Increased Diagnoses of Follicular Neoplasms Among Thyroid Nodules Submitted to Fine-Needle Aspiration With Ultrasound-Classification Indication and Adoption of the 3rd Edition of the Bethesda System
Funding: The authors received no specific funding for this work.
ABSTRACT
Objectives
The increasing percentage of follicular neoplasms among thyroid nodules submitted to fine-needle aspiration (FNA) cytology raises concerns regarding diagnostic uncertainty and clinical management. This study aims to investigate the factors contributing to this phenomenon, including the influence of the implementation of ultrasound (US) risk stratification systems and of the third edition of the Bethesda system for reporting thyroid cytopathology.
Methods
A retrospective cohort of 1255 patients (1470 nodules) diagnosed during the first 9 months of 2023, in a single institution, was analysed.
Results
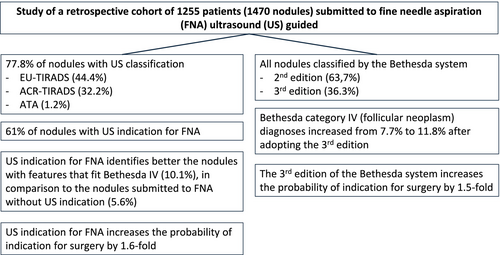
US classifications are used in 77.8% of the reported nodules, predominantly EU-TIRADS (44.4%) and ACR-TIRADS (32.2%). Bethesda category IV (follicular neoplasm) diagnoses increased from 7.7% to 11.8% after adopting the third edition, although without statistical significance. Notably, 39% of nodules were submitted to FNA without US indication, emphasising the need for improved adherence to guidelines.
Conclusions
The study highlighted the significant increase in follicular neoplasms among nodules with US indication for FNA. The transition from the 2nd to the 3rd edition of the Bethesda system increases the probability of indication for surgery by 1.5-fold, while the US indication for FNA increases the probability of indication for surgery by 1.6-fold, and demonstrated that the nodules with surgical indications (Bethesda categories IV, V, VI) increased from 5.3% in 2021 to 11.4% in 2023. Despite limitations, this work underscores the importance of aligning FNA practices with evidence-based recommendations to optimise patient care and minimise overdiagnosis.
Graphical Abstract
The study highlights the increase in follicular neoplasms among nodules with ultrasound (US) indication for fine needle aspiration due to the adoption of the 3rd edition of the Bethesda system (increases indication for surgery by 1.5-fold) and US classifications (increasing the indication for surgery by 1.6-fold).
Conflicts of Interest
C.E. consulted in the last 2 years for MSD, Diapath, Diaceutics, Epredia and Biocartis. A.P. consulted in the last 2 years to Indica Labs. The other authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.