Junction plakoglobin regulates and destabilizes HIF2α to inhibit tumorigenesis of renal cell carcinoma
Abstract
Background
Increased hypoxia-inducible factor 2α (HIF2α) activation is a common event in clear cell renal cell carcinoma (ccRCC) progression. However, the function and underlying mechanism of HIF2α in ccRCC remains uninvestigated. We conducted this study to access the potential link between junction plakoglobin (JUP) and HIF2α in ccRCC.
Methods
Affinity purification and mass spectrometry (AP-MS) screening, glutathione-s-transferase (GST) pull-down and co-immunoprecipitation (Co-IP) assays were performed to detect the interacting proteins of HIF2α. Quantitative PCR (qPCR) and Western blotting were used to detect the expression of JUP in human ccRCC samples. Luciferase reporter assays, chromatin immunoprecipitation (ChIP), cycloheximide chase assays, and ubiquitination assays were conducted to explore the regulation of JUP on the activity of HIF2α. Cell Counting Kit-8 (CCK-8) assays, colony formation assays, transwell assays, and xenograft tumor assays were performed to investigate the effect of JUP knockdown or overexpression on the tumorigenicity of renal cancer cells.
Results
We identified JUP as a novel HIF2α-binding partner and revealed an important role of JUP in recruiting von Hippel-Lindau (VHL) and histone deacetylases 1/2 (HDAC1/2) to HIF2α to regulate its stability and transactivation. JUP knockdown promoted and overexpression suppressed the tumorigenicity of renal cell carcinoma in vitro and in vivo. Importantly, the low expression of JUP was found in clinical ccRCC samples and correlated with enhanced hypoxia scores and poor treatment outcomes.
Conclusion
Taken together, these data support a role of JUP in modulating HIF2α signaling during ccRCC progression and identify JUP as a potential therapeutic target.
Abbreviations
-
- JUP
-
- Junction plakoglobin
-
- HIF2α
-
- Hypoxia-inducible factor 2 alpha
-
- EGLN1
-
- Egl-9 family hypoxia inducible factor 1
-
- ccRCC
-
- Clear cell renal cell carcinoma
-
- RCC
-
- Renal cell carcinoma
-
- AP-MS
-
- Affinity purification coupled with mass spectrometry
-
- qPCR
-
- Quantitative PCR
-
- RT-PCR
-
- Real time PCR
-
- ChIP
-
- Chromatin immunoprecipitation
-
- VHL
-
- von Hippel–Lindau
-
- PTMs
-
- Post-translational modifications
-
- CBP
-
- cAMP-response element binding protein
-
- HDACs
-
- Histone deacetylases
-
- HREs
-
- Hypoxia response elements
-
- ARNT
-
- Aryl hydrocarbon receptor nuclear translocator
-
- TCGA
-
- The Cancer Genome Atlas
-
- PAS
-
- Per-Arnt-Sim
-
- DM
-
- Double mutant
-
- Co-IP
-
- Co-Immunoprecipitation
-
- CCK-8
-
- Cell Counting Kit-8
-
- SDS-PAGE
-
- sodium dodecyl sulfate polyacrylamide gel electrophoresis
-
- GST
-
- Glutathione-S-transferases
-
- CUL2
-
- Cullin 2
-
- EGFP
-
- Enhanced Green Fluorescent Protein
-
- TCF-4
-
- Transcription Factor 4
-
- TBP
-
- TATA-Box Binding Protein
-
- IH
-
- inhibitory
-
- PAS
-
- the Per-Arnt-Sim
-
- PAC
-
- PAS-associated C-terminal
1 BACKGROUND
Renal cell carcinoma (RCC) is a common urologic tumor, accounting for more than 144,000 deaths each year [1], and clear cell renal cell carcinoma (ccRCC) is the most common histological type. Most ccRCC cases are due to loss of the tumor suppressor gene von Hippel-Lindau (VHL) [2]. The best-known function of VHL is as an E3 ubiquitin ligase that targets hypoxia-inducible factor 1α (HIF1α) and HIF2α for degradation [3]. However, HIF1α inhibits the initiation and/or progression of ccRCC, whereas HIF2α acts as an oncogene [4]. Extensive studies have revealed that hyperactivation of HIF2α signaling is a central module of cell survival and metastasis in ccRCC [5]. Apart from VHL-mediated ubiquitination, HIF2α activity is also regulated at various levels involving other post-translational modifications (PTMs) and cofactors. In hypoxic cells, cAMP-response element binding protein (CBP) catalyzes the acetylation of lysine residues of HIF2α, which acts in conjunction with sirtuin 1 to promote HIF2α signaling [6, 7]. Epigenetic regulators are also involved in HIF2α-mediated transactivation. For example, HIFs recruit histone deacetylases (HDACs) and p300/CBP to regulate the expression of some HIF-responsive genes [8, 9]. In addition, zinc finger MYND-type containing 8 promotes the transcriptional activity of both HIF1α and HIF2α by recruiting bromodomain-containing protein 4 to the hypoxia response elements (HREs), which induces the release of paused RNA polymerase II [10]. Importantly, there is evidence for crosstalk between HIF2α signaling and many other pathways, such as nuclear factor kappa-B (NF-κB) [11, 12], Notch signaling [13, 14], and Wnt/β-catenin signaling [15, 16], in various cancers including ccRCC.
Due to the fundamental role of HIF2α in ccRCC, structure-based drug design and rational modification has led to the development of a novel series of drugs, such as PT2385, PT2399, and PT2977, which can disrupt HIF2α/aryl hydrocarbon receptor nuclear translocator (ARNT) heterodimerization and inhibit HIF2α target gene expression [17-19]. Additionally, a phase I trial showed PT2385 had a favorable safety profile and was active in patients with advanced ccRCC who had previously taken a tyrosine kinase inhibitor [20]. However, many challenges still exist, such as differential sensitivity and the development of therapy resistance to HIF2α antagonists [18]; the function and underlying mechanism of HIF2α in tumorigenesis remains uninvestigated.
Given that HIF2α is a good therapeutic target for ccRCC treatment and the regulatory factors of HIF2α are still not clear, we sought to investigate the key molecules that could regulate the activity of HIF2α. Therefore, we screened the interaction proteins of HIF2α by affinity purification-MS (AP-MS). Our study aimed to investigate the interaction between JUP and HIF2α and whether HIF2α signaling is regulated by JUP during ccRCC progression.
2 MATERIALS AND METHODS
2.1 Human samples
ccRCC and corresponding adjacent normal tissues were obtained from 12 ccRCC patients treated in the Department of Urology at Tongji Hospital (Wuhan, Hubei, China) between January 2013 and August 2013 after they provided written informed consent. All samples were kept in liquid nitrogen before RNA and protein extraction. A ccRCC tissue array was purchased from Shanghai Superchip (HKidCRC030PG01; Shanghai, China).
2.2 Antibodies
The following antibodies were used in the experiments: mouse anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (G8795, 1:10,000) and anti-Flag (F3165, 1:2000) from Sigma-Aldrich (St. Louis, MO, USA); mouse anti-Myc (11667149001, 1:2000) and mouse anti-hemagglutinin (HA) antibody (11583816001, 1:2000) from Roche Applied Science (Penzberg, Germany); rabbit anti-Myc (562, 1:2000), rabbit anti-HA antibody (561, 1:2000), and anti-Strep-tag II (M211-3, 1:3000) from MBL Life Science (MBL, Nagoya, Japan); anti-HIF2α (NB100-122, 1:1000) from Novus Biologicals (Centennial, CO, USA); anti-histone H3 (acetyl K27) (H3K27ac, 1:100 for ChIP) (ab4729) from Abcam (Cambridge, MA, USA); anti-HDAC1 (GTX100513) from GeneTex (Irvine, CA, USA); rabbit anti-junction plakoglobin (JUP) (#75550, 1:1000) and anti-p300 (#86377, 1:100 for ChIP) from Cell Signaling Technology (Danvers, MA, USA); mouse anti-JUP (#MA5-15905, 1:1000), goat anti-mouse IgG secondary antibody (31430, 1:20,000), and goat anti-rabbit IgG-horseradish peroxidase (HRP) secondary antibody (31460, 1:20,000) from Thermo Fisher Scientific (Waltham, MA, USA).
2.3 Cell lines, cell culture, transfection, virus infection and treatments
The 786-O, OSRC-2, SN12-PM6, ACHN, and HEK293T cell lines were purchased from the Cell Bank of Shanghai Institute of Cell Biology (Chinese Academy of Medical Science, Shanghai, China). These cells were cultured in Dulbecco's Modified Eagle's Medium (DMEM, HyClone, Logan, Utah, USA) with 10% fetal bovine serum (HyClone) in the presence of 5% CO2 at 37°C in a humidified incubator (Thermo Fisher Scientific). Transfection was performed using Lipofectamine™2000 (Thermo Fisher Scientific). For lentiviral infection, HEK293T cells were transfected with packaging vectors (pRSV-Rev, pMD2.G, and pCMV-VSV-G) and LacZ shRNA, JUP shRNA, HIF2α shRNA plasmids, or psi-HA-JUP plasmids; the viruses were collected 60 h after transfection and used to infect 786-O or ACHN cells. Stably transduced cells were screened using puromycin (Sigma) at a final concentration of 2 mg/mL for 3 days. Cells were treated with 10 μg/mL MG132 (S1748, Beyotime Biotechnology, Beijing, China) for 6 h before being harvested for immunoblotting with anti-HIF2α antibody or control antibodies.
2.4 RT-PCR analysis
Total RNAs were extracted by TRIzol (Invitrogen, Carlsbad, CA, USA) and cDNAs were synthesized using the ReverTra Ace qPCR RT Kit (Toyobo, Osaka, Japan). Real-time PCR (qPCR) was performed following standard protocols using SYBR Green Mix (Roche). The primer sequences used are shown in Supplementary Table S1.
2.5 Luciferase assay
HEK293T or 786-O cells were grown in 48-well plates to 50%-70% confluency and transfected with 6xHRE-Luc, pRL-TK, Flag-HIF2α, and HA-JUP overexpressed plasmid combinations using Lipofectamine™2000 (Thermo Fisher Scientific). Approximately 36 h after transfection, cells were lysed and analyzed using the Dual-Luciferase Reporter System (Promega, Fitchburg, WI, USA).
2.6 Plasmids
VHL, Myc-ubiquitin, EGFP-HIF1α, TK-Rluc, and 6xHRE-Luc plasmids have been previously described [21, 22]. All the other plasmids in this study were constructed by standard molecular biology techniques. psi-StrepII, which contains Strep-tag-II, was constructed by inserting two Strep-tag sequences into the psi-Flag plasmid. PCDH-StrepII-GST-C1, containing fusion tags including Strep-tag-II and GST-tag, was constructed by subcloning Strep-tag-II and GST-tag into the XbaI and NotI sites of PCDH-CD513B-1 (System Biosciences, Palo Alto, CA, USA). The 12xHis-tag sequence was synthesized and cloned into PCDH-StrepII-GST-C1 to obtain PCDH-12xHis. Wild-type (WT) HIF2α cDNA was PCR-amplified using primers HIF2α-5’ and HIF2α-3’, digested by BamHI and SalI, and ligated into pEGFP-myc-C1, psi-StrepII, PCDH-12xHis, and psi-Flag to create EGFP-myc-HIF1α, StrepII-HIF2α, 12xHis-HIF2α, and Flag-HIF2α, respectively. JUP cDNA was PCR-amplified using primers JUP-5’ and JUP-3’, digested by BamHI and XhoI, and ligated into psi-HA, pGEX-4T-1, and PCDH-StrepII-GST-C1 to create HA-JUP, GST-JUP (bacterial expression plasmid), and StrepII-GST-JUP (mammalian expression plasmid). Mammalian expression plasmids for human ARNT, HDAC1, HDAC2, and deletion mutants of HIF2α and JUP were constructed using standard molecular biology techniques. The PCDH-H1 short hairpin RNA (shRNA) cloning vector was constructed by enzymatically deleting the cytomegalovirus (CMV) promoter of the PCDH-CD513B-1 plasmid, and then inserting the H1 promoter of pSilencer5.1-H1 Retro plasmid (Thermo Fisher Scientific). The shRNA target sequence was synthesized and inserted into the BamHI and NotI sites of PCDH-H1. The target sequences for shRNA JUP, HIF2α, or HDAC1 were as follows: sh-JUP-1#, 5’-GCTTCAGACTCAAGTACCCA-3’; sh-JUP-2#, 5’-GATCATGCGTAACTACAGTTA-3’; sh-HIF2α-1#, 5’-GATGGACTTACCTGGCAGAC-3’; sh-HIF2α-2#, 5’-GCTGACCAGCAGATGGACAAC-3’; and sh-HDAC1, 5’-GCAGATGCAGAGATTCAACG-3’. The primers used are shown in Supplementary Table S1.
2.7 Co-immunoprecipitation (Co-IP)
To analyze protein interactions, Co-IP experiments were performed using HEK293T or 786-O cells after 48-hour transfection. Then, the cells were lysed in NETN lysis buffer (50 mmol/L Tris-HCl at pH 8.0, 0.15 M NaCl, 1 mmol/L EDTA, 0.5% NP-40) with a 1× protease inhibitor cocktail (Roche). Specified antibody and Protein G Agarose (Roche) were incubated with the cell lysates overnight at 4°C. The resins were washed four times with wash buffer (50 mmol/L Tris-HCl at pH 8.0, 0.5 mol/L NaCl, 1 mmol/L EDTA, 0.5% NP-40). After elution by loading buffer, the bound proteins were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE).
2.8 Cycloheximide chase assays
For pulse-chase analysis, cells were aliquoted to 35 mm dishes. When achieved 80%–90% confluence, the cells were treated with cycloheximide (CHX, A10036, AdooQ BioScience, Irvine, CA, USA) at a final concentration of 100 μmol/L. At the indicated time points after CHX treatment, the cells were harvested for immunoblotting with anti-HIF2α antibody or control antibodies.
2.9 Ubiquitination assays
ACHN cells transfected with 12 × HIS-HIF2α, Myc-ubiquitin (Myc-UB), and HA-JUP were treated with 10 μg/mL MG132 for 6 h before being lysed with UREA buffer (10 mmol/L Tris-HCl at pH 8.0, 100 mmol/L NaH2PO4, 8 mol/L urea, 10 mmol/L imidazole). After sonication, the lysate was centrifuged at 12,000 × g for 10 min at 4°C. Then, supernatant was incubated with Ni-NTA Superflow agarose (Thermo Fisher Scientific) for 4 h at room temperature. The beads were washed four times with UREA buffer containing 20 mmol/L imidazole.
2.10 AP-MS
Cells (∼100 million) stably expressing StrepII or StrepII-HIF2α were lysed in NETN lysis buffer consisting of 50 mmol/L Tris-HCl at pH 8.0, 0.15 mol/L NaCl, 1 mmol/L ethylenediaminetetraacetic acid (EDTA), 0.5% NP-40, and a 1x protease inhibitor cocktail (Roche). Prior to complex purification, avidin (20 μg/mL extracts, A9275; Sigma) was added to the extracts to remove biotinylated molecules that may bind nonspecifically to the Strep-Tactin XT superflow resin (2–4010; IBA Lifesciences, Gottingen, Germany). Then the superflow resin (80 μL) was added to the extracts followed by incubation for 4 h at 4°C. Beads were washed five times with wash buffer and then eluted with NuPAGE™ LDS Sample Buffer (NP0007; Thermo Fisher Scientific). The proteins were separated by SDS-PAGE (NP0322BOX; Thermo Fisher Scientific) and cut from the Coomassie blue-stained gels into three portions according to their molecular weights (10–35 kDa, 35–70 kDa, and 70–180 kDa). Then the In-Gel proteins were digested and analyzed using the HPLC-Orbitrap-ELite Mass Spectrometer (Thermo Fisher Scientific) as previously described [23].
2.11 Chromatin immunoprecipitation (ChIP) assay
The ChIP assay was performed using antibodies against human JUP, HDAC1, HIF2α, p300, and H3K27ac as previously described [21]. The precipitated DNA was subjected to qPCR using primers described in Supplementary Table S1. Sonicated DNA was normalized for each sample of cells before immunoprecipitation.
2.12 Colony formation, proliferation, migration, and invasion assays
Colony formation was measured two weeks after seeding 1000 cells per well in 6-well plates. Cell proliferation was measured using the Cell Counting Kit-8 (CCK-8) assay (Dojindo Laboratories, Kumamoto, Japan) according to the manufacturer's instructions. Migration and invasion assays were conducted using uncoated and Matrigel-coated Transwell® inserts (Corning, NY, USA) according to the manufacturer's instructions. At 12–24 h after the cell seeding into Transwell® inserts, migrating and invading cells were stained with 0.5% crystal violet solution and imaged with a microscope.
2.13 Animal experiments
For the subcutaneous xenograft model, 5 × 106 cells in 100 μL phosphate-buffered saline (PBS) were injected subcutaneously in 7-week-old male BALB/c nude mice which were purchased from Beijing Huafukang Biotechnology (Beijing, China). Tumor volume was measured with calipers weekly and calculated according to the formula: volume = 0.5 × length × height × width.
For the tail intravenous injection assay, 5 × 106 cells in 100 μL PBS were injected into the tail vein of nude mice. The number and volume of metastases were examined weekly by an in vivo imaging system from day 28 after cell injection. All experiments were approved by the Animal Care and Use Committee of Tongji Medical College of Huazhong University of Science and Technology.
2.14 The cancer genome atlas (TCGA) analysis
TCGA data of mRNA expression levels and clinical characteristics were downloaded from an integrated TCGA Pan-Cancer Clinical Data Resource [24]. Hypoxia scores for TCGA patients were calculated by Bhandari et al. [25] using the method developed by Ragnum et al. [26]. Kaplan-Meier analyses were conducted using GraphPad Prism (San Diego, CA, USA). The clinical characteristics of patients with different JUP expression levels were compared by using two-tailed chi-square test with the SPSS 20 software (SPSS Inc., Chicago, IL, USA). A P value <0.05 was considered significant.
2.15 RNA sequencing and differential gene expression analysis
RNA sequencing of 786-O cells stably expressing sh-JUP or sh-LacZ was performed by Ouyi Biomedical Technology (Shanghai, China). The transcriptome of oligo-dT enriched mRNA was sequenced using HiSeqTM 2500 (Illumina, Watertown, MA, USA). Differential gene expression was determined by the R package edgeR [27] using Trimmed Mean of M-values (TMM) normalization.
3 RESULTS
3.1 Identification of JUP as an HIF2α-associated protein
We first screened interaction proteins of HIF2α using AP-MS. These experiments identified not only VHL, ARNT, Egl-9 family hypoxia-inducible factor 1 (EGLN1) and Cullin 2 (CUL2), which were expected, but also several unreported proteins including JUP (data not shown). For validation, Flag-HIF2α was co-expressed with HA-tagged JUP in HEK293T cells, and results showed HA-JUP efficiently co-precipitated with Flag-HIF2α in HEK293T cells (Figure 1A and B). Additionally, bacterially expressed GST-JUP associated with Flag-HIF2α (Figure 1C), indicating that HIF2α directly interacted with JUP. Furthermore, endogenous JUP and HIF2α co-precipitated in 786-O and OSRC-2 cells (Figure 1D). Given that both HIF1α and HIF2α sharing many common interaction proteins (e.g., β-catenin [15] and c-Myc [28]), we examined whether JUP also binds to HIF1α. As expected, Co-IP assays showed that EGFP-HIF1α was co-precipitated efficiently with endogenous JUP (Figure 1E). These results indicate that JUP associates with both HIF1α and HIF2α.
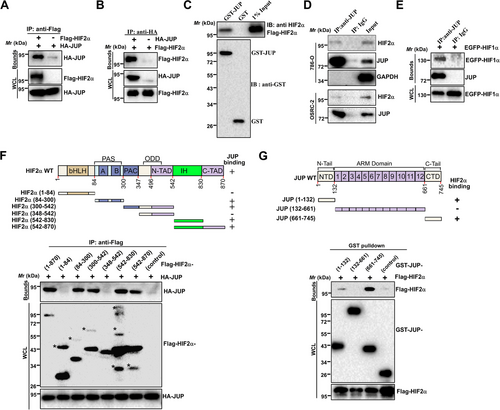
3.2 Identification of domains for JUP-HIF2α interaction
Next, we determined the structural domains required for HIF2α-JUP interaction. A series of truncated HIF2α constructs were co-expressed with HA-JUP in HEK293T cells for AP and analysis. As shown in Figure 1F, the Per-Arnt-Sim (PAS) and PAS-associated C-terminal (PAC) regions (spanning amino acids 84–347) and the inhibitory (IH) domain of HIF2α were sufficient for interaction with JUP. Similarly, JUP truncations were co-expressed with Flag-HIF2α, revealing that both the N- and C-terminal domains of JUP were sufficient for HIF2α interaction (Figure 1G).
3.3 JUP expression in ccRCC tissues
JUP is a member of the Armadillo protein family and is a structural and functional homolog of β-catenin. Previous studies have indicated that JUP with diverse functions are involved in human cancer [29-35]. We next assessed the role of JUP expression on renal cancer development and progression. Twelve specimens of ccRCC tissues with adjacent normal tissues were collected to examine the mRNA levels of JUP by qPCR. JUP was significantly downregulated in these ccRCC samples compared with the corresponding adjacent normal tissues (Figure 2A). We also evaluated the protein level of JUP in 5 specimens of ccRCC, 4 of which showed low expression of JUP protein (Figure 2B). We further JUP expression in a ccRCC tissue array from Shanghai Superchip (Supplementary Fig. S1, Figure 2C). Weak JUP staining was detected in 13 (86.7%) of 15 ccRCC tissues. In contrast, most normal tissues (14 of 15) exhibited strong JUP staining.
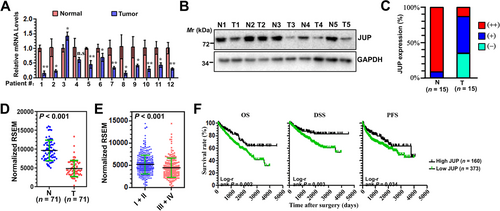
We further examined JUP expression in a TCGA cohort and found it was significantly downregulated in ccRCC tumors compared with normal tissues (Figure 2D). Additionally, JUP expression differed significantly in patients according to pathologic grade (P < 0.001), TNM stage (P < 0.001), and distant metastasis (P = 0.041) (Supplementary Table S2). We also found that JUP was significantly decreased in stages III and IV ccRCC compared with stages I and II tissues (Figure 2E). Furthermore, Kaplan-Meier survival analysis demonstrated patients with low JUP expression had significantly poorer OS, disease-specific survival, and progression-free survival compared to those with high JUP expression (Figure 2F), indicating JUP might be an independent prognostic factor for ccRCC survival.
3.4 The effects of JUP on the proliferation and metastasis of RCC cells
Next, we examined the possible role of JUP in ccRCC progression. We first stably expressed JUP in ACHN and 786-O cells and found that JUP inhibited colony formation (Figure 3A). Additionally, JUP suppressed the migration of these cells in a wound healing assay (Figure 3B). Consistent with the wound healing results, overexpression of JUP also inhibited the migration and invasion of these cells in the transwell assays (Figure 3C). Together, these results suggest that JUP may function as a tumor suppressor in ccRCC.
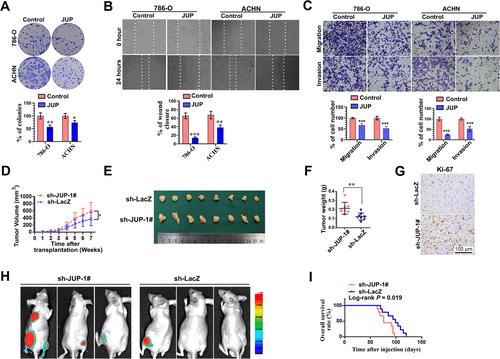
Next, we determined whether JUP depletion could promote tumor growth in a xenograft mouse model. Although JUP knockdown (JUP-KD) had no effect on the in vitro growth of ACHN and 786-O cells (Supplementary Fig. S2), tumors from subcutaneously transplanted ACHN cells with stable knockdown of JUP grew more rapidly compared with the controls (Figure 3D–F). Immunohistochemical staining showed that the expression of Ki-67, a cell proliferation marker, was significantly increased in JUP-KD tumors compared with sh-LacZ tumors (Figure 3G), suggesting increased cell proliferation in tumors by JUP-KD.
Finally, we studied the effect of JUP-KD on RCC cell metastasis to other parts of the body. In intravenous injection assay with EGFP quantitative imaging, we noticed that the mice injected with JUP-KD cells had a marked increase in fluorescence signal compared with those injected with control cells. Of the nine mice injected with JUP-KD cells, five developed detectable lesions, as observed in a live-animal imaging system, whereas only one mouse injected with sh-LacZ cells developed detectable lesions. Representative fluorescence images at eight weeks post-injection showed that more metastasis occurred in the leg and lung after JUP-KD (Figure 3H). Moreover, the overall survival (OS) curves showed the JUP-knockdown group had a worse prognosis than the sh-LacZ group (Figure 3I). Together, these findings indicate JUP is a potent tumor suppressor that inhibits RCC progression.
3.5 JUP plays a key role in regulating the HIF2α transcriptional activity
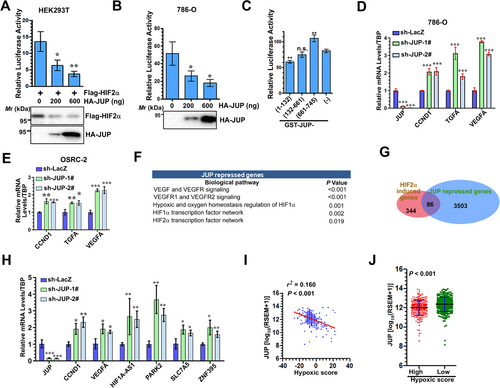
We next determined whether JUP affected the transactivation of HIF2α. Using 6xHRE-driven luciferase reporter, our results showed JUP decreased the transcriptional activity of both transfected and endogenous HIF2α in HEK293T and 786-O cells, respectively (Figure 4A and B). Moreover, when JUP fragments were expressed, the N-terminal of JUP decreased the transactivation of HIF2α, whereas the C-terminal of JUP had the opposite effect (Figure 4C). We also observed JUP-KD resulted in upregulation of HIF2α target genes in both 786-O and OSRC-2 cells (Figure 4D and E). To confirm the role of JUP in HIF2α activation, we performed genome-wide expression profiling of 786-O cells after JUP-KD (Supplementary Table S3). Gene Ontology analysis revealed JUP regulated cell communication, adhesion, and growth and ion transport genes (Supplementary Table S4). Additionally, biological pathway analysis demonstrated JUP repressed distinct pathways including the vascular endothelial growth factor (VEGF)/VEGF receptor, HIF1α, and HIF2α (Figure 4F and Supplementary Table S5). Furthermore, the overlapping data from JUP and HIF2α transcriptome analysis revealed that 85 HIF2α-activated genes were repressed by JUP (Figure 4G), indicating JUP inhibits the expression of a subset of HIF2α target genes. qPCR further validated six differentially expressed genes in two JUP-stable knockdown cell lines (Figure 4H). Given that HIF2α plays an important role in the adaptive cellular response to hypoxia in ccRCC, we examined the correlation between JUP expression and hypoxia gene signature in the TCGA cohort. Consistent with the negative regulation of HIF2α activity by JUP, we found a negative correlation between JUP mRNA levels and hypoxia gene signature in ccRCC samples (r2 = 0.160, P < 0.001; Figure 4I), but not in other cancer types (data not shown). Furthermore, hypoxia grading in ccRCC samples revealed JUP was significantly decreased in the ccRCC tissues with a high hypoxic signature score (Figure 4J).
3.6 Mechanism of regulation of HIF2α stability by JUP
HIF2α stability is critical for inducing the expression of HIF2α target genes, such as vascular endothelial growth factor A (VEGFA) [5] and zinc finger protein 395 (ZNF395) [36], so we tested whether JUP regulates HIF2α stability. When JUP was overexpressed, we found endogenous HIF2α protein level was decreased (Figure 5A). The HIF2α decrease induced by JUP overexpression was reversed by the proteasome inhibitor MG132 (Figure 5B). In parallel, shRNA-induced downregulation of JUP increased HIF2α levels in VHL-proficient ACHN and SN12-PM6 cells (Figure 5C and D). Additionally, JUP overexpression reduced the half-life of endogenous HIF2α in a cycloheximide chase experiment (Figure 5E and F), whereas JUP-KD led to the opposite effect (Figure 5G and H). Next, we examined whether JUP regulates HIF2α stability by promoting HIF2α ubiquitination. We found overexpression of JUP increased HIF2α ubiquitination (Figure 5I). This indicates JUP binds to and promotes HIF2α ubiquitination. Next, we examined whether promotion of HIF2α ubiquitination by JUP required a functional VHL protein. Overexpression of JUP in VHL-deficient 786-O cells had little effect on endogenous HIF2α levels (Supplementary Fig. S3A). In addition, the inhibitory effect of JUP on HIF2α levels was restored by co-expression of VHL WT but not by the VHL (Y98N) mutant (Supplementary Fig. S3B), indicating VHL is required for this effect. Consistent with this observation, overexpression of JUP had little effect on exogenously produced hydroxylation-defective mutant HIF2α (P405A/P531A) levels (Supplementary Fig. S3C). Furthermore, downregulation of JUP in VHL-null 786-O cells with shRNA resulted in no measurable effects on HIF2α stability (Supplementary Fig. S3D). This indicated JUP regulates HIF2α ubiquitination in a VHL-dependent way. Therefore, we investigated whether JUP interact with VHL. Endogenous JUP protein was observed after incubation with GST-VHL (Figure 5J). Co-IP assays showed that Flag-VHL efficiently co-precipitated with endogenous and exogenous JUP in HEK293T cells (Figure 5K and L). We next tried to identify the interacting site of JUP with VHL. Co-IP results indicate that VHL co-precipitated with the N-terminal domains of JUP (Figure 5M). Then we examined the possibility that JUP facilitates the binding of VHL to HIF2α. Ectopically expressed JUP was found to strengthen VHL-HIF2α interaction (Figure 5N). Taken together, these findings suggest that JUP promotes the ubiquitination of HIF2α by facilitating the VHL-HIF2α interaction.
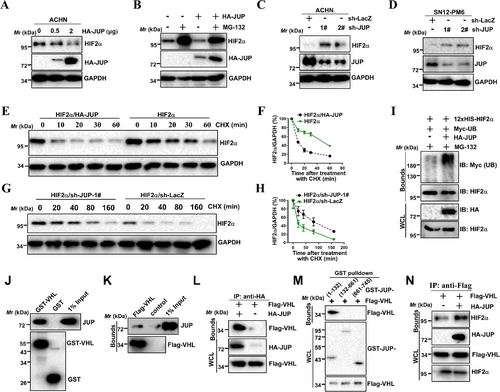
3.7 JUP regulates HIF2α transactivation through the recruitment of HDACs
Because JUP interacts with the HIF2α PAS domain, which is required for heterodimerization with ARNT, we speculated that JUP may interfere with HIF2α-ARNT interaction. To test this hypothesis, Co-IP assays using HIF2α, JUP, and ARNT constructs were performed in HEK293T cells. Unexpectedly, JUP overexpression had a minimal effect on HIF2α-ARNT interactions (Supplementary Fig. S4A). Instead, the JUP-ARNT interaction was independent of HIF2α (Supplementary Fig. S4B). Therefore, we hypothesized that JUP serves as a co-repressor to inhibit transactivation of the HIF2α/ARNT heterodimer.
We noticed that JUP also inhibited the transcriptional activity of endogenous HIF2α in VHL-null 786-O cells (Figure 4), suggesting an alternate, VHL-independent mechanism for JUP-mediated inhibition of HIF2α. Overexpression of JUP still repressed the transcriptional activity of the HRE reporter induced by HIF2α mutant (P405A and P531A double mutant [DM]) (Supplementary Fig. S5). As expected, JUP inhibition of HIF2α-DM signaling was weaker than WT HIF2α signaling for the HRE luciferase reporter (Supplementary Fig. S5), indicating JUP may inhibit HIF2α signaling in both VHL-dependent and VHL-independent manners.
Considering that HDACs usually act as co-repressors for many transcription factors and given HIF2α also interacted with HDAC1/2 (data not shown), we determined whether JUP interacts with HDAC1/2. To this end, we performed Co-IP experiments with Flag-tagged-HDAC1/2 to test the association of HDAC1/2 with JUP and HIF2α and revealed that endogenous JUP and HIF2α associated with both HDAC1 and HDAC2 in 786-O cells (Figure 6A). We next tried to identify the JUP site for HDAC1 binding. Co-IP analyses showed that the JUP C-terminus is responsible for binding HDAC1 (Figure 6B). Because the JUP sites for HDAC1 and HIF2α binding are not identical, JUP might regulate the HIF2α-HDAC1/2 interaction. To test this possibility, we co-expressed HDAC1/2 with or without JUP and performed Co-IP analysis. JUP overexpression strengthened both HIF2α-HDAC1 and HIF2α-HDAC2 interactions (Figure 6C). To further understand the JUP-mediated enhancement of the HIF2α-HDAC1/2 interaction, we investigated whether JUP directly interacted with HDAC1 or HDAC2 independent of HIF2α. Flag-HDAC1/2 proteins were observed after incubation with GST-JUP (Figure 6D), indicating JUP can recruit HDAC1/2 without HIF2α.
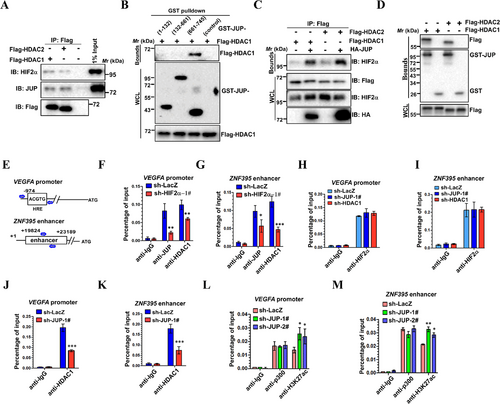
These studies suggest JUP may act as a corepressor by recruiting HDACs to HIF2α.
We next performed the ChIP assays to test whether JUP affects HDAC1/2 binding to the VEGFA and ZNF395 loci, two well-known HIF2α-responsive genes upregulated upon JUP depletion. HIF2α bound the VEGFA promoter and ZFN395 enhancer sites (Figure 6E). ChIP analysis indicated that the binding of JUP and HDAC1 to the VEGFA promoter and ZNF395 enhancer were decreased by HIF2α depletion (Figure 6F and G); this interaction is likely HIF2α-dependent. By contrast, HIF2α binding to these sites was unchanged when either JUP or HDAC was depleted in 786-O cells, suggesting that HIF2α binding to these sites is JUP- and HDAC1-independent (Figure 6H and I). As mentioned above, we demonstrated that JUP enhanced the HIF2α-HDAC1 interaction. Thus, we asked whether the binding of HDAC1 to these sites is regulated by JUP. As expected, JUP depletion decreased binding of HDAC1 to HIF2α target sites compared with the control (Figure 6J and K). Given that p300/CBP-mediated H3K27ac is a well-defined marker of active enhancers and promoters, we next tested whether JUP can regulate localization of p300 and H3K27Ac at these sites. Interestingly, we did not detect any change of p300 binding to these sites when JUP was knocked down (Figure 6L and M). However, the H3K27ac signals in these HIF2α-binding sites were slightly increased upon JUP depletion (Figure 6L and M). Collectively, these data suggest JUP promotes recruitment of HDAC1 to deacetylation of H3K27 at HIF2α target sites and further inhibits transcription of these target genes.
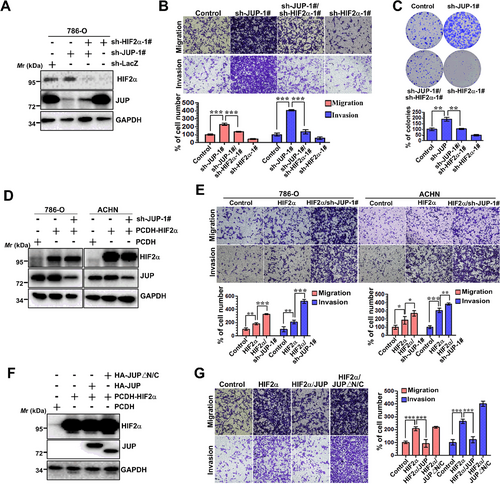
3.8 JUP regulates RCC migration and invasion via HIF2α
As HIF2α plays key roles at all stages of metastasis, we next investigated whether JUP affects cell migration and invasion by functionally repressing HIF2α. The effects of JUP-KD on migration and invasion were partially reversed by concomitant HIF2α knockdown (Figure 7A and B), suggesting the effects of JUP on cell migration and invasion are mediated, at least in part, through HIF2α. As a control, knockdown of HIF2α alone resulted in a marked decrease in migration and invasion (Figure 7B). Consistently, 786-O cells with HIF2α and JUP co-silencing grew significantly slower than cells with JUP silencing alone (Figure 7C). In addition, cell migratory and invasive abilities were increased in HIF2α-overexpressing cells, and further enhanced by JUP-KD (Figure 7D and E), again suggesting JUP is a potent tumor suppressor that inhibits HIF2α function. Finally, we focused on the effects of JUP and mutant JUP (△N/C, lacking both N- and C-terminal domains) on HIF2α-mediated migration and invasion. Our result showed the migration and invasion activity were increased in HIF2α-overexpressing cells, an effect that was partially reversed by the expression of full-length JUP, but not of mutant JUP (Figure 7F and G). Together, these data show JUP inhibits the HIF2α-enhanced migration and invasion of RCC cells.
4 DISCUSSION
In this study, we identified JUP as a novel HIF2α binding partner, and found that HIF2α signaling was inhibited by the JUP protein. Importantly, the low expression of JUP occurred in clinical ccRCC samples and was correlated with enhanced hypoxia scores.
JUP, also named plakoglobin and γ-catenin, is a structural and functional homolog of β-catenin. The best-known function of these catenin proteins is to regulate cell-cell adhesion [37, 38]. There is crosstalk between β-catenin signaling and HIF signaling in cancers. The interaction of β-catenin with HIF1α and HIF2α has been observed in various cancer cells, and β-catenin can enhance both HIF1α- and HIF2α-mediated transcription [15, 39, 40]. However, HIF1α-β-catenin interaction dissociates transcription factor 4 from β-catenin and inhibited β-catenin activity, which is enhanced by the HIF2α-β-catenin interaction [15, 16, 39, 41]. The opposite effects of HIF1α and HIF2α on β-catenin signaling may be caused by the different domains of β-catenin responsible for the interactions with HIF1α and HIF2α [15, 39]. Here, we found JUP associates with HIF2α through its N- and C-terminal transactivation domains, whereas only the N-terminal domain-truncated mutant decreased HIF2α transactivation. Interestingly, it was reported that β-catenin binds to HIF2α via its N-terminal domain (amino acids 1-259) [15], indicating the N-terminal domains of both JUP and β-catenin are critical for the regulation of HIF2α function. We also found the C-terminal domain of JUP could interact with HIF2α. However, when this domain was overexpressed in 786-O cells, HIF2α transcriptional activity was enhanced. Solanas et al. [42] demonstrated the C-terminal domain of JUP interacts with the armadillo repeat domain and regulates the ability of this region to complex with other cofactors such as E-cadherin, α-catenin, and TATA-box binding protein (TBP). Yin et al. [31] showed the C-terminal domain is required for the interaction of JUP with Src. Thus, it is most likely the overexpressed C-terminal domain of JUP competes with endogenous JUP for binding to HIF2α and other cofactors and perturbs the inhibitory effect of JUP.
In addition to interacting with HIF2α, in the present study, JUP was found to associate with VHL and HDAC1/2 and to decrease the stability and transactivity of HIF2α. A previous study showed JUP interacts with CUL2 by large-scale immunoprecipitation experiments [43], but the molecular mechanism of this interaction has not been studied. Given JUP facilitates the pVHL-HIF2α interaction, JUP is likely to directly recruit the pVHL E3 ligase complex to ubiquitinate HIF2α. The hydroxylation of the oxygen-dependent degradation domain of HIF2α in normoxic conditions is a prerequisite for VHL binding [44]. However, we found that JUP associated with the PAS/PAC and IH domains of HIF2α, indicating JUP may be sufficient to promote HIF2α ubiquination under both normoxic and hypoxic conditions. HIFα activity is regulated by interacting with the transcriptional coactivator p300/CBP and co-repressor HDACs [8, 9]. HDAC1/2, the catalytic core of different co-repressor complexes, is associated with histone deacetylation and silenced genes [45]. In addition, HIF1α-p300 interaction can be disrupted by HDAC1 [46]. In this context, we found JUP interacted with HDAC1/2 and promoted HDAC1-HIF2α interaction but was unable to bind p300 (data not shown). Zhao et al. [47] found JUP can interact with CBP in imatinib-resistant chronic myeloid leukemia cells. However, it remains unknown if JUP is involved in the HIF2α switch between association with p300/CBP and HDAC1/2. Various studies have indicated HIFα itself can also be acetylated by p300/CBP and deacetylated by HDACs. For example, Geng et al. [46] demonstrated HIF1α can be acetylated by p300 at Lys-709, which increases its protein stability. They also showed HIF1α can be deacetylated at Lys-709 by HDAC1. Acetylation also occurs on specific lysine residues of HIF2α [6, 7]. A critical question is whether HIF2α acetylation is regulated by the binding of JUP and HDAC1/2. Identification of the components of the JUP complex associated with each functional subset would provide valuable insight into the regulation of HIF2α signaling.
To date, many studies have investigated the function of JUP in tumorigenesis. Unlike β-catenin, which has well documented oncogenic activities, JUP typically acts as a tumor/metastasis suppressor [29-35]. Loss of total JUP gene expression is frequently observed in primary non-small cell lung cancer [29] and breast cancer [48]. However, some evidence has shown JUP is overexpressed in AML and promotes β-catenin signaling [49], suggesting JUP can also act as an oncogene in a cell-specific context. Most importantly, we analyzed the data from a TCGA ccRCC cohort and found the downregulation of JUP was associated with tumor grade and stage, distant metastasis, and poorer patient survival. Thus, JUP may act as a tumor suppressor in ccRCC development and serve as a potential prognostic marker for ccRCC patients.
Although β-catenin and HIF1α were not the major subjects of this research, the effects of JUP on β-catenin and HIF1α activity cannot be ignored. In addition, whether JUP competes with β-catenin for HIF2α binding is still unknown. The interplay between JUP and ccRCC remains complex, so additional experiments are needed in the future.
5 CONCLUSIONS
In summary, we propose a mechanism by which the tumor suppressor JUP interacts with the HIF2α transcription factor in ccRCC cells. JUP downregulation is likely to trigger the aberrant upregulation of HIF2α stability and transactivity, which further exerts effects on tumorigenesis in ccRCC. These results have important implications in both the diagnosis and treatment of RCC.
ACKNOWLEDGMENTS
We thank all the patients for their contribution in this study. We thank our group members for all the assistance they provided for this study.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study was approved by the Institutional Review Board of Huazhong University of Science and Technology, Tongji Medical College, Tongji Hospital. All human tumor tissues were obtained with written informed consent from patients prior to participation in the study.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST
No potential conflicts of interest were disclosed.
FUNDING
This work was supported by National Natural Science Foundation of China (grant number 81772721, 81874089, 81602236, 81702522), Natural Science Foundation of Jiangxi Province of China (20202BABL216060), and National Major Scientific and Technological Special Project for “Significant New Drugs Development” (2017ZX09304022). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
AUTHORS' CONTRIBUTIONS
HX and KC designed and supervised the project. KC, JZ and YS performed most experiments and data analyses, and helped writing the manuscript. WOY, GY, HZ, WMY, WX, and JHH assisted in performing animal experiments. YJZ and KFX conducted bioinformatic analysis. JCX, LW, ZQC and ZQY proofread this manuscript.




