIT
-
- photoimmunotherapy
-
- PpIX
-
- protoporphyrin IX
-
- PTA
-
- photothermal agent
-
- PTT
-
- photothermal therapy
-
- RES
-
- reticuloendothelial system
-
- rGO
-
- reduced graphene oxide
-
- ROS
-
- reactive oxygen species
-
- SWNT
-
- single-walled nanotubes
-
- TAA
-
- tumor-associated antigen.
-
- TME
-
- tumor microenvironment
-
- Treg
-
- T regulatory cell
1 BACKGROUND
Cancer remains a dominated cause of death that threatens the public health over the past decades. Currently, the major treatment modalities to cancer in clinic are surgery, chemotherapy, and radiotherapy. These cancer therapies purpose to eliminate cancer cells but do so at the same expense of normal cells, which sometimes causes serious adverse effects, accelerating the cancer progression in turn. In addition, the metastasis of cancer is the leading cause of treatment failure and cancer-related deaths. Therefore, a lot of newly developed treatment strategies, including targeted therapy, immunotherapy, gene therapy, and phototherapy, have been practically adopted in clinic or in clinical trials to treat cancer metastasis [1, 2]. In particular, cancer immunotherapy, which can either instigate or elevate the host immune response to track and clear away cancer cells, has gained much attention. Recently, many immunotherapy approaches, including checkpoint blockade therapy, cytokine therapy, monoclonal antibody therapy, tumor vaccination, and chimeric antigen receptor T-cell therapy, have been explored both pre-clinically or in clinical studies to treat cancer metastasis [3]. However, most cancer types are inherently immunosuppressive and are difficult to be controlled through the current immunotherapy [4]. Hence, the combination of topical therapy like phototherapy can cleverly overcome the limitations of immunotherapy as well as maximize the merits of both therapy modalities, which can not only activate immune system and achieve long-term immunity against cancer but also increase the selectivity of the overall treatment [5].
Phototherapy is known as a non-invasive or slightly invasive treatment approach that utilizes laser to transport energy to certain target tissue, providing an alternative solution for primary tumor elimination due to its high selectivity in light transportation, low degree of trauma as well as effectiveness in clearing up target tumor [6]. Near-infrared (NIR) light serves as the commonly used light source for phototherapy due to its deep penetration ability into biological tissues. The application of NIR light in phototherapy is often accompanied with in situ administration of natural absorbance agents such as photoactive agents or photosensitizers [7]. These photoagents can convert absorbed photon energy into thermal energy, which is termed as photothermal therapy (PTT) for the hyperthermia treatment of cancers, or absorb the specific light energy to generate cytotoxic reactive oxygen species (ROS), which is known as photodynamic therapy (PDT) [8]. It was also found that the immune response triggered by PDT treatment contributed to a significant role in inhibiting tumor recurrence [9]. Therefore, to reach the goal of destroying primary tumors and relieving metastasis, a strategy called photoimmunotherapy (PIT) that combines phototherapy and immunotherapy has emerged [10]. PIT is a synergistic treatment approach, of which the merits of both phototherapy and immunotherapy will be strengthened, while the inherent shortcomings are mitigated. The principal working mechanisms that enable phototherapy to largely improve immunotherapeutic efficacy can be concluded as below. Phototherapy serves as the first line of defense against tumor, which is able to effectively eliminate the primary tumors. Subsequently, the released tumor-associated antigens (TAAs) and damage-associated molecular patterns through the induction of immunogenic cell death (ICD) or the promotion of immune cell accumulation in target tumors will trigger immune response and strengthen the immunotherapy effect [11]. Besides, the number of pro-inflammatory cytokines that are able to activate the immune system is also increased [7]. Therefore, PIT is capable of circumventing the difficulties of tumor heterogeneity, tumor mutation, and tumor immune escape, meanwhile, enhancing tumor microenvironment's (TME) immunogenicity, eventually recruiting more antigen-presenting cells (APCs) or reducing immunoregulatory inhibition. This combined therapy modality can not only eliminate primary tumors and clear up residual tumor cells but also track the metastatic or distant sites, providing more possibilities for patients with advanced tumors [12].
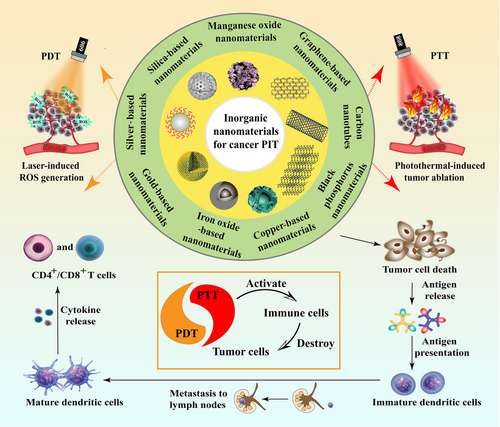
Nanomaterial-based platforms have been extensively investigated in cancer treatment owing to their unique physiochemical properties. They have also captured the researchers’ attention worldwide in the field of both immunotherapy and phototherapy. For instance, nanomaterials that incorporate immunomodulatory agents can stimulate immune cells and remodel the TME to elevate anti-tumor immunity [13-17]. In addition, nanomaterials also protect sensitive antigens or proteins from degradation or inactivation in the complex physiological environment during cancer immunotherapy. There are many kinds of novel nanomaterial-based platforms that possess excellent phototherapeutic performance. For instance, organic nanomaterials like polymeric micelle, nanogel, liposome and dendrimer show great potential in phototherapy-synergized cancer immunotherapy [9, 18, 19]. Inorganic-organic hybrid nanomaterials, such as gold nanocluster and metal-organic framework, also exhibit promising research prospect in cancer PIT [20]. In particular, inorganic nanomaterials, which can directly adsorb light energy or serve as carriers to deliver photoactive agents, are widely explored in PIT. From all of them, metallic, silica-based or carbon-related materials are the most commonly employed nanomaterials in PTT-mediated cancer immunotherapy because of their excellent optical properties as well as convenient surface modification possibility [21]. Black phosphorus (BP) and manganese dioxide have been widely evaluated in PDT-based cancer immunotherapy. These nanomaterials serve as carriers of photosensitizers in PDT, causing tumor cell apoptosis, autophagy, and necrosis [10]. Moreover, several other inorganic nanomaterials like cobalt sulfide nanospheres and zinc oxide nanoparticles also show great research potential in phototherapy-induced immunotherapy [22, 23]. Importantly, both PTT and PDT can significantly inhibit primary tumors, trigger immune response, and induce systemic anti-tumor effect, which largely improve the therapeutic efficacy of cancer immunotherapy. Therefore, this review mainly focuses on the recent advances of inorganic nanomaterial-mediated drug delivery in cancer PIT. Various inorganic nanomaterials classified according to their biodegradability will be discussed in detail (Figure 1). Moreover, an overview of inorganic nanomaterial-mediated cancer photoimmunotherapy is summarized in Table 1.
| Type of nanomaterials | Phototherapy model | Immunomodulator | Additional photosensitizer/photothermal agent | Tumor model | Effectiveness | Ref. |
|---|---|---|---|---|---|---|
| Black phosphorus | PTT + PDT | N/A | N/A | Murine breast cancer | Remodel TAMs phenotype, inhibit original tumor growth and prolong the survival time | [31] |
| PTT + PDT | TGF-β inhibitor LY364947 (LY) | N/A | Murine breast cancer | Induce potent immune activation and inhibit lung metastasis | [32] | |
| PTT | Anti-PD-1 | N/A | Murine breast cancer | Inhibit primary and secondary tumor growth | [35] | |
| Silica-based | PTT | N/A | ICG | Murine hepatocellular carcinoma | Induce a robust immune response and prevent recurrence | [44] |
| PDT | CpG ODN and neoantigen peptides | Ce6 | Murine colon cancer | Produce potent anti-tumor efficacy against local and distant tumor | [47] | |
| PDT | Anti-PD-1 | Ce6 | Murine breast cancer | Induce robust anti-tumor immunities and suppress the growth of tumor | [50] | |
| Manganese oxide | PDT | PD-L1-targeting siRNA | ICG | Murine lung cancer | Enhance PDT and anti-tumor immune responses, inhibit tumor cell growth | [59] |
| PDT | Anti-PD-L1 | Ce6 | Murine breast cancer | Inhibit primary tumors and prevent metastasis | [11] | |
| PDT + PTT | N/A | CyI | Murine breast cancer | Eliminate primary tumors and inhibit metastasis | [60] | |
| Iron oxide-based | PTT | CpG ODN | N/A | Murine breast cancer | Inhibit primary tumors and prevent metastasis | [68] |
| PTT | Anti-CTLA-4 | N/A | Murine breast cancer | Overcome Treg-mediated immunosuppression and inhibit tumor growth | [69] | |
| PTT | Anti-PD-L1 and R837 | N/A | Murine breast cancer | Eliminate the primary tumor and prevent tumor metastasis to lungs and liver, inhibit untreated distant tumor growth | [71] | |
| Graphene-based | PIT | CpG OND | N/A | Murine colon cancer | Inhibit original tumor growth and prolong the survival time of mice | [83] |
| PTT | TGF-β inhibitor(SB-431542) | N/A | Murine breast and colon cancer | Inhibit primary tumors and prolong the survival time of mice | [88] | |
| PDT + PTT | CpG OND | IR820 | Murine breast cancer | Induce the mitochondrial collapse and irreversible cell apoptosis, inhibit tumor growth with negligible toxic effects | [89] | |
| Carbon nanotubes | PTT | Anti-CTLA-4 | N/A | Murine breast cancer | Destroy the primary tumors, inhibit cancer metastasis at distant sites | [96] |
| PTT | Immunoadjuvant glycated chitosan | N/A | Murine breast cancer | Enhance the tumor immunogenicity and improve the uptake and presentation of tumor antigens, induce tumor cell apoptosis | [97] | |
| Gold-based | PTT | Doxorubicin | N/A | Human breast cancer | Reduce system toxicity and enhance anti-tumor effect | [108] |
| PTT | OVA | N/A | EG.7-OVA tumor model | Inhibit tumor growth and prolong the survival time of mice, promote CD8+ T cell proliferation and IFN-γ+ CD8+ T cells population | [119] | |
| Silver-based | PTT | N/A | N/A | Murine breast cancer | Inhibit the proliferation of primary tumor cells and stimulate the systematic immune response | [129] |
| PTT | Bestatin | N/A | Murine breast cancer | Inhibit the growth of primary tumors and distal lung metastatic nodules | [130] | |
| Copper-based | PTT + PDT | Anti-PD-L1 and CpG ODN | N/A | Murine breast cancer | Cause remarkable damage to tumors, inhibit the tumor growth | [137] |
| PTT | OVA | N/A | Human colon cancer | Enhance anti-tumor immune response, block the rise of the distant tumor | [138] |
- Abbreviations: PD-1, programmed cell death-1; PD-L1, programmed cell death-ligand 1; CTLA-1, cytotoxic T-lymphocyte antigen-4; Ce6, chlorin e6; CpG OND, cytosine guanine dinucleotide oligodeoxynucleotide; ICG, indocyanine green; OVA, ovalbumin; PD-L1, programmed cell death-ligand 1; PDT, photodynamic therapy; PTT, photothermal therapy; TAMs, tumor-associated macrophages; TGF-β, transforming growth factor-β.
2 BIODEGRADABLE INORGANIC NANOMATERIALS
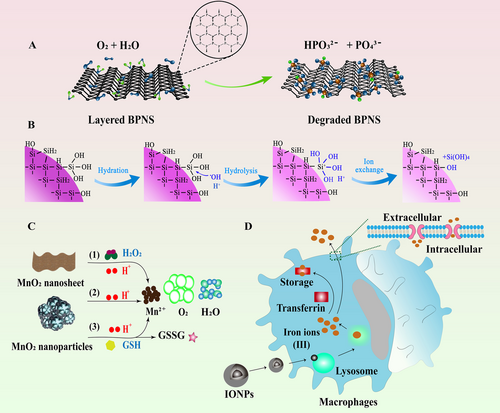
Inorganic nanomaterials serve as novel platforms that build the bridge between drugs and therapeutic targets. Due to their natural physicochemical properties including superior optical, thermal, and magnetic features, as well as their excellent performance in targeted drug delivery, controlled manner of drug release and imaging capability, they have been broadly used in the field of cancer theranostics [24]. However, in practical biomedical application, some inorganic nanomaterials are comparatively difficult to biodegrade in the body, resulting in a long residence time in the organism, thus triggering some adverse effects as a consequence. Therefore, the research and development of biodegradable inorganic nanomaterials plays an important role in facilitating their safe application in biomedical field. In this section, the application of four kinds of biodegradable inorganic nanomaterials, namely black phosphorus nanomaterials, silica-based nanomaterials, manganese oxide nanomaterials, and iron oxide-based nanomaterials, in cancer PIT will be outlined. The different degradation mechanisms of these inorganic nanomaterials are also illustrated (Figure 2).
2.1 BP nanomaterials
BP nanomaterial is one of the emerging inorganic nanomaterials with excellent biocompatibility and biodegradability, exhibiting great potentials as a promising nanomaterial in biomedical field [25, 26]. BP is comprised of flexuous planes of P atoms, which are linked via strong intralayer P-P bonding and weak interlayer van der Waals force to constitute a unique puckered honeycomb structure [27]. Therefore, BP can be exfoliated into BP nanosheets (BPNSs) by breaking down the weak van der Waals forces. These exfoliated BPNSs possess high surface area to enable easy bio-conjugation and molecular loading for drug delivery. In addition, BP possesses other unique properties that endow them as an ideal candidate for anti-cancer application. For instance, BP can be applied as photothermal agent (PTA) for PTT due to its high photothermal conversion efficiency and large NIR extinction coefficients [28]. The local hyperpyrexia generated by BP leads to thermal elimination of tumors. Besides, BP is capable of generating singlet oxygen, which enables their utilization as photosensitizers for PDT [29]. Moreover, the lone-pair electrons on each phosphorus atom cause the relatively high reactivity of BP toward water and oxygen, therefore, BP can easily biodegrade into nontoxic phosphonates and phosphate, improving its safety as a nanocarrier in cancer therapy [30]. Recently, it has been reported that BP-mediated PTT was able to stimulate immune responses and relieve immunosuppressive TME by identification of T lymphocytes and a number of immunocytokines, indicating that BP-mediated nanocomplexes could not only act as effective PTAs to ablate large solid tumors but also function as an immunomodulatory agent to clear up discrete tumorlets (Figure 3) [31]. Therefore, PIT based on BP nanomaterials will provide more possibilities for anti-tumor therapy in the future.
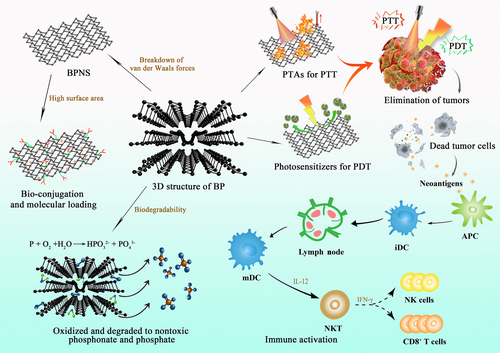
Zhang et al. [31] designed a kind of BP nanoparticles that were modified by PEGylated low molecular weight hyaluronic acid (HA) to improve the targeting specificity and stability of BP, endowing the nanoparticles with multifunctionality for application in PTT, PDT, and immunotherapy. In vitro results demonstrated that these nanoparticles could remodel the phenotype of tumor-associated macrophages by down-regulating M2 macrophage marker and up-regulating M1 macrophage marker. In addition, these multifunctional nanoparticles could remarkably suppress original tumor growth and trigger ICD to release damage-associated molecular patterns to induce the maturation of dendritic cells (DCs) and activate the function of T effector cells in 4T1 tumor model. This study confirmed the outstanding performance of BP nanomaterials in biomedical application and offered rational reference for investigating their potential in future cancer therapy. In our previous work, a biomimetic nanoplatform based on BP nanomaterials was fabricated to achieve light-induced multimodal tumor therapy to amplify the effects of PTT, PDT, and immunotherapy in a synergistic way [32]. The transforming growth factor-β (TGF-β) inhibitor LY364947 (LY) was cooperated to BPNSs through the modification of polyethyleneimine (PEI). Then, the LY-loaded BPNSs were clothed with the membrane of neutrophils (NE) to obtain the final NE/BP-PEI-LY nanoparticles. NE possessed high affinity to the inflammatory sites, and acute local inflammation could be induced after treatment with PTT and PDT. Therefore, NE-modified nanoparticles could precisely target to the tumor site after PTT and PDT. In addition, NE could protect BP from degradation within the body, which increased the stability of the obtained nanoparticles. More interestingly, with the enhanced amount of accumulated nanoparticles in tumor site, the local photothermal and photodynamic effect was further promoted, triggering more nanoparticles to tumor site, and eventually resulting in the positive feedback targeting effect. Moreover, this synergistic anti-cancer therapy based on PTT and PDT combined with LY effectively increased the infiltrating amount of CD4+ T cells and CD8+ T cells, while the amount of T regulatory cells (Tregs) in tumor was significantly decreased. Therefore, the idea to adopt a biological signal induced through anti-cancer immunotherapy to achieve both targeting efficiency and self-amplification of the nanoparticles opens a new world for BP-based targeted drug delivery. Black phosphorus quantum dots (BPQDs) are another existing form of BP nanomaterials that belong to zero-dimensional material and have achieved an increasing attention since their first synthesis [33, 34]. Liang et al. [35] designed a biomimetic formulation based on BPQD-erythrocyte membrane nanovesicle (BPQDs-RMNV), in which erythrocyte membranes were coated to BPQDs, resulting in a long circulation time in the body with enhanced tumor accumulation ability. BPQD-RMNV-mediated PTT was shown to promote 4T1 breast tumor's apoptosis and necrosis. In addition, when combined with anti-programmed cell death-ligand 1 (PD-1) antibody treatment, CD8+ T cells’ infiltration and activity were enhanced in the tumor, significantly delaying the tumor growth in residual and metastatic sites.
2.2 Silica-based nanomaterials
Silica-based nanomaterials are commonly used biodegradable and biocompatible inorganic nanomaterials which are regarded as excellent platforms for various biomedical applications [24, 36]. Mesoporous silica nanoparticles (MSNs), a type of silica-based nanomaterials, have drawn research interest due to their honeycomb-ordered porous structure, which presents their excellent absorption of multitude of bioactive molecules [37]. Moreover, MSNs have been intensively studied for their diagnostic applications to detect various cancers because of their tunable size, outstanding biocompatibility, tailorable surfaces, and great chemical and thermal stability. Recently, it has been demonstrated that MSNs serve as promising candidates for material-based immunotherapy [38, 39]. Their effects on monocyte-derived DCs were dependent on their size and concentration, which means that MSNs with larger particles and higher concentrations are more effective [40]. Besides, MSNs can be engineered to carry TAAs or adjuvants to produce immunomodulatory effects. Importantly, MSN-loaded vaccine can be internalized into APCs to deliver antigenic information due to their small size [41]. Furthermore, MSNs can incorporate with the metallic compounds to realize PTT, enabling the synergistic effect of immunotherapy and PTT with the best therapeutic outcome [42, 43].
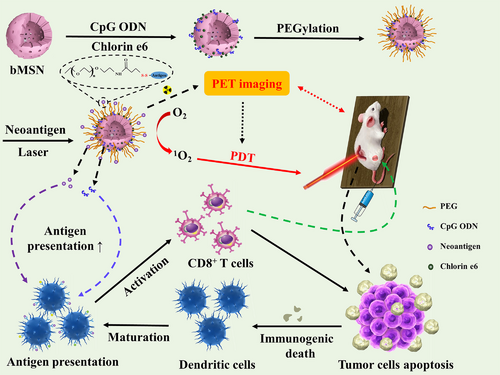
Yang et al. [44] applied mesoporous silica to co-load the PTT agent indocyanine green (ICG) and multikinase inhibitor sorafenib to achieve excellent imaging effect and therapeutic efficacy in hepatocellular carcinoma treatment. Triggered by NIR, this nanocomposite showed a significant PTT-mediated tumor-killing effect on H22 tumor-bearing mouse model. Meanwhile, it was found that this synergistic drug delivery system displayed a strong immune response and possessed great recurrence prevention ability with increased secretion of interferon-γ. Thus, efficient tumor destruction in vivo was achieved by the synergetic photothermal/immune-enhanced therapy. Positron emission tomography (PET) is a highly sensitive imaging tool with great penetration ability in a non-invasive way [45]. Multifunctional nanoparticles using PET imaging-guided PDT were able to facilitate the therapeutic selectivity of PDT through the imaging efficiency of PET [46]. For this purpose, Xu et al. [47] developed a kind of biodegradable MSNs that were composed of CpG adjuvant, chlorin e6 (Ce6) photosensitizer, and neoantigen peptides. The designed complexes could achieve neoantigen-based personalized cancer vaccination by integrating PET technology, PDT and immunotherapy. Based on PET imaging, these complexes accumulated effectively in MC-38 tumor sites after intravenous administration. In vivo results displayed that the synergistic effect of PDT and biodegradable mesoporous silica nanoparticle (bMSN)-mediated vaccination could induce the recruitment of DCs and evoke robust responses to neoantigen-specific CD8α+ cytotoxic T lymphocytes (CTLs). Therefore, bMSN-mediated drug delivery system (DDS) showed great prospect for personalized cancer immunotherapy (Figure 4). Hollow mesoporous silica (HMS) is another type of mesoporous silica materials that have drawn considerable attention recently [48]. Due to its large voids, HMS has a higher drug loading capacity compared to that of the traditional mesoporous silica materials [49]. Yang et al. [50] developed a smart nanoplatform using pH-responsive polymer-modified HMS with mitochondria-targeting ability due to the loaded therapeutic molecule (3-carboxypropyl)triphenylphosphonium bromide. Photosensitizer Ce6 and catalase (CAT) were also co-loaded on this nanoplatform. The PDT efficacy of this nanoplatform evaluated in 4T1 murine breast cancer cells displayed an enhanced PDT effect on solid tumors because of endogenous hydrogen peroxide's decomposition and hypoxia relief in tumor sites. Furthermore, by combining this PDT-generated nanoplatform with anti-PD-L1 checkpoint blockade immunotherapy, an obvious synergistic effect was obtained to significantly increase the infiltration of CTLs into the distant tumors, exhibiting a strong inhibitory effect on tumor metastasis.
2.3 Manganese oxide nanomaterials
Manganese oxide nanomaterials and their derivatives have received tremendous research interest in biomedical field due to their tunable structures and morphologies, unique physiochemical features, and excellent biosecurity [51]. Among them, manganese dioxide (MnO2), one of the redox active layered transition-metal dioxide nanomaterials, is being intensively explored as a novel delivery vehicle due to its distinctive properties [52]. For instance, MnO2 possesses a large surface area, providing it with great drug-loading capacity for anti-cancer drug delivery [53]. MnO2 is biodegradable because of its excellent catalytic activity and oxidation ability. Mn4+ in MnO2 is able to be reduced into Mn2+ by some reducing substances. Furthermore, it is well known that the TME is an acid environment with high glutathione (GSH) concentrations, which facilitates the degradation of MnO2 as well as the specific release of loaded drugs [54]. More importantly, the high H2O2 intensity in TME enables MnO2 to become a potential candidate in PIT field because MnO2 can effectively catalyze endogenous H2O2 into dissolved O2, serving as an enhancer for PDT-induced immunotherapy [52]. Additionally, MnO2 is able to consume GSH that can react with ROS, thus improving the effect of PDT (Figure 5) [55]. A series of studies have also testified that MnO2 can not only serve as a producer for enhanced PDT but also function as an outstanding PTA, which is helpful to boost the immunotherapy effect [56-58].
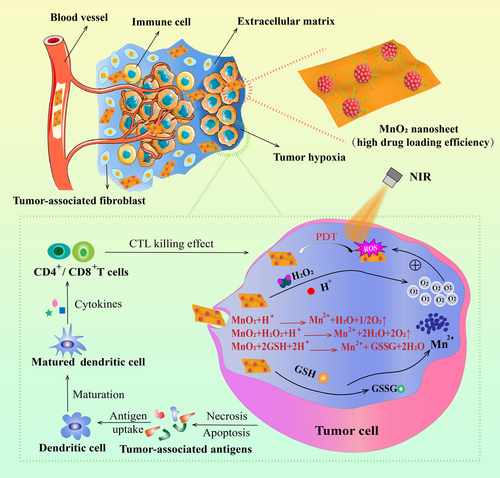
The unsatisfactory treatment effect of PDT is attributed to the tumor hypoxia within TME and the inherent immune resistance or immune escape of tumor cells. However, the currently booming checkpoint blockade therapies can recover the intrinsic immune capacities but are not able to directly attack tumor cells. To solve these problems, Liu et al. [59] designed Mn@CaCO3/ICG nanoparticles to load PD-L1-targeting small interfering RNA (siRNA), constructing an intelligent nanoplatform with multifunctionality to combine PDT with immunotherapy to promote the efficacy of PDT and prevent tumor cells from resistance and evasion. With the assistance of CaCO3 that protected ICG from inactivation and the oxygen generated by MnO2, a promoted PDT effect was observed in vitro in Lewis lung cancer cells. Moreover, in vivo experiments showed that this nanoplatform was able to effectively transport the carried cargos to tumor sites and remarkably alleviate the hypoxic condition of tumor cells, which largely strengthened the PDT efficacy. Additionally, the silence of PD-L1 gene that controls the immune evasion and resistance through loaded siRNA contributed to an outstanding synergistic therapeutic effect along with immune system activation. Similarly, Yang et al. [11] developed a smart nanoplatform based on hollow MnO2 (H-MnO2). H-MnO2 was functionalized with PEG to carry Ce6 and doxorubicin (DOX) [11]. This obtained nanoplatform could dissociate under the acid environment of TME to release the carried therapeutic agents, which relieved the tumor hypoxia through decomposing H2O2. In vivo results exhibited that a cascade of anti-tumor immune responses was triggered through the combinational chemo-photodynamic therapy. Another case of MnO2 to promote immune response was raised by Shen et al. [60]. They designed a type of TME-sensitive O2-generating nanosystem called MnO2@Chitosan-CyI (MCC) by integrating MnO2, Chitosan, and CyI (a photosensitizer) through self-assemble method for enhanced phototherapy. Upon NIR irradiation, MCC resulted in elevated production of ROS and enhanced generation of heat. When this nanocomposite was endocytosed, MnO2 was able to reduce the amount of GSH in TME and simultaneously functioned as an in situ O2 producer with high efficiency. Meanwhile, the in vivo blood flow could be accelerated through the elevated temperature induced by heat, which effectively alleviated the tumor hypoxia in 4T1 tumor-bearing mice. Altogether, this enhanced PDT could not only trigger an acute immune response, but also result in synergistic PIT effects to both eliminate primary tumors and reduce metastasis.
2.4 Iron oxide-based nanomaterials
Iron oxide nanoparticles (IONPs), a class of magnetic nanoparticles, are manipulated as excellent drug carriers because of their biodegradable, biocompatible, and superparamagnetic features [61, 62]. IONPs can be internalized by macrophages in various mononuclear phagocytic system organs, especially the liver and spleen. Then, they can be dissolved in the lysosome compartments of these macrophages [63]. The excess of iron after IONPs’ degradation requires to be balanced through the innate clearance mechanisms of the body. IONPs utilize endogenous transferrin or adopt additional exogenous substances like EDTA to consume the excess iron ions [24]. In addition, IONPs can be magnetically guided to a specific area of interest and hold the capability as multifunctional drug carriers that can reduce the required dose of loaded drug and decrease systemic adverse effects [64]. Moreover, the magnetic feature of IONPs enables their employment as magnetic resonance imaging (MRI) contrast agents in cancer diagnosis [65]. IONPs can produce magnetic hyperthermia by applying an external alternating magnetic field, which endows IONPs with multiple capabilities to function as both magnetic agents and PTAs. More importantly, magnetic hyperthermia has been reported to enhance antigen presentation, DC maturation, and migration, as well as to recruit T lymphocytes into lymph nodes to stimulate innate and adaptive immunity [66, 67]. Hence, the above properties make IONPs an ideal candidate in cancer therapy via drug delivery, photothermal ablation, immune stimulation, and image monitoring (Figure 6).
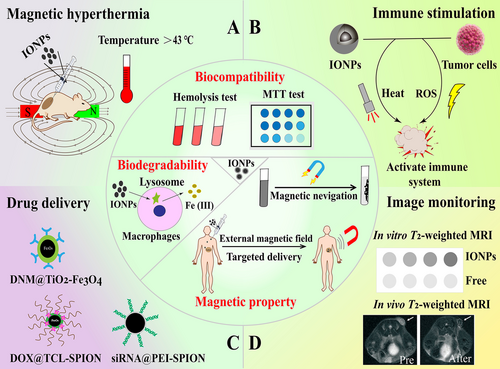
As IONPs possess excellent magnetic properties, they can be used as intelligent delivery vehicles to obtain local-regional drug release of therapeutic molecules and induce immune responses via magnetic navigation. For instance, Guo et al. [68] constructed a kind of magnetic-responsive immunostimulatory nanoagents (MINPs) that possessed magnetic-guided and immunostimulatory properties for cancer treatment. These nanoagents were mainly composed of superparamagnetic iron oxide nanoparticles as the core to load CpG immunoadjuvant. The applied external magnetic field could lead to MINP accumulation in tumor sites, facilitating the immunoadjuvant-boosted PTT-triggered anti-cancer immunotherapy with high efficiency [68]. NP-mediated hyperthermia as a noninvasive local anti-cancer treatment has been widely explored. Chen et al. [69] revealed that IONP-generated PTT could preferentially deplete tumor-associated Tregs. It was observed that an obvious 4T1 tumor growth inhibition could be achieved by combining PTT with anti-cytotoxic T-lymphocyte antigen-4 (CTLA-4) immunotherapy, offering a new therapeutic approach to overcome Treg-mediated immunosuppression and amplify the effect of cancer immunotherapy. Similarly, Fu et al. [70] developed a kind of IONPs-anchored titanium disulfide (TiS2) nanosheets that resulted in elevated NP accumulation at tumor sites compared to non-treated groups. Moreover, an enhanced population of immune cells with a reduced number of Tregs was observed after the combination treatment with anti-PD-1 and PTT. Ge et al. [71] designed a nanodrug carrier that loaded toll-like receptor 7 agonist R837 based on the copolymer-encapsulated Fe3O4 superparticles (Fe3O4-R837). The effect of NIR-irradiated PTT and the highly efficient magnetic attraction of this nanodrug carrier to tumor sites could generate TAAs to destroy the 4T1 breast tumor cells. In addition, R837 could be released from this nanodrug carrier through the trigger of PTT to produce a robust immune response against tumor. Moreover, with the combination of the anti-PD-1 therapy, the primary tumors could be effectively cleared up, and the corresponding lung and liver metastases could also be efficiently diminished, indicating the promising application potential of this nanodrug carrier [71].
3 NON-BIODEGRADABLE INORGANIC NANOMATERIALS
Although some inorganic nanomaterials cannot biodegrade within the body, they are still widely used in biomedical field due to their superior or irreplaceable properties. For instance, carbon-related nanomaterials, such as graphene and carbon nanotubes (CNTs), have been intensively explored because of their outstanding PTT potential for anti-cancer treatment. Through appropriate functionalization or modification, the biodegradability and biocompatibility of these carbon-related nanomaterials can be significantly enhanced with reduced toxicity to human body [72]. Metallic nanomaterials such as gold-, silver-, and copper-related nanomaterials also play an indispensable role in cancer theranostics due to their innate advantages like changeable size and excellent optical property. Thus, this section mainly focuses on these non-biodegradable nanomaterials with excellent performance in PIT. The distinct characteristics of these nanomaterials and their current applications in cancer therapy will be outlined.
3.1 Carbon-based nanomaterials
3.1.1 Graphene-based nanomaterials
Graphene, an amazing carbon-related two-dimensional nanomaterial with distinct electrical, thermal, and mechanical properties, has become extremely popular in biomedical field since its discovery in 2004 [73]. This newly proposed material possesses large surface area, great biocompatibility, good stability, distinctive thermal and optical properties, and superb conductivity [74]. Therefore, graphene and its derivatives, such as graphene oxide (GO), are being extensively utilized in the field of material science, micro-nano processing, biosensing, tissue engineering, drug delivery, PTT and PDT for tumor (Figure 7) [75-77]. Although it has been demonstrated that GO will reside for a long period in human body after administration, yet, they will not cause appreciable toxicity to our body even they cannot be cleared [78]. In addition, graphene and its functionalized derivatives can be used as carriers to transport both small-molecule chemotherapeutic drugs and biopharmaceutics, which are considered as revolutionary materials in the future.
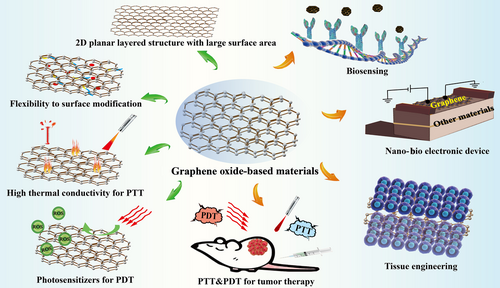
Recent studies have shown that graphene-based nanomaterials effectively activated macrophages and produced immune factors to improve the body's immune response [79, 80]. As a class of widely used therapeutic nucleic acids with outstanding immunostimulatory activities, CpG can be specifically recognized by mammalian toll-like receptors to secrete a variety of proinflammatory cytokines [81]. The functionalization using PEG could endow the nanomaterials with stealth characteristics to protect drugs from arresting by macrophages and diminish adverse immunological response [82]. Therefore, Tao et al. [83] designed a kind of GO-based nanocarrier (GO-PEG-PEI) by adopting PEG and PEI co-polymer to functionalize GO for effective CpG delivery. Owing to the photothermal property of GO, this carrier could enhance intracellular transportation of CpG under laser irradiation to achieve an effective regulation of immune response. In vivo results showed that the target experimental group exhibited the smallest CT26 colon tumor volumes than other control groups, indicating the promising application of GO as a nanomaterial in PIT for cancer. GO can serve as an immunostimulant to activate the host's immune system because they can reduce ROS production in cells, thus changing the repolarization of macrophages and regulating the immune response of the body [78, 84]. It has been demonstrated that the earlier shift of macrophage phenotypes to the M2 type and ROS regulation in inflammation-stimulated macrophages play significant roles in M1 macrophage treatment [85, 86]. Therefore, Wang et al. [87] formulated a hybrid nanocomposite by coating magnetite nanoparticles with reduced graphene oxide (rGO) and followed by PEG-NH2 modification on their surface. This nanocomposite could shift macrophage phenotypes to the M2 type and be further applied in MRI-guided PIT for metastatic cancers. Zhou et al. [88] developed a nanosystem that combined chemotherapeutic agent mitoxantrone and TGF-β inhibitor SB-431542 onto rGO. Upon non-invasive NIR irradiation, the system could not only destroy CT26 colon carcinoma cells and 4T1 breast cancer cells in vitro but also inhibit distant metastasis in the poorly immunogenic and highly metastatic 4T1 tumor model in vivo. Wu et al. [89] fabricated a type of graphene-based nanocomposite with mitochondria-targeting and NIR-activable ability by modifying triphenylphosphonium and photosensitizer IR820 on graphene to make the nanocomposite specifically home to mitochondria. This photoactive nanocomposite could produce a large amount of ROS and hyperthermia to destroy cancer cells by inducing the collapse of mitochondria to cause irreversible cell apoptosis. Besides, the nanocomposite could significantly elevate the immunogenicity of tumors by loading CpG immunoadjuvant. In vivo results demonstrated that the graphene-based nanocomposite displayed a good anti-tumor effect on EMT6 tumor-bearing mice, which remarkably inhibited tumor growth and caused negligible toxic effects on mice.
3.1.2 Carbon nanotubes
CNTs are allotropes from carbon and possess elongated and hollow cylindrical nanostructures. Single-walled nanotubes (SWNTs) and multi-walled nanotubes (MWNTs) are two representative types of CNTs. After their discovery by Sumio Iijima in 1991, CNTs have achieved a wide biomedical application due to their attractive structures and distinctive features like excellent electrical characteristic, good thermal transfer performance, and rich surface area for chemical functionalities [90]. By means of appropriate functionalization, CNTs can be utilized as nanocarriers to deliver chemotherapeutics, antibodies, proteins, peptide-based drugs, and therapeutic genes for cancer treatment [91]. The functionalization of CNTs can not only enhance their solubility and biocompatibility but also influence their cellular interaction pathways, contributing to much attenuated cytotoxic effects [92]. Typically, covalent and non-covalent functionalization with a variety of chemical groups or targeting ligands are two approaches to modify CNTs. Covalent modification like oxidation or carboxylation can provide a stable functionalized platform for drug delivery, while non-covalent functionalization causes minimal damage to the surface of CNTs [91]. Both functionalization can improve the biocompatibility and biodegradability of CNTs with reduced toxicity, enable their deep tumor penetration, and endow CNTs with active targeting ability [93] (Figure 8). Therefore, owing to their feasible surface modification possibility and easy controllability result from their unique physicochemical features, various immunoadjuvants and antigens are able to be either connected on CNTs’ surface through functionalization or encapsulated into their inner structure, showing great potential in cancer immunotherapy. In addition, CNTs have been regarded as a candidate material in PTT and PDT because of their excellent optical properties to produce cytotoxic singlet oxygen and heat upon NIR excitation. By using combinations of light energy, CNTs can be applied as effective tools in phototherapy to efficiently destroy and eliminate cancer cells [94]. Besides, CNTs are also reported to stimulate and activate immune responses, and drug-loaded CNTs are capable of entering APCs via various pathways rapidly, resulting in the elevated internalization between CNTs and APCs [13, 95]. Therefore, owing to the aforementioned shining points, CNTs exhibit great research potential in anti-cancer immunotherapy.
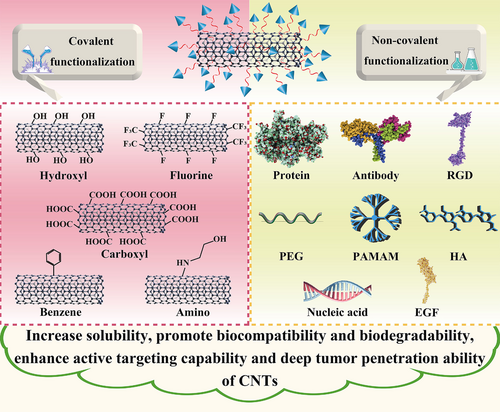
Wang et al. [96] modified SWNTs with a PEG-grafted amphiphilic polymer through a non-covalent approach. By combining a-CTLA-4 therapy with SWNT-mediated PTT, these polymer-coated SWNTs were able to not only ablate primary tumors through PTT-based hyperthermia but also acted as an immunostimulant to largely improve the maturation of DCs and the production of anti-tumor cytokines. Furthermore, in vitro and in vivo experiments proved that CTLA-4 blockade applied in this SWNT-mediated combinational therapy could induce effective T cell infiltration, while greatly inhibit Tregs at distant tumors, indicating the great potential in anti-cancer immunotherapy. Zhou et al. [97] adopted glycated chitosan (GC), an immunoadjuvant, to coat SWNTs. This SWNT-GC nanosystem could retain the optical features of SWNTs and the immunological properties of GC, which was also able to enter into cancer cells via SWNT-based delivery. In vitro results showed that EMT6 tumor cells could be destructed through the thermal ablation by cellular SWNTs. Meanwhile, cellular GC was able to elevate the tumor immunogenicity and improve tumor antigens’ uptake and presentation, resulting in specific anti-tumor immune responses. Besides, all the EMT6 tumor-bearing mice in the SWNT-GC group with laser irradiation survived with tumor remission, illustrating the great anti-tumor effect of SWNT-GC therapy in vivo.
3.2 Metallic nanomaterials
3.2.1 Gold-based nanomaterials
Gold nanoparticles (AuNPs), a kind of metallic nanoparticles, have long been used in drug delivery due to their special chemical and physical characteristics such as tunable size, optical properties, easy surface modification, stability, and biocompatibility [98]. A lot of studies have proved that AuNPs can be used as PTAs because of their high absorption property in second NIR window [99, 100]. This property endows AuNPs with the ability to produce hyperthermia in tumor sites. Au nanospheres, Au nanorods, Au nanocages, Au nanoshells, and Au nanostars are commonly used gold nanoplatforms for PTT to treat different cancers [98]. Recently, a number of researches have indicated that AuNPs could be a perfect carrier with good compatibility for immunotherapy to deliver therapeutic agents towards specific sites of interest, which can also achieve theranostic function with special drug release property [101-104]. Moreover, the application of AuNP-based vehicles can promote the safety of immunomodulators delivery, and AuNP-mediated immunotherapies are very suitable for combinational therapy with phototherapy [105]. Therefore, AuNPs are considered as promising candidates in cancer PIT (Figure 9).
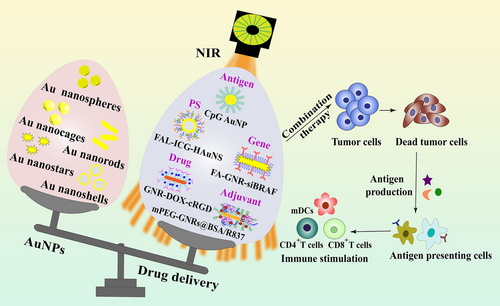
The unique properties of AuNPs enable their applications in cancer therapies, especially in light-triggered thermal therapy [98]. Hyperthermia can induce cell death through a number of pathways, for example, the high temperature at tumor tissue could promote the ICD and a series of immune responses [106]. Therefore, different types of AuNPs, such as gold-gold sulfide nanoparticles, hollow gold nanoshells, and gold-silica nanoshells, have been designed for PTT [107]. For instance, You et al. [108] designed DOX-encapsulated Au nanoshells to achieve photothermal/chemo combinational therapy, indicating that the combined treatment modality exhibited more effective therapeutic outcome compared to single treatment with attenuated toxicity of DOX in vivo. Furthermore, this treatment modality could potentially be applied in cancer metastasis treatment because a systemic anti-tumor immune reaction could be induced through the therapeutic agent DOX and PTT [109]. The capability of AuNPs to load and release nucleic acids exhibits great research potential in cancer immunotherapy, because PTT-induced hyperthermia or light-triggered drug release can be synergistically achieved with the delivery of nucleic acid immunoadjuvants, which protects these bio-therapeutics from inactivation or degradation [110]. For instance, it was reported that AuNPs can improve the effectiveness of CpG both in vitro and in vivo [111-113]. The delivery of CpG immunostimulant by AuNPs can result in better accumulation of nanoparticles, meanwhile, AuNPs are able to induce the infiltration of macrophages and DCs, inhibiting B16-ovalbumin (OVA) tumor growth in vivo [111]. In addition to immune adjuvant, cancer vaccines are another type of immunotherapy approach that leads to specific, strong, and persistent immune response [114]. Several studies have indicated the potential of AuNPs to deliver a large vaccine payload [115, 116]. AuNPs are able to be taken up by phagocytic cells and DCs, which greatly facilitates the antigen transportation to enhance the overall dosage of delivered vaccine to APCs [117]. In addition, most vaccines are injected subcutaneously, and AuNPs are easy to accumulate in the reticuloendothelial system (RES) such as the spleen when using intravenous injection. For other AuNP-mediated DDS, this RES accumulation is often regarded as toxic phenomenon to cause adverse effects. However, particle accumulation in RES is very beneficial to elevate the effectiveness of vaccines because the spleen is the largest immune organ with a great number of lymphocytes and APCs [118]. Thus, AuNPs are ideal platforms for vaccine delivery. Cao et al. [119] developed a kind of AuNP-involved nanovaccine decorated by HA and OVA, which integrated NIR-caused endo/lysosomal rupture with cytosolic OVA release through active targeting mediated by HA-CD44 receptor. HA-AuNPs inside endo/lysosomes could result in a photothermal effect due to the optical property of AuNPs upon NIR irradiation, which disrupted the endo/lysosomal membrane, and thereby released the OVA antigens into the cytosol because of the leaky endo/lysosomes. In vivo experiments using EG.7-OVA tumor-bearing mice by evaluating the tumor volume change demonstrated that HA-OVA-AuNP nanovaccine plus laser irradiation exhibited a remarkable tumor suppression effect with prolonged survival time than control groups. Moreover, this nanovaccine could induce the proliferation of CD8+ T cells and elevate the level of intracellular interferon-γ production by CD8+ T cells. All these data demonstrated the effectiveness of AuNPs as a potent antigen delivery vehicle in cancer immunotherapy.
3.2.2 Silver-based nanomaterials
Similar as gold-based nanomaterials, nanomaterials composed of silver, another noble metal, have also drawn attention in scientific research due to their versatility in cancer diagnosis and treatment [120, 121]. For instance, the surface plasmon response of silver is very strong when excited, which can convert photon energy into hyperthermia for the application in PTT [122, 123]. Silver tends to be more reactive in an environment where O2 is abundant and is usually employed as an adjuvant agent for wound treatment due to its antibacterial property [124]. Ag2S, a well investigated silver-containing nanomaterial, is serving as an ideal vehicle to transport anti-cancer therapeutic agents, which has also been reported for its application in PTT [125, 126]. Ag2S with an appropriate size is able to cause a photothermal-based tumor-killing effect. In addition, it can be used in imaging with better tissue penetration ability to provide more detailed information for cancer diagnosis [127, 128]. Moreover, compared to other commonly used photothermal materials like gold and copper sulfide, AgNPs represent a better in vivo stability and safer biological statistics with great photothermal performance [129]. Therefore, the application of Ag2S nanoparticles will offer more opportunities in cancer PIT.
Han et al. [129] synthesized a kind of Ag2S nanoparticles which were water-soluble with a small particle size for about 15 nm, and modified these nanoparticles with the cyclic arginine-glycine-aspartic peptide to elevate their tumor penetration and accumulation. In vivo and in vitro experiments showed that the photothermal effects generated by Ag2S nanoparticles elevated the effectiveness of cancer treatment, reduced the primary tumor recurrence, and alleviated metastasis in a breast cancer model. As the immunosuppressive TME is an obstacle that hinders the effect of immunotherapy, to address this tricky issue, Hou et al. [130] created an engineered hydrogel to encapsulate Ag2S QDs into its hydrophobic core. DOX and bestatin are two hydrophilic anti-cancer agents and were integrated into this hydrogel. The good photothermal performance of Ag2S QDs was able to trigger sustained release of DOX to initiate in situ vaccination. Bestatin is an immunoadjuvant drug that could amplify the system immune reaction. In vivo data showed that Ag2S QD-based hydrogel could stimulate the immune response to inhibit the primary tumor growth and distal lung metastasis after laser irradiation, indicating the promising application future of Ag2S QD-induced PTT in cancer treatment.
3.2.3 Copper-based nanomaterials
Copper-based nanomaterials, especially copper sulfide nanoparticles (CuS nanoparticles) are another type of nanoplatforms that have emerged recently with therapeutic and diagnostic applications in anti-cancer treatment [131]. CuS nanoparticles have been investigated widely due to their cheap price, low toxicity, simple preparation, and easy surface modification [132]. Owing to their stoichiometric transition metal semiconductor nanostructures, CuS is able to be employed as outstanding photoacoustic agents for imaging [133]. Additionally, CuS is a well-reported PTA, exhibiting wide absorption range in the NIR window [134]. It was also reported that CuS can produce a huge amount of ROS for PDT upon NIR irradiation, which is realized via the Fenton-like reaction or Haber-Weiss reaction [135, 136]. Thus, as promising candidates for phototherapy, CuS combined with immunotherapy can achieve very good immune responses for cancer treatment.
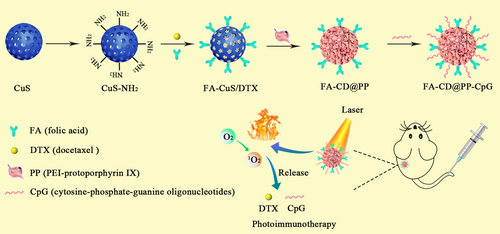
Chen et al. [137] successfully developed a nanoplatform that combined phototherapy with immunotherapy to enhance the effectiveness of docetaxel. As illustrated in Figure 10, mesoporous CuS nanoparticles were selected as the delivery vehicle and PTA due to their good photothermal performance and great biocompatibility. Then, FA was used as a targeting ligand for surface modification of CuS nanoparticles to achieve the active targeted delivery of docetaxel and enhance its transport efficiency at tumor sites. PEI-PpIX (protoporphyrin IX) conjugates were applied to increase the hydrophilicity of this nanoplatform to enable CpG delivery. PEI-PpIX and CpG were respectively cloaked and anchored on CuS nanoparticles to construct the final nanocomposite. Experimental results revealed that these nanocomposites exhibited great PTT and PDT efficacy under 808 and 650 nm irradiation, which resulted in a significant 4T1 tumor growth inhibition without obvious adverse effects. In addition, anti-PD-L1 was combined with this nanocomposite to achieve an enhanced therapeutic effect. This combinational therapy increased the infiltration of CTLs, prevented CTLs from dysfunction and exhaustion, suppressed myeloid-derived suppressor cells (MDSCs), altered MDSC phenotype to decrease tumor volume, thus causing considerable damage to 4T1 tumors in mice and providing great prospect for clinical breast cancer immune therapy. Chen et al. [138] designed a coreshell structured nanocomplex, in which OVA and CuS nanoparticles were incorporated into the poly(lactic-co-glycolic acid) (PLGA) nanoparticles. Prompting by the PTT produced by CuS nanoparticles, OVA was released to elicit vigorous immune response with the most abundant cytotoxic CD8+ T cells in the primary and distant tumor sites. Accordingly, it was proved that the irradiated nanocomplex could suppress the growth of primary tumors and effectively inhibited tumor metastasis in 4T1 tumor model.
4 CONCLUSIONS AND PERSPECTIVE
This review gives a summarized introduction of the recent advances of inorganic nanomaterial-mediated drug delivery in cancer PIT. Immunotherapy, phototherapy and multifunctional inorganic nanomaterials are considered as three promising technological revolutions in cancer treatment. Therefore, the combinational strategy of immunotherapy and phototherapy is able to not only eliminate the primary tumors directly but also induce a series of host immune response to clear up residual tumor cells and distant metastasis. Inorganic nanomaterials, owing to their unique physiochemical properties, play a crucial role in anti-cancer drug delivery. The strategy of PIT that uses multifunctional inorganic nanomaterials to deliver immunomodulators is greatly accelerating the development speed of clinical cancer treatment. Except for the targeted and controlled delivery of therapeutic agents, several inorganic nanomaterials can also serve as PTAs or photodynamic enhancers to promote the efficacy of both therapeutic approaches. In addition, accumulating experimental data have indicated that inorganic nanomaterial-based PIT have a significant influence on the treatment of metastatic tumors. Thus, this intelligent strategy through the combination of the three novel technologies opens a new era in treating both primary and metastatic tumors with minimal adverse effects, providing more survival opportunities for patients with advanced tumors.
Despite the success and merits mentioned above, clinical translation of such strategies still faces challenges. For instance, the boundedness of phototherapy is its insufficient tissue penetration of light, which limits its non-invasive application to treat tumors in deep organs. Therefore, phototherapy is more suitable for superficial cancers, such as osteosarcoma, melanoma, and squamous cell carcinoma. In addition, the distinct properties of multiple immune-modulating agents require the nanocarriers to satisfy their different requirements to achieve high loading efficiency and universality. However, very few studies concentrated on the systemic interaction between nanomaterials and their loaded therapeutic agents. Besides, from the aspect of inorganic nanomaterials, although they possess numerous advantages that do benefits to PIT, their inherent limitations cannot be ignored. For instance, BP is easy to be degraded in physiological environment. Therefore, BP is usually coated with a polymer or cell membrane to prevent its degradation. However, this extra procedure may increase the complexity of the whole drug delivery system to hinder the clinical application of BP nanomaterials. Furthermore, it is still challenging that the clearance of nanoparticles from the circulation by cells in the liver, spleen, and other parts of the RES may reduce the accumulation of the therapeutic agents in tumor sites. Last but not least, the safety issues of inorganic nanomaterials also impede their progress in clinical application. The long-term nanotoxicity of metallic and carbon-related nanomaterials should be carefully evaluated and monitored to ensure their safe application. Therefore, more investigations are required to confirm the clinical value of inorganic nanomaterial-based PIT. Although there are many challenges during the clinical translation of these inorganic nanomaterials and most of them are still in the stage of pre-clinical study, some inorganic nanomaterials like gold-silica nanoshells were in clinical trials, which indicates the promising translational potential in the future [139, 140]. Therefore, with the rapid development of modern technology, expanded preclinical and clinical studies, we are confident that PIT mediated by inorganic nanomaterials will have a bright future and the obstacles that hinder their clinical applications will be overcome one day.
ACKNOWLEDGEMENTS
The authors appreciate the financial support from National Nature Science Foundation of China (Nos. 31872756 and 32071387).
DECLARATIONS
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
Not applicable.
COMPETING INTERESTS
The authors declare that they have no competing interests.
AUTHORS’ CONTRIBUTIONS
Conceptualization, WW, LT; Original draft preparation, LT, AZ, ZZ, QZ, JL, YM, YY; Review and editing, WW, LT; Supervision, WW, LT, and AZ; Project administration, WW and LT, AZ; Funding acquisition, WW All authors have read and agreed to the published version of the manuscript.




