Neoadjuvant immunotherapy for non–small cell lung cancer: State of the art
Abstract
Lung cancer mortality has decreased over the past decade and can be partly attributed to advances in targeted therapy and immunotherapy. Immune checkpoint inhibitors (ICIs) have rapidly evolved from investigational drugs to standard of care for the treatment of metastatic non-small cell lung cancer (NSCLC). In particular, antibodies that block inhibitory immune checkpoints, such as programmed cell death protein 1 (PD-1) and programmed cell death 1 ligand 1 (PD-L1), have revolutionized the treatment of advanced NSCLC, when administered alone or in combination with chemotherapy. Immunotherapy is associated with higher response rates, improved overall survival (OS), and increased tolerability compared with conventional cytotoxic chemotherapy. These benefits may increase the utility of immunotherapy and its combinational use with chemotherapy in the neoadjuvant treatment of patients with NSCLC. Early findings from various ongoing clinical trials suggest that neoadjuvant ICIs alone or combined with chemotherapy may significantly reduce systemic recurrence and improve long-term OS or cure rates in resectable NSCLC. Here we further summarize the safety and efficacy of various neoadjuvant treatment regimens including immunotherapy from ongoing clinical trials and elaborate the role of neoadjuvant immunotherapy in patients with resectable NSCLC. In addition, we discuss several unresolved challenges, including the evaluations to assess neoadjuvant immunotherapy response, the role of adjuvant treatment after neoadjuvant immunotherapy, the efficacy of treatment for oncogenic-addicted tumors, and predictive biomarkers. We also provide our perspective on ways to overcome current obstacles and establish neoadjuvant immunotherapy as a standard of care.
Abbreviations
-
- AEs
-
- adverse events
-
- ALK
-
- anaplastic lymphoma kinase
-
- CD
-
- cluster of differentiation
-
- CEA
-
- carcinoembryonic antigen
-
- CI
-
- confidence interval
-
- CT
-
- computed tomography
-
- ctDNA
-
- circulating tumor DNA
-
- CTLA-4
-
- cytotoxic T-lymphocyte-associated protein 4
-
- CYFRA 21-1
-
- cytokeratin-19 fragments
-
- DFS
-
- disease-free survival
-
- EGFR
-
- epidermal growth factor receptor
-
- ICIs
-
- immune checkpoint inhibitors
-
- KEAP1
-
- Kelch Like ECH Associated Protein 1
-
- KRAS
-
- V-Ki-ras2 Kirsten ratsarcoma viral oncogene homolog
-
- LC
-
- lung cancer
-
- MPR
-
- major pathological response
-
- NSCLC
-
- non-small cell lung cancer
-
- OS
-
- overall survival
-
- ORR
-
- objective response rate
-
- PD-1
-
- programmed death 1
-
- PD-L1
-
- programmed death-ligand 1
-
- PET/CT
-
- positron emission tomography/computed tomography
-
- PFS
-
- progression-free survival
-
- pCR
-
- pathological complete response
-
- STK11
-
- serine/threonine kinase 11
-
- TMB
-
- tumor mutational burden
1 BACKGROUND
Lung cancer (LC) is one of the most common malignancies and has the highest rate of cancer-related death in both men and women worldwide [1]. Non-small cell lung cancer (NSCLC) accounts for approximately 80%-85% of newly diagnosed cases of LC annually [2]. Screening with low-dose spiral computed tomography (CT) has led to significant improvements in the identification of patients with early-stage LC [3]. Surgical resection with curative intent continues to be the mainstay treatment for early-stage NSCLC, however, its 5-year survival rates remain unsatisfactory, ranging from 36% for stage IIIA disease to 60% for stage IIA, due to the high rates of recurrence and metastasis. It is commonly recognized that early-stage NSCLC patients should not be treated with surgery alone as evidence from randomized trials have shown that the addition of neoadjuvant or adjuvant chemotherapy could have superior outcomes compared with resection alone [4]. Conventional adjuvant chemotherapy may improve 5-year survival rates by approximately 5%, which provides only a modest survival benefit over surgery [5].
Neoadjuvant therapy is a promising approach for improving long-term survival and increasing the chance of cure rates for patients with early-stage LC. In a retrospective study, neoadjuvant biologic therapy was associated with improved overall survival (OS) compared to adjuvant biologic therapy (P = 0.006), with 5-year OS being 56.2% and 33.0%, respectively [6]. A study of 1769 patients reported that the long-term survival of patients with stage I–IIIA NSCLC aged <75 years was significantly higher with neoadjuvant chemoradiotherapy compared with adjuvant chemoradiotherapy (5-year OS rate: 38.1% vs 27.0%; hazard ratio, 0.74; P < 0.001) [7]. However, these previous studies [6, 7] were retrospective in nature and were not randomized controlled trials. In a multicenter, phase III, randomized trial of preoperative chemotherapy or adjuvant chemotherapy in patients with early-stage NSCLC [8], disease-free survival (DFS) did not differ significantly between patients receiving neoadjuvant treatment and those receiving only surgery (5-year DFS rate: 38.3% vs 34.1%; hazard ratio, 0.92; P = 0.176). Multiple preoperative treatments have been extensively studied. However, in recent years, newer immunotherapies with immune checkpoint inhibitors (ICIs), including antibodies that modulate cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) [9], programmed cell death protein 1 (PD-1) [10], and programmed death receptor-ligand 1 (PD-L1) [11], have demonstrated remarkable therapeutic efficacy against advanced NSCLC and may shed new light on potential therapeutic breakthroughs. ICIs have revolutionized treatment strategies and patient prognoses in several solid tumors and their efficacies have been sufficiently striking to change current treatment paradigms. Ongoing trials evaluating these agents are rapidly moving from advanced NSCLC to earlier-stage disease and from palliative to curative intent [12]. Neoadjuvant immunotherapies continue to evolve at a rapid pace, continually producing new clinical trial data and biologic discoveries. A meta-analysis of seven studies comprising of 252 patients supported the safety and efficacy of neoadjuvant immunotherapy in resectable NSCLC [13]. In addition, multiple forms of combination therapy, such as neoadjuvant chemoimmunotherapy, neoadjuvant immunotherapy plus radiotherapy/chemoradiotherapy, and neoadjuvant immunotherapy plus antiangiogenic therapy, are currently being investigated [14].
In this review, we examine (1) existing preclinical data from emerging immunotherapy trials, (2) the combination of chemotherapy with immunotherapy in a neoadjuvant setting, and (3) potential biologic mechanisms that impact OS in NSCLC patients. In addition, we discuss potential caveats of neoadjuvant immunotherapy and ongoing challenges associated with these approaches.
2 RATIONALE FOR NEOADJUVANT IMMUNOTHERAPY AGAINST NSCLC
Current immunotherapy regimens include cytokine therapy, adoptive T-cell transfer, and the use of oncolytic viruses [15]. ICIs can activate previously suppressed T cell-mediated antitumor immune responses by blocking intrinsic downstream regulators of immunity while maintaining dynamic interactions among the cluster of differentiation (CD)8+ T cells, antigen-presenting cells, and tumor cells [16].
In a preclinical study by Liu et al. [17], using two immunocompetent murine models of triple-negative breast cancer, the authors found that when a variety of immunotherapies (anti-CD25, anti-PD-1 alone, or anti-PD-1 in combination with anti-CD137) were used in a neoadjuvant setting, improved long-term survival and enhanced antitumor immune responses to metastatic disease compared with adjuvant therapy could be observed. Neoadjuvant immunotherapy was associated with an increased number of tumor-specific CD8+ T cells, which may account for the improved efficacy. Two additional studies using murine models of triple-negative breast cancer demonstrated that the combination of neoadjuvant or oncolytic virotherapy administered with curative intent prior to surgery could prolong the survival of the murine models [18, 19]. Further, a treatment regime composed of neoadjuvant chemoimmunotherapy–resection–adjuvant anti-CD96 and chemotherapy successfully treated a proportion of mice with pancreatic ductal adenocarcinoma [20].
PD-1 and CTLA-4 are known to inhibit T cell activation; however, pharmacologic agents against PD-1 or CTLA-4, or the combination of them, have had distinct immunologic effects in preclinical studies [21-23]. The efficacy of dual blockade therapy of CTLA-4 and PD-1 was found to be superior to monotherapies [24]. Anti-CTLA-4 therapy is involved in the activation and proliferation of T cells while anti-PD-1 therapy aids existing T cells in detecting tumor cells. Although these therapies employ different mechanisms, they may act synergistically [25]. Moreover, the efficacy of combination anti-CTLA-4 and anti-PD-1 blockade therapy against metastatic melanoma, advanced renal cell carcinoma, advanced NSCLC, and metastatic colorectal cancer with high microsatellite instability or mismatch repair has been confirmed in a number of clinical trials [26-29]. A preclinical study by Cascone et al. [30] was designed to examine the treatment of early-stage NSCLC using a murine model. Syngeneic mice were implanted with 344SQ-OVA + NSCLC cells. Some mice received three doses of neoadjuvant anti-PD-1, anti-CTLA-4, or anti-PD-1 plus anti-CTLA-4 therapy, while some mice received three doses of adjuvant anti-PD-1, anti-CTLA-4, or anti-PD-1 plus anti-CTLA-4 therapy. Mice that received the anti-PD-1 plus anti-CTLA-4 regimen in a neoadjuvant setting demonstrated significantly longer survival than mice in an adjuvant setting (P < 0.05). Moreover, the most significant reduction in lung metastases occurred in the neoadjuvant combined immunotherapy group. These preclinical findings imply that the efficacy of neoadjuvant combined immunotherapy was superior to that of any adjuvant immunotherapy or neoadjuvant single-agent immunotherapy. Multiple clinical studies are evaluating these results [31-33]. The pharmacodynamics of pre-operative PD1 checkpoint blockade and receptor activator of nuclear factor-kappa-b ligand inhibition in NSCLC (POPCORN) trial is providing a unique platform for translational research to (1) determine the mechanism of action of novel combination immunotherapy (PD1 checkpoint blockade and receptor activator of nuclear factor kappa-B ligand inhibition) for NSCLC and (2) define the tumor-immune correlation of combination therapy compared with monotherapy [34]. The POPCORN trial may elucidate mechanisms that contribute to the efficacy of neoadjuvant combined immunotherapy against NSCLC.
3 RATIONALE FOR CHEMOIMMUNOTHERAPY
The observation that chemoimmunotherapy may significantly improve the progression-free survival (PFS) and OS of patients with advanced NSCLC [35, 36] is supported by the mechanistic rationale that chemoimmunotherapy acts synergistically to reinforce the anti-tumor immune response. Chemotherapy inhibits the proliferation of tumor cells and has immunostimulatory effects. It inhibits the activities of immunosuppressive and/or activated effector cells, increases immunogenicity and T-cell infiltration, and induces the expression of favorable immunizing antigens in the tumor microenvironment. These processes convert tumors with no detectable inflammation (cold tumors) to tumors with marked inflammation (hot tumors) [37, 38]. Chemotherapy may potentiate immunotherapy efficacy via these mechanisms (1) blockade of signal transducer and activator of transcription 6 (STAT6) signalings, (2) downregulation of PD-L1 and induction of mannose-6-phosphate receptor expression, and (3) stimulation of adenosine triphosphate production and activation of high-mobility group protein box-1 to promote the death of immunogenic tumor cells [39, 40].
4 RATIONALE FOR COMBINING IMMUNOTHERAPY AND RADIOTHERAPY
Integrating radiotherapy and immunotherapy is a promising strategy for treating early-stage NSCLC due to the abscopal effect, whereby radiotherapies generate immune-mediated anti-tumor effects that cause the regression of non-irradiated metastases that are distant from the primary irradiated site. The abscopal effect is believed to be a systemic anti-tumor immune response that may be stimulated by immunotherapy [41, 42]. Radiotherapy has the capacity to overcome several mechanisms that allow tumors to escape the immune response. For example, under certain conditions, radiotherapy may liberate immunogenic tumor antigens which may stimulate inflammatory cytokines and chemokines to attract immune cells. In addition, radiotherapy can induce the infiltration of leukocytes and increase the susceptibility of tumor cells. These complex interaction mechanisms contribute to reprograming the anti-immunologic tumor microenvironment and increasing the functionality of antigen-presenting cells and T cells [43]. Radiotherapy permits the immune system to more easily recognize and eradicate tumor cells, thereby augmenting both the local and systemic immune response. In the phase III, randomized, double-blind, placebo-controlled, multi-center, international study of MEDI4736 as sequential therapy in patients with locally advanced, unresectable NSCLC (stage III) who had not progressed following definitive, platinum-based, concurrent chemoradiation therapy [44], patients with locally advanced, unresectable NSCLC after definitive concurrent chemoradiation were randomly assigned (ratio, 2:1) to receive durvalumab or placebo every two weeks for a maximum of 12 months. Durvalumab after concurrent chemoradiation resulted in significantly longer PFS and OS than placebo [PFS: 17.2 months vs 5.6 months, stratified hazard ratio, 0.51, 95% confidence interval (CI), 0.41 to 0.63; 24-month OS rate: 66.3% vs 55.6%, two-sided P = 0.005]. Combining radiotherapy and immunotherapy reduced local recurrence and metastasis and enhanced overall systemic therapeutic effects.
Palliative radiation (6 Gray X5 or 9 Gray X3) plus ipilimumab has been reported to be effective against chemo-refractory metastatic NSCLC [45], which may provide evidence for the abscopal response that radiation-induced exposure of immunogenic mutations could enhance the immune system in vivo. Increased levels of serum interferon-β following radiation and early dynamic changes in blood T cell lineages were identified as predictors of a positive response to such combination regimens.
5 CURRENT CLINICAL TRIALS
Multiple clinical trials are exploring the efficacy of immunotherapy against operable NSCLC in the neoadjuvant setting. Initially, clinical trials examined single-drug neoadjuvant immunotherapies [46-48] and now recent trials have begun to evaluate neoadjuvant chemoimmunotherapy [49, 50], neoadjuvant immunotherapy plus antiangiogenic therapy [14], and neoadjuvant immunotherapy plus radiotherapy [14, 51]. Current clinical trials on neoadjuvant immunotherapies are mainly single-arm studies with a small sample [46, 47, 52].
Short-period efficacy indicators, such as major pathological response (MPR), are usually used as the endpoints in the clinical trials [52, 53]. Translational researches are included in neoadjuvant immunotherapy trials and in the exploratory stage [54, 55]. There are still many issues to be addressed. In addition, the outcomes of these trials are immature. Phase III, randomized, double-blind, multi-center, international studies about neoadjuvant immunotherapies in early-stage LC should be carried out to confirm the results. The main available results of neoadjuvant immunotherapy trials are presented in Table 1.
| Trial | Identifier | Phase | NSCLC stages | Sample size | Primary endpoint | Treatment | Surgery | MPR | pCR | TRAEs ≥ 3 |
|---|---|---|---|---|---|---|---|---|---|---|
|
Forde et al. Ref. [47] |
NCT02259621 | II | IB-IIIA | 21 | Safety, Feasibility | Nivolumab: 3 mg/kg, Q2W, 2 cycles | 95% | 45% | 15% | 4.5% |
|
MK3475-223 Ref. [108] |
NCT02938624 | I | I-II | 15 | Toxicity, MPR | Pembrolizumab: 200 mg, 1 cycle or Q3W, 2 cycles | 87% | 31% | 15% | NR |
|
LCMC3 Ref. [48] |
NCT02927301 | II | IB-IIIB | 181 | MPR | Atezolizumab: 1200 mg, Q3W, 2 cycles | 88% | 21% | 7% | 6% |
NEOSTAR Ref. [53] |
NCT03158129 | II |
I-IIIA (N2 single) |
23 | MPR | Nivolumab: 3mg/kg, Q2W, 3 cycles | 96% | 17% | 9% | 13% |
| 21 |
Nivolumab: 3mg/kg, Q2W, 3 cycles Ipilimumab: 1 mg/kg, day 1 |
81% | 33% | 29% | 23% | |||||
|
Gao Ref. [59] |
ChiCTR-OIC-17013726 | IA-IIIA | 40 | Safety | Sintilimab: 200 mg, Q3W, 2 cycles | 93% | 41% | 16% | 10% | |
|
NADIM Ref. [56] |
NCT03081689 | II | IIIA (N2 orT4) | 46 | Safety, Feasibility | Nivolumab (360 mg) + chemotherapy Q3W, 3 cycles | 89% | 83% | 59% | 30% |
|
Shu et al. Ref. [52] |
NCT02716038 | II | IB-IIIA | 30 | MPR | Atezolizumab 1200 mg + chemotherapy, Q3W, 2 cycles | 87% | 57% | 33% | 6% |
|
PRINCEPS Ref. [46] |
NCT 02994576 | II | I-IIIA | 30 | Toxicity | Atezolizumab: 1200 mg, 1 cycle | 97% | 14% | NR | NR |
|
IFCT-1601 IONESCO Ref. [58] |
NCT03030131 | II | IB-IIIA non-N2 | 46 | % of R0 | Durvalumab: 750mg, day 1,15,29 | 93% | 19% | NR | 0 |
|
Hong et al. Ref. [55] |
NCT03694236 | Ib |
III non-N3 |
24/39 | Toxicity |
Neoadjuvant durvalumab 1500mg, day 1, 29 + chemotherapy, QW, 5 cycles + RT (45 Gy-25 Fraction) Adjuvant durvalumab 1500 mg, Q4W, 13 cycles |
75% | 78% | 39% | 17% |
- Abbreviations: Gy, gray; MPR, major pathological response; NSCLC, non-small cell lung cancer; NR, not reported; pCR, pathological complete response; R0, complete surgical resection; RT, radiotherapy; TRAEs, treatment-related adverse events.
6 CLINICAL TRIALS OF NEOADJUVANT IMMUNOTHERAPY AND CHEMOIMMUNOTHERAPY
Ongoing clinical trials indicate that neoadjuvant immunotherapy could play a significant role in multimodality therapy for early-stage NSCLC. A phase II trial conducted by the Lung Cancer Mutation Consortium (LCMC3 trial) [48] reported that two cycles of neoadjuvant atezolizumab for resectable NSCLC did not increase toxicity, however, 6% of the patients developed ≥ grade 3 pre-operative treatment-related adverse events (AEs). One hundred and fifty-nine (88%) of 181 patients underwent surgery and 22 (12%) did not undergo surgery. Of the 159 NSCLC patients who underwent surgery, 15 (8%) were diagnosed with oncogene-addicted tumors and 30 (21%) achieved MPR. Of the 144 patients in the primary efficacy population, 10 (7%) achieved a pathological complete response (pCR). The MPR rate of PD-L1 protein expression ≥ 50% patients (SP142 antibody) was up to 33%, while that of PD-L1 protein expression < 50% patients was only 11% (P = 0.004). Pathologic regression was weakly correlated with tumor mutational burden (TMB) determined by exon sequencing (P = 0.047). Serine/threonine kinase 11 (STK11)-mutated tumors trended toward less pathologic regression (P = 0.008). Multiplex immunofluorescence analysis showed that patients with pre-existing enrichment of CD68 + cells (P = 0.005) and CD3 + / PD-1 + T cells (P = 0.049) trended towards achieving MPR. The preliminary data of the LCMC3 trial suggested that artificial intelligence quantification could provide meaningful biological and clinically relevant insights [54].
Chemoimmunotherapy has dramatically improved the objective response rate (ORR) and OS of patients with metastatic NSCLC [35, 50]. Chemoimmunotherapy in the neoadjuvant setting may improve pathological response, thereby prolonging OS. In a phase II trial by Shu et al. [52], 30 patients with stage IB–IIIA NSCLC received neoadjuvant atezolizumab, carboplatin, and nab-paclitaxel. Seventeen (56.7%) patients achieved MPR and 10 (33.3%) had pCR. The median DFS was 17.9 months (95% CI, 14.3 to not reached). Among patients with oncogenic-addicted NSCLC, two patients with epidermal growth factor receptor (EGFR) mutations achieved pCR while three with STK11 mutations did not.
Current evidence supports the use of ICIs with nivolumab in the neoadjuvant setting. In a single-arm trial (NCT02259621) of 21 patients with stage I–IIIA NSCLC who received two cycles of nivolumab every two weeks before surgery, there was no delay to surgery and the MPR rate was 45% (9/20) [47]. The neoadjuvant chemotherapy and nivolumab in resectable NSCLC (NADIM) trial assessed the activity and safety of three cycles of neoadjuvant nivolumab and chemotherapy before surgical resection followed by adjuvant nivolumab monotherapy for one year in stage IIIA NSCLC patients. No AEs associated with surgical delays or deaths were reported during a median follow-up duration of 24.0 months (interquartile range, 21.4-28.1). Following tumor resection, 35 (85.4%) of 41 patients survived without recurrence. Down-staging occurred in 90% of cases [56].
The phase II study of induction checkpoint blockade for untreated stage I-IIIA NSCLC amenable for surgical resection (NEOSTAR)[53] evaluated a neoadjuvant immunotherapy regimen comprising of three doses of nivolumab every two weeks with or without ipilimumab in 44 patients with stage I–IIIA NSCLC (nivolumab group, n = 23; nivolumab with ipilimumab group, n = 21). MPR rates in the nivolumab and nivolumab plus ipilimumab groups were 17% and 33%, respectively. Although sample sizes were small, the results suggested that the combination of nivolumab and ipilimumab could be superior to nivolumab only. In a different study [32], neoadjuvant nivolumab plus ipilimumab appeared to be feasible and the long-term survival of patients who achieved pCR was initially encouraging. However, the treatment regimen was terminated ahead of schedule by the investigators’ consensus due to toxicity in nine of 15 patients. Among the nine enrolled patients, six patients experienced treatment-related AEs and three patients experienced grade ≥ 3 treatment-related AEs. Of three patients who experienced tumor progression during neoadjuvant therapy, two had tumors with V-Ki-ras2 Kirsten ratsarcoma viral oncogene homolog (KRAS) /STK11/Kelch Like ECH Associated Protein 1 (KEAP1) co-mutations [32]. Therefore, biomarkers such as KRAS/STK11/KEAP1 co-mutations could be useful for identifying patients who would not benefit from neoadjuvant nivolumab plus ipilimumab therapy. The ipilimumab plus nivolumab and chemoradiotherapy followed by surgery in patients with resectable and borderline resectable T3-4N0-1 non-small cell lung cancer trial (Netherlands trial register number: NL8435) is evaluating the safety and efficacy of four combination treatments in patients with resectable, T3-4N0-1 NSCLC [51]. Although this trial is ongoing, the results could provide insights into whether pCR rates would be further improved by adding short-course immunotherapy (ipilimumab and nivolumab) to induction chemoradiotherapy. Nivolumab is also being explored in combination with BMS-813160 (CC chemokine receptor2/5-inhibitor) or BMS-986253 (anti- interleukin -8) in the neoadjuvant setting (NCT04123379).
Pembrolizumab has been approved for use in patients with advanced NSCLC but has only been recently evaluated in the neoadjuvant setting. The DFS and 5-year survival of NSCLC patients with stage IIA-IIIA disease who received surgery alone are unsatisfactory. These patients are prone to relapse within five years. Surgical interventions do not remove distant micrometastases, and chemotherapy in the neoadjuvant or adjuvant setting often results in AEs. Effective treatments with low toxicity, such as monotherapy using pembrolizumab, should be explored in the neoadjuvant setting. A phase II trial is exploring two cycles of pembrolizumab as neoadjuvant immunotherapy in stage IIA-IIIA NSCLC patients. The primary objectives are to assess the safety and feasibility of treatment as well as clinical and pathological tumor responses [57]. Similarly, the immune neoadjuvant therapy in early-stage non-small cell lung cancer (IONESCO) phase II study (NCT03030131) is examining the rate of complete surgical resection after a maximum of three cycles of neoadjuvant durvalumab in patients with stage I–IIIA NSCLC [58].
In addition to the numerous ongoing clinical trials on ICIs produced by various pharmaceutical companies, similar drugs produced in China are currently under study. For example, a study by Gao et al. [59] which used two doses of sintilimab (a PD-L1 inhibitor approved in China) as a neoadjuvant treatment for stage IA–IIIB NSCLC reported that surgery was delayed in 7.5% (3/40) of patients. Of 37 patients who underwent surgery, 15 (40.5%) achieved MPR, and six (16.2%) achieved pCR. A decrease in the maximum standardized uptake value by positron emission tomography/computed tomography (PET/CT) after sintilimab administration was predictive of the pathological response. Various trials are examining the efficacy of tislelizumab (NCT03745222), SHR-1316 (NCT04316364), camrelizumab (NCT04541251, NCT04338620), toripalimab (NCT04304248, NCT04158440), and cemiplimab (NCT03916627) and the effects of combining ICIs with different chemotherapy regimens. More importantly, the number of chemoimmunotherapy trials has increased faster than the number of immunotherapy trials [14] as applications of chemoimmunotherapy have shown the potential to expand benefits for a wider range of patients.
7 CLINICAL TRIALS OF NEOADJUVANT COMBINATION THERAPY INCLUDING IMMUNOTHERAPY
Ongoing clinical trials are evaluating a combination of antiangiogenic therapy and immunotherapy in neoadjuvant settings such as apatinib plus camrelizumab (NCT04506242, NCT04379739, NCT04133337), pembrolizumab plus ramucirumab (NCT04040361), and sintilimab plus bevacizumab and chemotherapy (NCT03872661). However, some of these trials remain in the recruitment phase. Although antiangiogenic therapy plus immunotherapy is efficacious against advanced NSCLC [36], the efficacy of this regimen against early-stage NSCLC remains unclear. A growing number of neoadjuvant trials of immunotherapy and radiotherapy/chemoradiotherapy are also ongoing. The interim analysis of neoadjuvant chemoradiotherapy and durvalumab for potentially resectable Stage III NSCLC trial showed that 18 patients undergo surgery among 24 eligible patients included and received neoadjuvant treatment. The MPR rate was 77.8% (14/18, 95% CI: 54.3% - 91.5%) and the pCR rate was 38.9% (7/18, 95% CI: 20.2% - 61.5%) [55]. Similarly, a randomized single-center, phase II, open-label clinical trial of neoadjuvant pembrolizumab with or without low-dose stereotactic radiation therapy has been initiated for patients with stage I–IIIA NSCLC who plan to undergo surgical resection (NCT03217071). Similar studies, such as nivolumab plus radiation and durvalumab plus tremelimumab (anti-CTLA-4) plus radiation, are ongoing.
8 ADVANTAGES AND DISADVANTAGES OF IMMUNOTHERAPY AS A NEOADJUVANT STRATEGY
The advantages and disadvantages of neoadjuvant immunotherapies are summarized in Table 2. Neoadjuvant immunotherapies may reduce the size of, and downstage the primary tumor, and increase the likelihood of complete surgical resection after neoadjuvant therapy for patients with large tumors or tumors in anatomically inaccessible locations. Neoadjuvant immunotherapies may aid patients in producing antigens against the primary tumor and distant micro-metastases at the earliest time, thus hopefully preventing tumor recurrence and migration. Antigens induced by neoadjuvant immunotherapies may induce a strong and prolonged antitumor T cell immune response and may remain functional sustainably even after surgery.
| Advantages | Disadvantages |
|---|---|
| Downstaging and increased likelihood of complete surgical resection | Immune-therapy related adverse events lead to delay surgery delays or death |
| Attack the primary tumor and eliminate micrometastases early in treatment | |
| Improve tolerance and compliance | Potential for immune hyper-progression |
| Assess sensitivity and resistance to drugs | |
| Provides additional time to identify unsuspected metastases and comorbidities, and to achieve smoking withdrawal | Increased number of complications, increased duration, and difficulty of surgery. |
| Shorten trial timelines and accelerate the evidence for pCR or MPR as surrogate predictors of OS |
- Abbreviations: MPR, major pathological response; pCR, pathological complete response; OS, overall survival.
In addition, earlier assessment of patient responses to drug therapies may provide valuable prognostic information to improve tolerance and compliance. Assessing drug sensitivity and resistance in a neoadjuvant setting would provide useful evidence for improving treatment regimens following surgery and would benefit patients by identifying the most sensitive treatment for recurrent disease. The effectiveness of neoadjuvant treatment should be evaluated by PET/CT over time to identify unsuspected metastases and comorbidities, and to achieve smoking withdrawal [60]. Neoadjuvant treatment may shorten the length of clinical trials and accelerate the use of pCR or MPR as surrogate predictors of OS. Although neoadjuvant immunotherapies have shown high efficacy for treating patients with NSCLC, these regimens are often accompanied by undesirable side effects [61].
Different ICIs have different toxicity profiles involving multiple organs, such as myocarditis, colitis, pituitary inflammation, rash, pneumonia, neuromuscular toxicity, hypothyroidism, and joint pain [61, 62]. Although ICI-related cardiotoxicity is rare, it is clinically significant due to high mortality rates [63]. Compared with conventional chemotherapy, neoadjuvant immunotherapies may limit the impact of treatment-related toxicities following surgical resection. Immunotherapy-related AEs may delay surgery and/or increase the risk of intraoperative complications. In some cases, extended delays in surgical resection due to toxicity may result in tumor progression [64]. In addition, physical fatigue induced by neoadjuvant therapy may prolong recovery from surgery. Importantly, delayed surgery is associated with poorer outcomes. Hyper-progression induced by immunotherapy may result in the delay or cancelation of surgery. Neoadjuvant immunotherapy and chemoimmunotherapy may destroy the primary tumor vascularization and microenvironment resulting in adhesions and fibrosis, increasing the difficulty and duration of surgery. At present, there is no evidence that the rate of conversion from minimally invasive resection to open thoracotomy increases as the complication rate increases.
9 CHALLENGES AND UNANSWERED QUESTIONS
9.1 Imaging techniques to assess neoadjuvant immunotherapy response
CT is an imaging technique commonly used in clinical practice to objectively assess the tumor response after therapy. However, a recent retrospective study reported no relationship between the response assessed by imaging and the pathologic response [65]. Therefore, the utility of CT for assessing the total response of neoadjuvant immunotherapies is limited [47]. PET/CT is usually recommended for preoperative staging [66] and perioperative PET/CT has been used in some neoadjuvant trials [67]. For example, in the trial (NCT04586465), dynamic PET/CT was used to evaluate the response of stage Ⅱa–IIIb NSCLC to neoadjuvant pembrolizumab combined with chemotherapy. The change in the maximum standard uptake value (or metabolic activity) of primary tumors on PET/CT after neoadjuvant therapy has a near-linear relationship with pathologic response. Using PET/CT to assess responses to immunotherapy is necessary to evaluate tumor response and to distinguish immune-related side effects [68]. PET/CT may be useful for predicting a MPR to neoadjuvant immunotherapy in resectable NSCLC patients [69] and for distinguishing from pseudo-progression [70, 71]. Although PET/CT is a valuable modality, financial considerations may limit its applicability.
9.2 Timing of surgery
A preclinical study reported that the timing of surgery influences the efficacy of neoadjuvant immunotherapy as timely surgery following neoadjuvant treatment dramatically affects long-term survival. Shortening the duration of neoadjuvant therapy may improve immune activity and reduce the incidence of immunotherapy-related AEs. Therefore, it might be advantageous for patients to receive fewer cycles of immunotherapy prior to surgery to shorten the time to surgery. To date, based on the comparability between individual trials and to prevent the deterioration of non-responders, a timeframe of six to eight weeks is recommended for patients with melanoma receiving neoadjuvant therapy [72]. At present, however, most neoadjuvant trials of NSCLC administer only two or three cycles as there is no standard consensus for this issue.
9.3 Surrogate endpoints for OS
Prolonging OS is the primary goal of neoadjuvant immunotherapy. However, OS is not a feasible endpoint in some clinical trials due to the extended time periods required to monitor results. Therefore, DFS is often used as a well-established surrogate endpoint in adjuvant clinical trials. Nevertheless, DFS must be measured over a period of several years, which does not benefit the development of new drugs or regimens. In contrast, radiologic and pathologic responses can be documented within a few weeks after treatment. The ORR is useful for radiographically determining antitumor activity directly in a timely and practical manner, but the ORR does not reflect the pathologic response. Pseudo-progression of the tumor following immunotherapy adds a degree of unreliability and controversy to the use of the ORR as a surrogate of OS [70, 71]. In addition, missing tumor measurements may impact the ORR, but not significantly alter the predictive ability of the ORR [73]. Some studies have shown that ORR is not a reliable surrogate of OS in NSCLC [74, 75], thus investigators do not pay much attention to ORR in clinical trials. Conversely, pCR is considered an ideal therapeutic goal after neoadjuvant therapy [76]. Although pCR after neoadjuvant therapy may be an effective alternative measurement for OS [77, 78], pCR is rarely used as the primary endpoint in clinical trials [52, 53]. MPR, defined as ≤10% of viable tumor cells in a surgically resected specimen, is more commonly used in clinical trials because it has a higher incidence than pCR [79]. Many studies have reported that MPR is predictive of the OS of patients with early-stage NSCLC [77, 80, 81].
10 SURGICAL APPROACH AFTER NEOADJUVANT IMMUNOTHERAPY
The feasibility and safety of pulmonary resection after neoadjuvant immunotherapy warrants further investigation. Multiple clinical trials suggest that surgical treatment is often delayed following neoadjuvant immunotherapy. Neoadjuvant immunotherapy may lead to mediastinal and hilar fibrosis [82]. Bott et al. [83] pointed out that although technically challenging, pulmonary resection is feasible without excessive morbidity or mortality. Likewise, Yang et al. [84] confirmed the feasibility and safety of pulmonary resection after neoadjuvant ipilimumab plus chemotherapy. Another study noted the possibility of an unexpected transformation from lobectomy to thoracotomy during surgery [85]. Overall, neoadjuvant chemotherapy and minimally invasive lobectomy are feasible for stage IIIA (N2) NSCLC [86], however, only 25.7% of these patients undergo lobectomy [87]. Nevertheless, thoracotomy does not appear to significantly affect morbidity and early mortality rates [83]. After pulmonary resection, the pathologist can determine the pathological response and identify predictive factors of MPR as well as potential impacts on survival.
11 ONCOGENIC-ADDICTED NSCLC
Administering neoadjuvant immunotherapy is challenging in patients with NSCLC, particularly for patients with oncogenic-addicted NSCLC. It is currently unclear whether patients with targeted genes, such as EGFR, anaplastic lymphoma kinase (ALK), should be enrolled in neoadjuvant immunotherapy trials. Although these patients may benefit from neoadjuvant targeted therapy, data on outcomes of neoadjuvant immunotherapy or chemoimmunotherapy are very limited. Patients with early-stage NSCLC may not be offered genomic testing because genomic analysis is not currently considered a standard of care. In the ongoing study of neoadjuvant/adjuvant durvalumab for the treatment of patients with resectable NSCLC trial (NCT03800134), EGFR/ALK testing and tumor PD-L1 status are required because 20% of patients recruited into the study will have EGFR mutations. Patients with oncogenic-addicted NSCLC have been excluded from some studies such as the phase III Checkmate 77T trial (NCT04025879). In the LCMC3 [48] and Cornell single-agent [88] trials, no patients with an EGFR mutation achieved MPR. Three Patients with EGFR or KRAS mutations also did not achieve MPR or pCR in the trial by Hong et al [55]. Hyper-progression might be one reason why patients with EGFR-mutated NSCLC benefit less from immunotherapy than wild-type patients. EGFR mutation status may help cancer cells avoid immune attack by up-regulating the expression of PD-1, PD-L1, and CTLA-1 [89]. Approximately 20% of NSCLCs carrying EGFR mutations undergo hyper-progression. EGFR mutations are associated with decreased immune infiltration or a lack of infiltrating immune cells in the NSCLC microenvironment [90], suggesting a higher incidence of progression for EGFR-mutated NSCLC. In addition, the incidence of AEs is significantly higher in NSCLC patients carrying EGFR mutations. Hence, neoadjuvant targeted therapies may be more suitable for most patients with oncogenic-addicted NSCLC but neoadjuvant chemoimmunotherapy should still be considered for a small subset of these patients. We have noted that two patients carrying EGFR mutations achieved pCR with a neoadjuvant regimen consisting of atezolizumab, carboplatin, and nab-paclitaxel [52]. The small sample size of the study does not explain this problem but rather presents the existence of various possibilities. Further exploratory researches may help us identify the proportion of oncogenic-addicted NSCLC patients who most likely benefit from neoadjuvant chemoimmunotherapy.
12 PREDICTIVE BIOMARKERS FOR NEOADJUVANT IMMUNOTHERAPY
12.1 PD-L1/TMB
Additional data on biomarkers that facilitate patient selection and risk stratification are critical for exploring the role of immunotherapy in early-stage NSCLC. Numerous studies have examined the predictive utility of PD-L1 expression or high TMB for immunotherapy in advanced NSCLC. Mono-immunotherapy or chemoimmunotherapy may be selected as a first-line strategy for advanced NSCLC based on PD-L1 expression, however, there is no consensus on the use of PD-L1 expression and TMB to assess the efficacy of neoadjuvant ICI. Zaric et al. [91] reported a potential relationship between prolonged survival and PD-1 expression as an independent prognostic factor for cancer recurrence and death. For early-stage squamous cell lung carcinoma, however, PD-L1 expression, the TMB, and T-effector, and interferon-gamma gene signatures were not associated with OS [92]. Similarly, Tsao et al. [93] found no relationship between PD-L1 expression and prognosis of early-stage NSCLC patients. Based on these conflicting reports, the utility of PD-L1 as a predictor of efficacy for neoadjuvant or adjuvant immunotherapy requires further exploration.
The TMB is an approximate measure of all gene mutations in tumor tissues and an emerging biomarker for response to immunotherapy. Although a high TMB tends to be associated with a better response to immunotherapy [94], there are still pros and cons associated with the clinical utility of TMB. Owada-Ozaki et al. [95] reported a statistically significant correlation between TMB >62 and poor prognosis in resected NSCLC. Conversely, in a retrospective analysis of two large randomized trials, patients treated with atezolizumab with a blood-based high TMB (≥ 16) demonstrated better PFS than patients treated with docetaxel (the interaction P = 0.036) [96]. Some researchers are attempting to clarify the correlation between PD-L1/TMB and pathological response. MPR was not correlated with PD-L1 expression and the TMB was identified as a potential predictive biomarker for MPR in the neoadjuvant immunotherapy setting [47].
12.2 Circulating tumor DNA (ctDNA)
Advances in liquid biopsy technologies have resulted in increased interest in exploiting ctDNA as a promising tool to monitor response to anti-cancer treatments. ctDNA is a type of cell-free DNA in the peripheral blood that originates from tumors and is detectable in 50%–95% of stage I–III LCs [97, 98], suggesting it may be widely used as a biomarker in clinical practice. The role of ctDNA has been investigated in the adjuvant therapeutic setting. Comparing ctDNA levels before and after surgery may help to identify patients at high risk for disease recurrence [99]. In a recent study of localized NSCLC, preoperative ctDNA was found to be a strong predictor of relapse-free survival and OS after complete resection of pulmonary tumors [100]. These observations suggest that ctDNA could be useful to stratify patients for further adjuvant or neoadjuvant therapy.
A current clinical trial (NCT04367311) is assessing concomitant chemotherapy plus atezolizumab in the adjuvant setting for patients with stage I-select IIIA NSCLC and detectable ctDNA after surgery. Clearance of ctDNA may serve as a surrogate marker for long-term DFS and OS in these patients. Similarly, in a breast cancer study, ctDNA was reported to be a highly accurate indicator of molecular responses and residual disease after neoadjuvant therapy [101]. Similar studies to exploring the clinical utility of ctDNA in NSCLC patients receiving neoadjuvant immunotherapy should be emerging soon.
12.3 Other potential biomarkers
Routine blood testing, including the neutrophil to lymphocyte ratio and platelet to lymphocyte ratio, are useful for predicting patient prognosis. Prognosis is often poor in patients with relatively higher ratios at the baseline of immunotherapy treatment. Peripheral blood tumor markers, such as carcinoembryonic antigen (CEA) and cytokeratin-19 fragments (CYFRA 21-1), have been used to monitor the efficacy of anti-tumor treatment for decades [102]. The usefulness of CEA and CYFRA 21-1 has been confirmed to monitor the response to chemotherapy by a meta-analysis [102]. The usefulness of or CEA and CYFRA21-1 might also be a reliable biomarker to predict nivolumab efficacy in NSCLC patients [103]. The immune microenvironment of LC prior to immunotherapy is different from the immune microenvironment after immunotherapy. By comparing gene expression profiles in surgically resected specimens with normal lung samples, the NSCLC immune microenvironment was shown to be predictive of prognosis after surgery [104]. In this case, the overall immune microenvironment could be considered as a biomarker for the efficacy of immunotherapy. Accumulating evidence suggests that the microbiome is relevant to ICIs and affects the therapeutic efficacy of immunotherapy [105]. Future studies should monitor the microbiome before and after neoadjuvant immunotherapies and after surgery. The gut microbiota, galectin-3, and the intensity of CD8 cell infiltration may be useful as predictive biomarkers [106]. Finally, Zhang et al. [107] recommended compartmental analysis of T-cell clonal dynamics as a biomarker of the pathologic response to neoadjuvant anti-PD-1 immunotherapy in resectable NSCLC. T cell receptor sequencing in the periphery also may prove useful for predicting the response to immunotherapy.
13 FUTURE PERSPECTIVES
ICIs represent an important advance in the treatment of LC. ICIs will likely move forward to the neoadjuvant setting for patients diagnosed with NSCLC, however, several challenges are to overcome (Figure 1), including (1) determining whether oncogenic-addicted NSCLC can be excluded from single immunotherapy, (2) selecting the appropriate set of biomarkers, (3) using the optimal number of cycles prior to surgery to achieve maximum benefits, (4) using neoadjuvant ICIs, chemotherapy, or other specialized treatments, (5) determining the optimal timing of surgery and the best indicators for follow-up after surgery, (6) defining the role of adjuvant treatment and detailed treatment regimens, (7) adopting the appropriate surrogate endpoint of long-term survival in neoadjuvant trials and more.
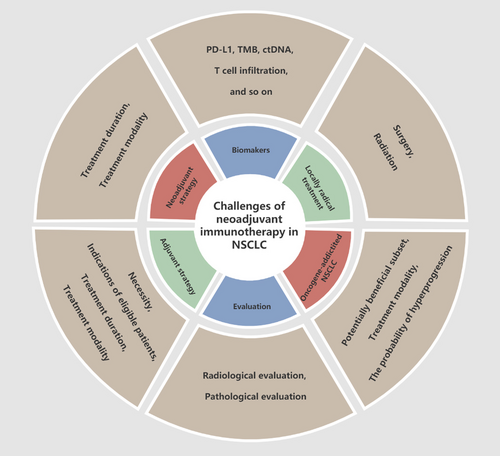
Challenges of neoadjuvant immunotherapy in NSCLC. Abbreviations: ctDNA, circulating tumor DNA; NSCLC, non-small cell lung cancer; PD-L1, programmed cell death 1 ligand 1; TMB, tumor mutational burden
Based on ongoing clinical trials, we envision the potential neoadjuvant and adjuvant strategy for NSCLC patients presented in Figure 2. For potentially resectable NSCLC (Figure 2), genetic testing will be necessary for screening prior to neoadjuvant treatment. Oncogenic-addicted NSCLC is more suitable for neoadjuvant chemoimmunotherapy or neoadjuvant tyrosine kinase inhibitors. For wild-type NSCLC, defining the optimum predictive biomarkers will be important for developing a personalized medicine approach, in which different treatment options will be selected based on biomarkers. Following surgery, tumors are usually restaged to assess pathologic regression and determine the need for adjuvant treatment. We look forward to additional evidence supporting pCR as a surrogate predictor of OS. In this case, patients achieving a pCR would only require regular follow-up examinations after surgery. Patients who achieve a MPR or other pathological response would be eligible for adjuvant treatment. All of these should be further investigated.
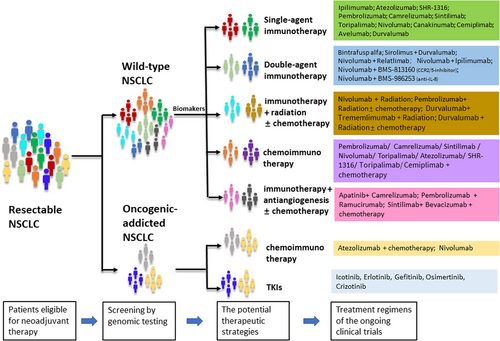
The potential neoadjuvant strategy in NSCLC and the treatment regimens of the ongoing clinical trials. Abbreviations: CCR, CC chemokine receptor; IL, interleukin; NSCLC, non-small cell lung cancer; SRT, stereotactic radiation therapy; TKIs, tyrosine kinase inhibitors
As thoracic surgical oncologists, we should actively participate in the development and execution of neoadjuvant trials to address these concerns. When possible, we should conduct real-world studies to overcome the limitations of clinical trials. Results of clinical trials and real-world studies may provide answers to immunologic, clinical, and translational questions, and establish neoadjuvant immunotherapy as a standard of care in the near future.
14 CONCLUSIONS
The treatment landscape for patients diagnosed with NSCLC has changed dramatically in recent years due to the success of novel immunotherapies. With the potential to improve the OS of patients with early-stage NSCLC, moving immunotherapy to the neoadjuvant setting is just around the corner. Current data suggest that lung resection following neoadjuvant immunotherapy is safe and feasible. Numerous ongoing clinical trials of ICIs and the novel combination therapies suggest that immunotherapies may be an optimal treatment strategy for early-stage NSCLC in the neoadjuvant setting. Although clinical trial data are still emerging, many factors remain to be determined, including identifying predictors of response, determining patients who will most benefit from it, and exploring mechanisms of primary and secondary resistance to immunotherapies. We eagerly await the results of multiple ongoing trials which are essential for determining how to best integrate this model for the treatment of early-stage NSCLC. Given the favorable efficacy and safety profiles, we believe that neoadjuvant immunotherapy and/or combination therapies will change the paradigm of NSCLC treatment in the near future.
DECLARATIONS
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
Not applicable.
COMPETING INTERESTS
The authors declare that they have no competing interests.
FUNDING
This study was supported by the High-Level Hospital Construction Project (grant No. Dfjh201801), the National Natural Science Foundation of China (grant No. 81872510 and 81802266), the Guangdong Provincial People's Hospital Young Talent Project (grant No. Gdpphytp201902), the Guangdong Provincial Key Laboratory of Lung Cancer Translational Medicine (grant No. 2017b030314120), and the Guangdong Basic and Applied Basic Research Foundation (grant No. 2019b1515130002).
AUTHORS’ CONTRIBUTIONS
All authors conceived, directed, and supervised this review. J.K. drafted the manuscript and W.Z.Z. revised the manuscript. All authors read and approved the final version of the manuscript.
ACKNOWLEDGMENTS
None.




