Zn2[(S)-O3PCH2NHC4H7CO2]2: A Homochiral 3D Zinc Phosphonate with Helical Channels†
Xin Shi Dr.
State Key Laboratory of Inorganic Synthesis & Preparative Chemistry, Department of Chemistry, Jilin University, Changchun 130012, China, Fax: (+86) 431-5168589
Search for more papers by this authorGuangshan Zhu Dr.
State Key Laboratory of Inorganic Synthesis & Preparative Chemistry, Department of Chemistry, Jilin University, Changchun 130012, China, Fax: (+86) 431-5168589
Search for more papers by this authorShilun Qiu Prof.
State Key Laboratory of Inorganic Synthesis & Preparative Chemistry, Department of Chemistry, Jilin University, Changchun 130012, China, Fax: (+86) 431-5168589
Search for more papers by this authorKunlin Huang Dr.
State Key Laboratory of Inorganic Synthesis & Preparative Chemistry, Department of Chemistry, Jilin University, Changchun 130012, China, Fax: (+86) 431-5168589
Search for more papers by this authorJihong Yu Prof.
State Key Laboratory of Inorganic Synthesis & Preparative Chemistry, Department of Chemistry, Jilin University, Changchun 130012, China, Fax: (+86) 431-5168589
Search for more papers by this authorRuren Xu Prof.
State Key Laboratory of Inorganic Synthesis & Preparative Chemistry, Department of Chemistry, Jilin University, Changchun 130012, China, Fax: (+86) 431-5168589
Search for more papers by this authorXin Shi Dr.
State Key Laboratory of Inorganic Synthesis & Preparative Chemistry, Department of Chemistry, Jilin University, Changchun 130012, China, Fax: (+86) 431-5168589
Search for more papers by this authorGuangshan Zhu Dr.
State Key Laboratory of Inorganic Synthesis & Preparative Chemistry, Department of Chemistry, Jilin University, Changchun 130012, China, Fax: (+86) 431-5168589
Search for more papers by this authorShilun Qiu Prof.
State Key Laboratory of Inorganic Synthesis & Preparative Chemistry, Department of Chemistry, Jilin University, Changchun 130012, China, Fax: (+86) 431-5168589
Search for more papers by this authorKunlin Huang Dr.
State Key Laboratory of Inorganic Synthesis & Preparative Chemistry, Department of Chemistry, Jilin University, Changchun 130012, China, Fax: (+86) 431-5168589
Search for more papers by this authorJihong Yu Prof.
State Key Laboratory of Inorganic Synthesis & Preparative Chemistry, Department of Chemistry, Jilin University, Changchun 130012, China, Fax: (+86) 431-5168589
Search for more papers by this authorRuren Xu Prof.
State Key Laboratory of Inorganic Synthesis & Preparative Chemistry, Department of Chemistry, Jilin University, Changchun 130012, China, Fax: (+86) 431-5168589
Search for more papers by this authorWe are grateful for financial support from the State Basic Research Project (G2000077507) and the National Nature Science Foundation of China (Grant no. 29873017, 20101004 and 20371020). We thank Professor Guangdi Yang and Dr. Guanghua Li (Department of Chemistry, Jilin University) for their help in the analysis of the single-crystal structure.
Graphical Abstract
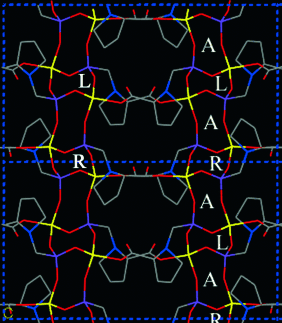
Drei Arten von Kanälen zeichnen die Struktur der Titelverbindung aus (siehe Bild): rechts- (R) und linksgängige (L) helicale sowie achirale Kanäle (A). Die katalytisch aktiven Gruppen des 1-Phosphonomethylprolin-Liganden ragen ins Innere der Kanäle, sodass das Material möglicherweise als „Heterogen-Organokatalysator“ dienen kann.
Supporting Information
Supporting information for this article is available on the WWW under http://www.wiley-vch.de/contents/jc_2001/2004/z460724_s.pdf or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aC. J. Kepert, T. J. Prior, M. J. Rosseinsky, J. Am. Chem. Soc. 2000, 122, 5158–5168;
- 1bH. Kumagai, K. Inoue, Angew. Chem. 1999, 111, 1694–1696;
10.1002/(SICI)1521-3757(19990601)111:11<1694::AID-ANGE1694>3.0.CO;2-N Google ScholarAngew. Chem. Int. Ed. 1999, 38, 1601–1603;10.1002/(SICI)1521-3773(19990601)38:11<1601::AID-ANIE1601>3.0.CO;2-# CAS Web of Science® Google Scholar
- 1cR. Andrés, M. Brissard, M. Gruselle, C. Train, J. Vaissermann, B. Malézieux, J.-P. Jamet, M. Verdaguer, Inorg. Chem. 2001, 40, 4633–4640;
- 1dT. J. Prior, M. J. Rosseinsky, Inorg. Chem. 2003, 42, 1564–1575;
- 1eG. Seeber, A. L. Pickering, D.-L. Long, L. Cronin, Chem. Commun. 2003, 2002–2003;
- 1fK. Inoue, H. Imai, P. S. Ghalsasi, K. Kikuchi, M. Ohba, H. Ōkawa, J. V. Yakhmi, Angew. Chem. 2001, 113,
4372–4375; Angew. Chem. Int. Ed. 2001, 40, 4242–4245.
10.1002/1521-3773(20011119)40:22<4242::AID-ANIE4242>3.0.CO;2-H Google Scholar
- 2aM. Minguet, D. Luneau, E. Lhotel, V. Villar, C. Paulsen, D. B. Amabilino, J. Veciana, Angew. Chem. 2002, 114, 606–609;
10.1002/1521-3757(20020215)114:4<606::AID-ANGE606>3.0.CO;2-I Google ScholarAngew. Chem. Int. Ed. 2002, 41, 586–589;
- 2bC. Qin, L. Xu, Y. Wei, X. Wang, F. Li, Inorg. Chem. 2003, 42, 3107–3110;
- 2cU. Siemeling, I. Scheppelmann, B. Neumann, A. Stammler, H.-G. Stammler, J. Frelek, Chem. Commun. 2003, 2236–2237;
- 2dM. Vázquez, A. Taglietti, D. Gatteschi, L. Sorace, C. Sangregorio, A. M. González, M. Maneiro, R. M. Pedrido, M. R. Bermejo, Chem. Commun. 2003, 1840–1841;
- 2eA. Jouaiti, M. W. Hosseini, N. Kyritsakas, Chem. Commun. 2003, 472–473;
- 2fE. Coronado, C. J. Gómez-García, A. Nuez, F. M. Romero, E. Rusanov, H. Stoeckli-Evans, Inorg. Chem. 2002, 41, 4615–4617.
- 3U. Knof, A. von Zelewsky, Angew. Chem. 1999, 111, 312–333;
10.1002/(SICI)1521-3757(19990201)111:3<312::AID-ANGE312>3.0.CO;2-D Google ScholarAngew. Chem. Int. Ed. 1999, 38, 302–322.10.1002/(SICI)1521-3773(19990201)38:3<302::AID-ANIE302>3.0.CO;2-G CAS PubMed Web of Science® Google Scholar
- 4L. Pérez-García, D. B. Amabilino, Chem. Soc. Rev. 2002, 31, 342–356.
- 5aE. Coronado, J. R. Galán-Mascarós, C. J. Gómez-García, J. M. Martínez-Agudo, Inorg. Chem. 2001, 40, 113–120;
- 5bE. Gao, S. Bai, Z. Wang, C. Yan, J. Am. Chem. Soc. 2003, 125, 4984–4985;
- 5cJ. Sun, L. Weng, Y. Zhou, J. Chen, Z. Chen, Z. Liu, D. Zhao, Angew. Chem. 2002, 114, 4651–4653;
Angew. Chem. Int. Ed. 2002, 41, 4471–4473.
10.1002/1521-3773(20021202)41:23<4471::AID-ANIE4471>3.0.CO;2-9 CAS PubMed Web of Science® Google Scholar
- 6
- 6aM. Hernández-Molina, F. Lloret, C. Ruiz-Pérez, M. Julve, Inorg. Chem. 1998, 37, 4131–4135;
- 6bS. Han, J. L. Manson, J. Kim, J. S. Miller, Inorg. Chem. 2000, 39, 4182–4185;
- 6cE. Gao, Y. Yue, S. Bai, Z. He, C. Yan, J. Am. Chem. Soc. 2004, 126, 1419–1429.
- 7
- 7aK. Inoue, K. Kikuchi, M. Ohba, H. Ōkawa, Angew. Chem. 2003, 115, 4958–4961; Angew. Chem. Int. Ed. 2003, 42, 4810–4813;
- 7bP. Grosshans, A. Jouaiti, V. Bulach, J.-M. Planeix, M. W. Hosseini, J.-F. Nicoud, Chem. Commun. 2003, 1336–1337;
- 7cY. Cui, S. J. Lee, W. Lin, J. Am. Chem. Soc. 2003, 125, 6014–6015.
- 8
- 8aJ. D. Ranford, J. J. Vittal, D. Wu, Angew. Chem. 1998, 110, 1159–1162;
10.1002/(SICI)1521-3757(19980420)110:8<1159::AID-ANGE1159>3.0.CO;2-J Google ScholarAngew. Chem. Int. Ed. 1998, 35, 1114–1116;
- 8bC. Wu, C. Lu, S. Lu, H. Zhuang, J. Huang, J. Chem. Soc. Dalton Trans. 2003, 3192–3198;
- 8cY. Cui, O. R. Evans, H. L. Ngo, P. S. White, W. Lin, Angew. Chem. 2002, 114, 1207–1210;
Angew. Chem. Int. Ed. 2002, 41, 1159–1160.
10.1002/1521-3773(20020402)41:7<1159::AID-ANIE1159>3.0.CO;2-5 CAS PubMed Web of Science® Google Scholar
- 9J. S. Seo, D. Whang, H. Lee, S. I. Jun, J. Oh, Y. J. Jeon, K. Kim, Nature 2000, 404, 982–986.
- 10R. Xiong, X. You, B. F. Abrahams, Z. Xue, C. Che, Angew. Chem. 2001, 113, 4554–4557;
Angew. Chem. Int. Ed. 2001, 40, 4422–4425.
10.1002/1521-3773(20011203)40:23<4422::AID-ANIE4422>3.0.CO;2-G CAS PubMed Web of Science® Google Scholar
- 11O. R. Evans, H. L. Ngo, W. Lin, J. Am. Chem. Soc. 2001, 123, 10 395–10 396.
- 12A. Bøgevig, K. Juhl, N. Kumaragurubaran, W. Zhuang, K. A. Jørgensen, Angew. Chem. 2002, 114, 1868–1871;
10.1002/1521-3757(20020517)114:10<1868::AID-ANGE1868>3.0.CO;2-P Google ScholarAngew. Chem. Int. Ed. 2002, 41, 1790–1793.10.1002/1521-3773(20020517)41:10<1790::AID-ANIE1790>3.0.CO;2-Y CAS PubMed Web of Science® Google Scholar
- 13A. G. M. Barrett, A. S. Cook, A. Kamimura, Chem. Commun. 1998, 2533–2534.
- 14Reviews: R. O. Duthaler, Angew. Chem. 2003, 115, 1005–1008;
10.1002/ange.200390228 Google ScholarAngew. Chem. Int. Ed. 2003, 42, 975–978; P. Dalko, L. Moisan, Angew. Chem. 2004, 116, 5248–5286;10.1002/ange.200400650 Google ScholarAngew. Chem. Int. Ed. 2004, 43, 5138–5175.
- 15
- 15aO. R. Evans, D. R. Manke, W. Lin, Chem. Mater. 2002, 14, 3866–3874;
- 15bH. L. Ngo, W. Lin, J. Am. Chem. Soc. 2002, 124, 14 298–14 299.
- 16
- 16aF. Fredoueil, M. Evain, M. Bujoli-Doeuff, B. Bujoli, Eur. J. Inorg. Chem. 1999, 1077–1079;
10.1002/(SICI)1099-0682(199907)1999:7<1077::AID-EJIC1077>3.0.CO;2-5 CAS Web of Science® Google Scholar
- 16bF. Fredoueil, M. Evain, D. Massiot, M. Bujoli-Doeuff, B. Bujoli, J. Mater. Chem. 2001, 11, 1106–1110;
- 16cA. Turner, P.-A. Jaffrès, E. J. MacLean, D. Villemin, V. Mckee, G. B. Hix, J. Chem. Soc. Dalton Trans. 2003, 1314–1319;
- 16dB. Yang, J. Mao, Y. Sun, H. Zhao, A. Clearfield, Eur. J. Inorg. Chem. 2003, 4211–4217.
- 17J. Liang, Y. Wang, J. Yu, Y. Li, R. Xu, Chem. Commun. 2003, 882–883.
- 18G. M. Sheldrick, SHELXS-97, Program for Crystal Structure Solution, Göttingen University, Germany, 1997.
- 19G. M. Sheldrick, SHELXL-97, Program for Crystal Structure Refinement, Göttingen University, Germany, 1997.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.