Quantitative phase microscopy monitors subcellular dynamics in single cells exposed to nanosecond pulsed electric fields
Corresponding Author
Zachary A. Steelman
National Research Council Research Associateship Program, Washington, District of Columbia, USA
Correspondence
Zachary A. Steelman, Air Force Research Laboratory, San Antonio, TX, USA.
Email: [email protected]
Search for more papers by this authorZachary N. Coker
Department of Physics and Astronomy, Texas A&M University, College Station, Texas, USA
SAIC, San Antonio, Texas, USA
Search for more papers by this authorAllen Kiester
711th Human Performance Wing, Airman Systems Directorate, Bioeffects Division, JBSA Fort Sam Houston, San Antonio, Texas, USA
Search for more papers by this authorBennett L. Ibey
711th Human Performance Wing, Airman Systems Directorate, Bioeffects Division, JBSA Fort Sam Houston, San Antonio, Texas, USA
Search for more papers by this authorJoel N. Bixler
711th Human Performance Wing, Airman Systems Directorate, Bioeffects Division, JBSA Fort Sam Houston, San Antonio, Texas, USA
Search for more papers by this authorCorresponding Author
Zachary A. Steelman
National Research Council Research Associateship Program, Washington, District of Columbia, USA
Correspondence
Zachary A. Steelman, Air Force Research Laboratory, San Antonio, TX, USA.
Email: [email protected]
Search for more papers by this authorZachary N. Coker
Department of Physics and Astronomy, Texas A&M University, College Station, Texas, USA
SAIC, San Antonio, Texas, USA
Search for more papers by this authorAllen Kiester
711th Human Performance Wing, Airman Systems Directorate, Bioeffects Division, JBSA Fort Sam Houston, San Antonio, Texas, USA
Search for more papers by this authorBennett L. Ibey
711th Human Performance Wing, Airman Systems Directorate, Bioeffects Division, JBSA Fort Sam Houston, San Antonio, Texas, USA
Search for more papers by this authorJoel N. Bixler
711th Human Performance Wing, Airman Systems Directorate, Bioeffects Division, JBSA Fort Sam Houston, San Antonio, Texas, USA
Search for more papers by this authorFunding information: Air Force Office of Scientific Research, Grant/Award Numbers: 17RHCOR483, 20RHCOR051; Air Force Research Laboratory, Grant/Award Number: FA8650-C-6024; Oak Ridge Institute for Science and Education, Grant/Award Number: DE-SC0014664
Abstract
A substantial body of literature exists to study the dynamics of single cells exposed to short duration (<1 μs), high peak power (~1 MV/m) transient electric fields. Much of this research is limited to traditional fluorescence-based microscopy techniques, which introduce exogenous agents to the culture and are only sensitive to a single molecular target. Quantitative phase imaging (QPI) is a coherent imaging modality which uses optical path length as a label-free contrast mechanism, and has proven highly effective for the study of single-cell dynamics. In this work, we introduce QPI as a useful imaging tool for the study of cells undergoing cytoskeletal remodeling after nanosecond pulsed electric field (nsPEF) exposure. In particular, we use cell swelling, dry mass and disorder strength measurements derived from QPI phase images to monitor the cellular response to nsPEFs. We hope this demonstration of QPI's utility will lead to a further adoption of the technique for the study of directed energy bioeffects.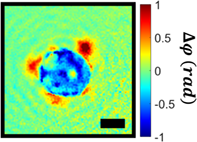
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Supporting Information
| Filename | Description |
|---|---|
| jbio202100125-sup-0001-Supinfo.docxWord 2007 document , 388.7 KB | Appendix S1: Supplementary Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
REFERENCES
- 1K. H. Schoenbach, S. J. Hargrave, R. P. Joshi, J. F. Kolb, R. Nuccitelli, C. Osgood, A. Pakhomov, M. Stacey, R. J. Swanson, J. A. White, IEEE Trans. Dielectr. Electr. Insul. 2007, 14(5), 1088.
- 2A. G. Pakhomov, R. Shevin, J. A. White, J. F. Kolb, O. N. Pakhomova, R. P. Joshi, K. H. Schoenbach, Arch. Biochem. Biophys. 2007, 465(1), 109.
- 3M. Stacey, P. Fox, S. Buescher, J. Kolb, Bioelectrochemistry 2011, 82(2), 131.
- 4A. G. Pakhomov, S. Xiao, O. N. Pakhomova, I. Semenov, M. A. Kuipers, B. L. Ibey, Bioelectrochemistry 2014, 100, 88.
- 5L. Carr, S. M. Bardet, R. C. Burke, D. Arnaud-Cormos, P. Leveque, R. P. O'Connor, Sci. Rep. 2017, 7(1), 1.
- 6G. P. Tolstykh, G. L. Thompson, H. T. Beier, Z. A. Steelman, B. L. Ibey, Biochem. Biophys. Rep. 2017, 9, 36.
- 7E. B. Sözer, Y.-H. Wu, S. Romeo, P. T. Vernier, J. Membr. Biol. 2017, 250(1), 21.
- 8O. M. Nesin, O. N. Pakhomova, S. Xiao, A. G. Pakhomov, Biochim. Biophys. Acta 2011, 1808(3), 792.
- 9S. Romeo, Y.-H. Wu, Z. A. Levine, M. A. Gundersen, P. T. Vernier, Biochim. Biophys. Acta 2013, 1828(8), 1715.
- 10S. J. Beebe, N. M. Sain, W. Ren, Cell 2013, 2(1), 136.
- 11W. Ren, S. J. Beebe, Apoptosis 2011, 16(4), 382.
- 12P. Zhou, F. He, Y. Han, B. Liu, S. Wei, Bioelectrochemistry 2018, 124, 7.
- 13P. T. Vernier, Y. Sun, L. Marcu, S. Salemi, C. M. Craft, M. A. Gundersen, Biochem. Biophys. Res. Commun. 2003, 310(2), 286.
- 14A. G. Pakhomov, A. M. Bowman, B. L. Ibey, F. M. Andre, O. N. Pakhomova, K. H. Schoenbach, Biochem. Biophys. Res. Commun. 2009, 385(2), 181.
- 15A. G. Pakhomov, J. F. Kolb, J. A. White, R. P. Joshi, S. Xiao, K. H. Schoenbach, Bioelectromagnetics 2007, 28(8), 655.
- 16S. Yin, X. Chen, C. Hu, X. Zhang, Z. Hu, J. Yu, X. Feng, K. Jiang, S. Ye, K. Shen, Cancer Lett. 2014, 346(2), 285.
- 17S. J. Beebe, X. Chen, J. A. Liu, K. H. Schoenbach. 2011 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, IEEE, 2011, p. 6861.
10.1109/IEMBS.2011.6091692 Google Scholar
- 18R. Chen, X. Chen, S. J. Beebe, Surgery 2013, 12, 1.
- 19G. S. Munavalli, B. D. Zelickson, M. M. Selim, S. L. Kilmer, T. E. Rohrer, J. Newman, L. Jauregui, W. A. Knape, E. Ebbers, D. Uecker, Dermatol. Surg. 2020, 46(6), 803.
- 20M. Casciola, S. Xiao, A. G. Pakhomov, Sci. Rep. 2017, 7(1), 1.
- 21J. E. Azarov, I. Semenov, M. Casciola, A. G. Pakhomov, J. Cardiovasc. Electrophysiol. 2019, 30(3), 392.
- 22X. Chen, J. F. Kolb, R. J. Swanson, K. H. Schoenbach, S. J. Beebe, Pigment Cell Melanoma Res. 2010, 23(4), 554.
- 23A. Rossi, O. N. Pakhomova, P. A. Mollica, M. Casciola, U. Mangalanathan, A. G. Pakhomov, C. Muratori, Cancers (Basel) 2019, 11(12), 2034.
- 24E. C. Gianulis, M. Casciola, C. Zhou, E. Yang, S. Xiao, A. G. Pakhomov, Sci. Rep. 2019, 9(1), 1.
- 25E. B. Sözer, S. Haldar, P. S. Blank, F. Castellani, P. T. Vernier, J. Zimmerberg, Biophys. J. 2020, 119(9), 1724.
- 26O. N. Pakhomova, V. A. Khorokhorina, A. M. Bowman, R. Rodaitė-Riševičienė, G. Saulis, S. Xiao, A. G. Pakhomov, Arch. Biochem. Biophys. 2012, 527(1), 55.
- 27V. Nesin, A. G. Pakhomov, Bioelectromagnetics 2012, 33(6), 443.
- 28G. P. Tolstykh, H. T. Beier, C. C. Roth, G. L. Thompson, J. A. Payne, M. A. Kuipers, B. L. Ibey, Bioelectrochemistry 2013, 94, 23.
- 29R. Nuccitelli, K. Lui, M. Kreis, B. Athos, P. Nuccitelli, Biochem. Biophys. Res. Commun. 2013, 435(4), 580.
- 30O. N. Pakhomova, B. Gregory, I. Semenov, A. G. Pakhomov, Biochim. Biophys. Acta 2014, 1838(10), 2547.
- 31A. G. Pakhomov, E. Gianulis, P. T. Vernier, I. Semenov, S. Xiao, O. N. Pakhomova, Biochim. Biophys. Acta 2015, 1848(4), 958.
- 32E. B. Sözer, Z. A. Levine, P. T. Vernier, Sci. Rep. 2017, 7(1), 1.
- 33E. C. Gianulis, C. Labib, G. Saulis, V. Novickij, O. N. Pakhomova, A. G. Pakhomov, Cell. Mol. Life Sci. 2017, 74(9), 1741.
- 34M. R. Arnison, K. G. Larkin, C. J. Sheppard, N. I. Smith, C. J. Cogswell, J. Microsc. 2004, 214(1), 7.
- 35Y. Park, C. Depeursinge, G. Popescu, Nat. Photonics 2018, 12(10), 578.
- 36M. Mir, B. Bhaduri, R. Wang, R. Zhu, G. Popescu, Prog. Opt. 2012, 57(133–37), 217.
- 37G. Popescu, Quantitative Phase Imaging of Cells and Tissues, McGraw-Hill Education, New York, NY 2011.
- 38H. S. Park, M. T. Rinehart, K. A. Walzer, J.-T. A. Chi, A. Wax, PLoS One 2016, 11(9), e0163045.
- 39W. J. Eldridge, Z. A. Steelman, B. Loomis, A. Wax, Biophys. J. 2017, 112(4), 692.
- 40G. L. Thompson, C. Roth, G. Tolstykh, M. Kuipers, B. L. Ibey, Bioelectromagnetics 2014, 35(4), 262.
- 41G. L. Thompson, C. C. Roth, M. A. Kuipers, G. P. Tolstykh, H. T. Beier, B. L. Ibey, Biochem. Biophys. Res. Commun. 2016, 470(1), 35.
- 42W. J. Eldridge, A. Sheinfeld, M. T. Rinehart, A. Wax, Opt. Lett. 2016, 41(2), 352. https://doi.org/10.1364/OL.41.000352.
- 43N. T. Shaked, Y. Zhu, N. Badie, N. Bursac, A. P. Wax, J. Biomed. Opt. 2010, 15(3), 030503.
- 44G. P. Tolstykh, H. T. Beier, C. C. Roth, G. L. Thompson, B. L. Ibey, Bioelectrochemistry 2014, 100, 80.
- 45Z. A. Steelman, G. P. Tolstykh, H. T. Beier, B. L. Ibey, Biochem. Biophys. Res. Commun. 2016, 478(3), 1261.
- 46I. Semenov, S. Xiao, A. G. Pakhomov, Biochim. Biophys. Acta 2013, 1828(3), 981.
- 47B. L. Ibey, C. C. Roth, A. G. Pakhomov, J. A. Bernhard, G. J. Wilmink, O. N. Pakhomova, PLoS One 2011, 6(1), e15642.
- 48M. Liebling, T. Blu, M. Unser, J. Opt. Soc. Am. A 2004, 21(3), 367.
- 49J. W. Cooley, J. W. Tukey, Math. Comput. 1965, 19(90), 297.
- 50Z. Zhao, H. Zhang, Z. Xiao, H. Du, Y. Zhuang, C. Fan, H. Zhao, Meas. Sci. Technol. 2018, 30(1), 015201.
- 51W. J. Eldridge, J. Hoballah, A. Wax, J. Biophotonics 2018, 11(12), e201800126.
- 52G. Popescu, K. Park, M. Mir, R. Bashir, Lab Chip 2014, 14(4), 646.
- 53M. Mir, Z. Wang, Z. Shen, M. Bednarz, R. Bashir, I. Golding, S. G. Prasanth, G. Popescu, Proc. Natl. Acad. Sci. U. S.A. 2011, 108(32), 13124.
- 54Z. A. Steelman, A. Sedelnikova, Z. N. Coker, A. Kiester, G. Noojin, B. L. Ibey, J. N. Bixler, Appl. Opt. 2021, 60(25), G10. https://doi.org/10.1364/AO.426147.
- 55A. Muralidharan, L. Rems, M. T. Kreutzer, P. E. Boukany, Biochim. Biophys. Acta 2021, 1863(1), 183468.
- 56Z. A. Steelman, W. J. Eldridge, J. B. Weintraub, A. Wax, J. Biophotonics 2017, 10(12), 1714.
- 57G. Charras, E. Paluch, Nat. Rev. Mol. Cell Biol. 2008, 9(9), 730.
- 58G. Charras, J. Microsc. 2008, 231(3), 466.
- 59V. L. Calin, M. Mihailescu, N. Mihale, A. V. Baluta, E. Kovacs, T. Savopol, M. G. Moisescu, Biomed. Opt. Express 2017, 8(4), 2222.
- 60V. L. Calin, M. Mihailescu, N. Tarba, A. M. Sandu, E. Scarlat, M. G. Moisescu, T. Savopol, Biomed. Opt. Express 2021, 12(4), 2519.
- 61S. J. Beebe, J. White, P. F. Blackmore, Y. Deng, K. Somers, K. H. Schoenbach, DNA Cell Biol. 2003, 22(12), 785.
- 62Z. A. Steelman, W. J. Eldridge, A. Wax, J. Biophotonics 2018, 11(6), e201800091.
- 63W. Choi, C. Fang-Yen, K. Badizadegan, S. Oh, N. Lue, R. R. Dasari, M. S. Feld, Nat. Methods 2007, 4(9), 717.
- 64K. H. Schoenbach, S. J. Beebe, E. S. Buescher, Bioelectromagnetics 2001, 22(6), 440.
- 65G. Popescu, Y. Park, N. Lue, C. Best-Popescu, L. Deflores, R. R. Dasari, M. S. Feld, K. Badizadegan, Am. J. Phys. Cell Phys. 2008, 295(2), C538.
- 66R. Barer, Nature 1952, 169(4296), 366.
- 67W. J. Eldridge, S. Ceballos, T. Shah, H. S. Park, Z. A. Steelman, S. Zauscher, A. Wax, Biophys. J. 2019, 117(4), 696.
- 68A. J. Traverso, J. V. Thompson, Z. A. Steelman, Z. Meng, M. O. Scully, V. V. Yakovlev, Anal. Chem. 2015, 87(15), 7519.
- 69Z. Meng, A. J. Traverso, C. W. Ballmann, M. A. Troyanova-Wood, V. V. Yakovlev, Adv. Opt. Photon. 2016, 8(2), 300.
- 70M.-P. Rols, J. Teissié, Biochim. Biophys. Acta 1992, 1111(1), 45.
- 71H. S. Park, S. Ceballos, W. J. Eldridge, A. Wax, APL Photonics 2018, 3(11), 110802.
- 72M. T. Rinehart, H. S. Park, A. Wax, Biomed. Opt. Express 2015, 6(6), 2067.
- 73S. Mues, S. Ketelhut, B. Kemper, J. Schnekenburger, European Conference on Biomedical Optics, Optical Society of America, 2017, 104140J.
- 74M. Mugnano, G. Lama, R. Castaldo, V. Marchesano, F. Merola, D. Del Giudice, A. Calabuig, G. Gentile, V. Ambrogi, P. Cerruti, ACS Appl. Nano Mater. 2019, 3(1), 428.
- 75M. Mugnano, P. Memmolo, L. Miccio, S. Grilli, F. Merola, A. Calabuig, A. Bramanti, E. Mazzon, P. Ferraro, J. Biophotonics 2018, 11(12), e201800099.
- 76A. Calabuig, M. Mugnano, L. Miccio, S. Grilli, P. Ferraro, J. Biophotonics 2017, 10(6–7), 919.
- 77J. Kühn, E. Shaffer, J. Mena, B. Breton, J. Parent, B. Rappaz, M. Chambon, Y. Emery, P. Magistretti, C. Depeursinge, Assay Drug Dev. Technol. 2013, 11(2), 101.
- 78B. L. Ibey, J. C. Ullery, O. N. Pakhomova, C. C. Roth, I. Semenov, H. T. Beier, M. Tarango, S. Xiao, K. H. Schoenbach, A. G. Pakhomov, Biochem. Biophys. Res. Commun. 2014, 443(2), 568.