Diagnosing Malignant Epithelial Neoplasms of the Lung in Cytological Specimens: Cytomorphology, Ancillary Studies and Management
Magdalena M. Brune
Institute of Medical Genetics and Pathology, University Hospital Basel, Basel, Switzerland
Search for more papers by this authorZubair Baloch
Department of Pathology, Hospital of the University of Pennsylvania, Philadelphia, Pennsylvania, USA
Search for more papers by this authorLukas Bubendorf
Institute of Medical Genetics and Pathology, University Hospital Basel, Basel, Switzerland
Search for more papers by this authorCorresponding Author
Spasenija Savic Prince
Institute of Medical Genetics and Pathology, University Hospital Basel, Basel, Switzerland
Correspondence:
Spasenija Savic Prince ([email protected])
Search for more papers by this authorMagdalena M. Brune
Institute of Medical Genetics and Pathology, University Hospital Basel, Basel, Switzerland
Search for more papers by this authorZubair Baloch
Department of Pathology, Hospital of the University of Pennsylvania, Philadelphia, Pennsylvania, USA
Search for more papers by this authorLukas Bubendorf
Institute of Medical Genetics and Pathology, University Hospital Basel, Basel, Switzerland
Search for more papers by this authorCorresponding Author
Spasenija Savic Prince
Institute of Medical Genetics and Pathology, University Hospital Basel, Basel, Switzerland
Correspondence:
Spasenija Savic Prince ([email protected])
Search for more papers by this authorFunding: The authors received no specific funding for this work.
ABSTRACT
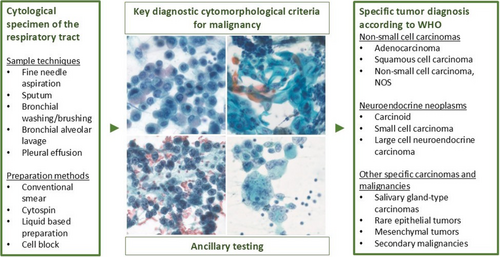
The World Health Organization's (WHO) Reporting System for Lung Cytopathology intends to standardise the diagnosing and reporting of cytology specimens from the lung and aims at enhancing the communication between clinicians and (cyto)pathologists. It is closely connected to the 5th edition of the WHO Classification of Thoracic Tumours. The system includes five diagnostic categories, among them the ‘Malignant’ diagnostic category that incorporates both primary malignant tumours and metastases. Advancements in bronchoscopy have notably improved the diagnostic capacity of cytological specimens that represent the sole source of tumour material in approximately 40% of all lung carcinoma cases. An accurate diagnosis of malignancy and treatment-guiding classification into specific tumour types and subtypes can reliably be achieved with cytology specimens. They additionally serve as an excellent source for predictive immunocytochemistry (ICC) and molecular testing for targetable oncogenic alterations. This review article provides an overview of the key cytopathological features defining the ‘Malignant’ category of the WHO Reporting System for Lung Cytopathology for non-small cell carcinomas, neuroendocrine neoplasms and other specific carcinomas and malignancies which can be encountered in cytological specimens of the lung. It further describes the application of ancillary techniques, such as ICC and molecular testing, that have been successfully incorporated into different cytological sample types using various preparation methods.
Graphical Abstract
This review provides an overview of the key cytopathological features defining the ‘Malignant’ category of the World Health Organization's Reporting System for Lung Cytopathology and discusses the application of ancillary techniques in cytological specimens from the lung, including immunocytochemistry and molecular testing.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analysed in this study.
Supporting Information
| Filename | Description |
|---|---|
| cyt13511-sup-0001-FigureS1.jpgJPEG image, 1.1 MB |
Figure S1. Air-dried specimens stained with various Romanowsky type stainings (May-Grünwald-Giemsa: A, B; Diff-Quik: C, D). (A, B) Two examples of lung adenocarcinoma (LUAD) with enlarged, variably sized nuclei, prominent nucleoli and vacuolar cytoplasmic changes (A: bronchoalveolar lavage, ×1000 and B: pleural effusion, ×630), (C) LUAD with enlarged nuclei and columnar, delicate cytoplasm (endobronchial ultrasound-guided transbronchial needle aspiration [EBUS-TBNA] of the lung, ×630), (D) Lung squamous cell carcinoma with dark nuclei and dense, keratinised cytoplasm (EBUS-TBNA, ×630). |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1J. L. Sauter and WHO Classification of Tumours Editorial Board, Thoracic Tumours, WHO classification of tumours series, 5th ed. (International Agency for Research on Cancer, 2021).
- 2A. Friedlaender, M. Perol, G. L. Banna, K. Parikh, and A. Addeo, “Oncogenic Alterations in Advanced NSCLC: A Molecular Super-Highway,” Biomarker Research 12, no. 1 (2024): 24.
- 3W. D. Travis, E. Brambilla, M. Noguchi, et al., “International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society International Multidisciplinary Classification of Lung Adenocarcinoma,” Journal of Thoracic Oncology 6, no. 2 (2011): 244–285.
- 4S. E. P. Kops, P. Heus, D. A. Korevaar, et al., “Diagnostic Yield and Safety of Navigation Bronchoscopy: A Systematic Review and Meta-Analysis,” Lung Cancer 180 (2023): 107196, https://doi.org/10.1016/j.lungcan.2023.107196.
- 5I. C. Ifeanyi, J. S. Heir, and O. Idowu, “Robotic Bronchoscopy: Evolution of Advanced Diagnostic Technologies for Pulmonary Lesions,” Best Practice & Research. Clinical Anaesthesiology 38, no. 1 (2024): 38–46.
- 6A. C. Chen, N. J. Pastis, A. K. Mahajan, et al., “Robotic Bronchoscopy for Peripheral Pulmonary Lesions: A Multicenter Pilot and Feasibility Study (BENEFIT),” Chest 159, no. 2 (2021): 845–852.
- 7O. Kalchiem-Dekel, J. G. Connolly, I. H. Lin, et al., “Shape-Sensing Robotic-Assisted Bronchoscopy in the Diagnosis of Pulmonary Parenchymal Lesions,” Chest 161, no. 2 (2022): 572–582.
- 8E. T. Sumner, J. Chang, P. R. Patel, H. Bedi, and B. D. Shaller, “State of the Art: Peripheral Diagnostic Bronchoscopy,” Journal of Thoracic Disease 16, no. 8 (2024): 5409–5421.
- 9M. Ueoka, R. Ronaghi, S. Khauli, and C. L. Channick, “Cryoprobe Biopsy Versus Mechanical Biopsies in Pulmonary Diagnostics,” Current Opinion in Pulmonary Medicine 31, no. 1 (2025): 19–27.
- 10S. Savic, C. Tapia, B. Grilli, et al., “Comprehensive Epidermal Growth Factor Receptor Gene Analysis From Cytological Specimens of Non-Small-Cell Lung Cancers,” British Journal of Cancer 98, no. 1 (2008): 154–160.
- 11P. N. Aguiar, R. A. De Mello, P. Hall, H. Tadokoro, and G. D. Lima Lopes, “PD-L1 Expression as a Predictive Biomarker in Advanced Non-Small-Cell Lung Cancer: Updated Survival Data,” Immunotherapy 9, no. 6 (2017): 499–506.
- 12D. König, S. Savic Prince, and S. I. Rothschild, “Targeted Therapy in Advanced and Metastatic Non-Small Cell Lung Cancer. An Update on Treatment of the Most Important Actionable Oncogenic Driver Alterations,” Cancers 13, no. 4 (2021): 804.
- 13E. D. Rossi, A. Wiles, and A. Vecchione, “Lung Cancer and Molecular Testing in Small Biopsies Versus Cytology: The Logics of Worlds,” Cancer Cytopathology 128, no. 9 (2020): 637–641.
- 14D. Jain and S. Roy-Chowdhuri, “Molecular Pathology of Lung Cancer Cytology Specimens: A Concise Review,” Archives of Pathology & Laboratory Medicine 142, no. 9 (2018): 1127–1133.
- 15C. Bellevicine, U. Malapelle, E. Vigliar, P. Pisapia, G. Vita, and G. Troncone, “How to Prepare Cytological Samples for Molecular Testing,” Journal of Clinical Pathology 70, no. 10 (2017): 819–826.
- 16D. Frankel, I. Nanni, L. Ouafik, et al., “Cytological Samples: An Asset for the Diagnosis and Therapeutic Management of Patients With Lung Cancer,” Cells 12, no. 5 (2023): 754.
- 17 International Academy of Cytology – International Agency for Research on Cancer – World Health Organization Joint Editorial Board, WHO Reporting System for Lung Cytopathology. IAC-IARC-WHO Cytopathology Reporting Systems Series, vol. 1, 1st ed. (International Agency for Research on Cancer, 2022), https://publications.iarc.fr/620.
- 18D. Dolezal, I. Kholová, and G. Cai, “The World Health Organization Reporting System for Lung Cytopathology—A Review of the First Edition,” Journal of Clinical and Translational Pathology 4, no. 1 (2024): 18–35.
- 19F. C. Schmitt, L. Bubendorf, S. Canberk, et al., “The World Health Organization Reporting System for Lung Cytopathology,” Acta Cytologica 67, no. 1 (2023): 80–91.
- 20W. Bulman, A. Saqi, and C. A. Powell, “Acquisition and Processing of Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration Specimens in the Era of Targeted Lung Cancer Chemotherapy,” American Journal of Respiratory and Critical Care Medicine 185, no. 6 (2012): 606–611.
- 21S. R. Turner, D. Buonocore, P. Desmeules, et al., “Feasibility of Endobronchial Ultrasound Transbronchial Needle Aspiration for Massively Parallel Next-Generation Sequencing in Thoracic Cancer Patients,” Lung Cancer 119 (2018): 85–90, https://doi.org/10.1016/j.lungcan.2018.03.003.
- 22P. Pisapia, U. Malapelle, G. Roma, et al., “Consistency and Reproducibility of Next-Generation Sequencing in Cytopathology: A Second Worldwide Ring Trial Study on Improved Cytological Molecular Reference Specimens,” Cancer Cytopathology 127, no. 5 (2019): 285–296.
- 23S. Roy-Chowdhuri, P. Pisapia, M. Salto-Tellez, et al., “Invited Review-Next-Generation Sequencing: A Modern Tool in Cytopathology,” Virchows Archiv 475, no. 1 (2019): 3–11, https://doi.org/10.1007/s00428-019-02559-z.
- 24L. Bubendorf, S. Lantuejoul, A. J. de Langen, and E. Thunnissen, “Nonsmall Cell Lung Carcinoma: Diagnostic Difficulties in Small Biopsies and Cytological Specimens: Number 2 in the Series ‘Pathology for the Clinician’ Edited by Peter Dorfmüller and Alberto Cavazza,” European Respiratory Review 26, no. 144 (2017): 170007.
- 25S. Canberk, D. Montezuma, O. Aydın, et al., “The New Guidelines of Papanicolaou Society of Cytopathology for Respiratory Specimens: Assessment of Risk of Malignancy and Diagnostic Yield in Different Cytological Modalities,” Diagnostic Cytopathology 46, no. 9 (2018): 725–729.
- 26K. L. Murray, E. Duvall, D. M. Salter, and H. Monaghan, “Efficacy and Pattern of Use of Sputum Cytology as a Diagnostic Test,” Cytopathology 13, no. 6 (2002): 350–354.
- 27L. J. Layfield and M. Esebua, “A Modified Papanicolaou Society of Cytopathology System for Reporting Respiratory Cytology Specimens: Implications for Estimates of Malignancy Risk and Diagnostic Accuracy,” Diagnostic Cytopathology 49, no. 11 (2021): 1167–1172.
- 28L. Righi, P. Graziano, A. Fornari, et al., “Immunohistochemical Subtyping of Nonsmall Cell Lung Cancer Not Otherwise Specified in Fine-Needle Aspiration Cytology: A Retrospective Study of 103 Cases With Surgical Correlation,” Cancer 117, no. 15 (2011): 3416–3423.
- 29L. J. Layfield, L. Pearson, B. S. Walker, S. K. White, and R. L. Schmidt, “Diagnostic Accuracy of Fine-Needle Aspiration Cytology for Discrimination of Squamous Cell Carcinoma From Adenocarcinoma in Non-Small Cell Lung Cancer: A Systematic Review and Meta-Analysis,” Acta Cytologica 62, no. 5–6 (2018): 318–326.
- 30D. Jain, A. Nambirajan, G. Chen, et al., “NSCLC Subtyping in Conventional Cytology: Results of the International Association for the Study of Lung Cancer Cytology Working Group Survey to Determine Specific Cytomorphologic Criteria for Adenocarcinoma and Squamous Cell Carcinoma,” Journal of Thoracic Oncology 17, no. 6 (2022): 793–805, https://doi.org/10.1016/j.jtho.2022.02.013.
- 31M. Nishino, M. P. Hoang, P. Della Pelle, et al., “Napsin A/p40 Antibody Cocktail for Subtyping Non-Small Cell Lung Carcinoma on Cytology and Small Biopsy Specimens,” Cancer Cytopathology 124, no. 7 (2016): 472–484.
- 32S. Sathiyamoorthy and Z. Maleki, “Cytomorphologic Overlap of Differentiated Thyroid Carcinoma and Lung Adenocarcinoma and Diagnostic Value of TTF-1 and TGB on Cytologic Material,” Diagnostic Cytopathology 42, no. 1 (2014): 5–10.
- 33D. Jain, A. Nambirajan, A. Borczuk, et al., “Immunocytochemistry for Predictive Biomarker Testing in Lung Cancer Cytology,” Cancer Cytopathology 127, no. 5 (2019): 325–339.
- 34C. Bontoux, V. Hofman, M. Abboute, et al., “c-Met Immunohistochemistry as Reflex Test at Diagnosis for Non-Small Cell Lung Cancer: A Real-World Experience From a Monocentric Case Series,” Journal of Clinical Pathology 78, no. 1 (2024): 35–41.
- 35N. I. Lindeman, P. T. Cagle, D. L. Aisner, et al., “Updated Molecular Testing Guideline for the Selection of Lung Cancer Patients for Treatment With Targeted Tyrosine Kinase Inhibitors: Guideline From the College of American Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology,” Archives of Pathology & Laboratory Medicine 142, no. 3 (2018): 321–346.
- 36J. A. Bishop, T. Ogawa, X. Chang, et al., “HPV Analysis in Distinguishing Second Primary Tumors From Lung Metastases in Patients With Head and Neck Squamous Cell Carcinoma,” American Journal of Surgical Pathology 36, no. 1 (2012): 142–148.
- 37L. Paz-Ares, A. Luft, D. Vicente, et al., “Pembrolizumab Plus Chemotherapy for Squamous Non-Small-Cell Lung Cancer,” New England Journal of Medicine 379, no. 21 (2018): 2040–2051.
- 38R. S. Herbst, G. Giaccone, F. de Marinis, et al., “Atezolizumab for First-Line Treatment of PD-L1-Selected Patients With NSCLC,” New England Journal of Medicine 383, no. 14 (2020): 1328–1339.
- 39A. Gagné, E. Wang, N. Bastien, et al., “Impact of Specimen Characteristics on PD-L1 Testing in Non-Small Cell Lung Cancer: Validation of the IASLC PD-L1 Testing Recommendations,” Journal of Thoracic Oncology 14, no. 12 (2019): 2062–2070, https://doi.org/10.1016/j.jtho.2019.08.2503.
- 40B. G. Skov and T. Skov, “Paired Comparison of PD-L1 Expression on Cytologic and Histologic Specimens From Malignancies in the Lung Assessed With PD-L1 IHC 28-8pharmDx and PD-L1 IHC 22C3pharmDx,” Applied Immunohistochemistry & Molecular Morphology 25, no. 7 (2017): 453–459, https://doi.org/10.1097/PAI.0000000000000540.
- 41M. D. Lozano, J. I. Echeveste, M. Abengozar, et al., “Cytology Smears in the Era of Molecular Biomarkers in Non-Small Cell Lung Cancer: Doing More With Less,” Archives of Pathology & Laboratory Medicine 142, no. 3 (2018): 291–298.
- 42B. Noll, W. L. Wang, Y. Gong, et al., “Programmed Death Ligand 1 Testing in Non-Small Cell Lung Carcinoma Cytology Cell Block and Aspirate Smear Preparations,” Cancer Cytopathology 126, no. 5 (2018): 342–352.
- 43G. Centonze, P. Maisonneuve, M. Simbolo, et al., “Lung Carcinoid Tumours: Histology and Ki-67, the Eternal Rivalry,” Histopathology 82, no. 2 (2023): 324–339.
- 44L. M. Rooper, R. Sharma, Q. K. Li, P. B. Illei, and W. H. Westra, “INSM1 Demonstrates Superior Performance to the Individual and Combined Use of Synaptophysin, Chromogranin and CD56 for Diagnosing Neuroendocrine Tumors of the Thoracic Cavity,” American Journal of Surgical Pathology 41, no. 11 (2017): 1561–1569.
- 45K. Viswanathan, M. T. Siddiqui, and A. C. Borczuk, “Insulinoma-Associated Protein 1 Is a Sensitive and Specific Marker for Lung Neuroendocrine Tumors in Cytologic and Surgical Specimens,” Journal of the American Society of Cytopathology 8, no. 6 (2019): 299–308.
- 46C. M. Gay, C. A. Stewart, E. M. Park, et al., “Patterns of Transcription Factor Programs and Immune Pathway Activation Define Four Major Subtypes of SCLC With Distinct Therapeutic Vulnerabilities,” Cancer Cell 39, no. 3 (2021): 346–360.e7.
- 47Y. Yatabe, S. Dacic, A. C. Borczuk, et al., “Best Practices Recommendations for Diagnostic Immunohistochemistry in Lung Cancer,” Journal of Thoracic Oncology 14, no. 3 (2019): 377–407, https://doi.org/10.1016/j.jtho.2018.12.005.
- 48L. M. Stoll, M. W. Johnson, F. Burroughs, and Q. K. Li, “Cytologic Diagnosis and Differential Diagnosis of Lung Carcinoid Tumors a Retrospective Study of 63 Cases With Histologic Correlation,” Cancer Cytopathology 118, no. 6 (2010): 457–467.
- 49F. Le Loarer, S. Watson, G. Pierron, et al., “SMARCA4 Inactivation Defines a Group of Undifferentiated Thoracic Malignancies Transcriptionally Related to BAF-Deficient Sarcomas,” Nature Genetics 47, no. 10 (2015): 1200–1205.
- 50D. N. Reisman, J. Sciarrotta, W. Wang, W. K. Funkhouser, and B. E. Weissman, “Loss of BRG1/BRM in Human Lung Cancer Cell Lines and Primary Lung Cancers: Correlation With Poor Prognosis,” Cancer Research 63, no. 3 (2003): 560–566.
- 51F. Le Loarer, D. Pissaloux, S. Watson, et al., “Clinicopathologic Features of CIC-NUTM1 Sarcomas, a New Molecular Variant of the Family of CIC-Fused Sarcomas,” American Journal of Surgical Pathology 43, no. 2 (2019): 268–276.
- 52B. C. Dickson, Y. S. Sung, M. K. Rosenblum, et al., “NUTM1 Gene Fusions Characterize a Subset of Undifferentiated Soft Tissue and Visceral Tumors,” American Journal of Surgical Pathology 42, no. 5 (2018): 636–645.
- 53S. Lantuejoul, D. Pissaloux, G. R. Ferretti, and A. McLeer, “NUT Carcinoma of the Lung,” Seminars in Diagnostic Pathology 38, no. 5 (2021): 72–82.
- 54F. Pezzuto, F. Fortarezza, M. Mammana, et al., “Immunohistochemical Neuroendocrine Marker Expression in Primary Pulmonary NUT Carcinoma: A Diagnostic Pitfall,” Histopathology 77, no. 3 (2020): 508–510.
- 55Y. P. Hung, A. L. Chen, M. S. Taylor, et al., “Thoracic Nuclear Protein in Testis (NUT) Carcinoma: Expanded Pathological Spectrum With Expression of Thyroid Transcription Factor-1 and Neuroendocrine Markers,” Histopathology 78, no. 6 (2021): 896–904.
- 56Y. Zhang, X. Liu, Y. Gu, and S. Zhang, “Clinical, Laboratory, Pathological, and Radiological Characteristics and Prognosis of Patients With Pulmonary Salivary Gland-Type Tumors,” Journal of Cancer Research and Clinical Oncology 149, no. 7 (2023): 4025–4039.
- 57N. Falk, A. Weissferdt, N. Kalhor, and C. A. Moran, “Primary Pulmonary Salivary Gland-Type Tumors: A Review and Update,” Advances in Anatomic Pathology 23, no. 1 (2016): 13–23.
Online Version of Record before inclusion in an issue