Identification of polystyrene nanoparticle penetration across intact skin barrier as rare event at sites of focal particle aggregations
Nadine Döge
Charité—Universitätsmedizin Berlin, Corporate Member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Clinical Research Center for Hair and Skin Science, Department of Dermatology and Allergy, Berlin, Germany
Institute for Pharmacy, Pharmacology and Toxicology, Freie Universität Berlin, Berlin, Germany
Search for more papers by this authorSabrina Hadam
Charité—Universitätsmedizin Berlin, Corporate Member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Clinical Research Center for Hair and Skin Science, Department of Dermatology and Allergy, Berlin, Germany
Search for more papers by this authorPierre Volz
Department of Physics, Freie Universität Berlin, Berlin, Germany
Search for more papers by this authorAlexander Wolf
Department of Physics, Freie Universität Berlin, Berlin, Germany
Search for more papers by this authorKarl-Heinz Schönborn
W.O.M. World of Medicine GmbH, Berlin, Germany
Search for more papers by this authorUlrike Blume-Peytavi
Charité—Universitätsmedizin Berlin, Corporate Member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Clinical Research Center for Hair and Skin Science, Department of Dermatology and Allergy, Berlin, Germany
Search for more papers by this authorCorresponding Author
Ulrike Alexiev
Department of Physics, Freie Universität Berlin, Berlin, Germany
Correspondence
Annika Vogt, Charité—Universitätsmedizin Berlin, Corporate Member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Clinical Research Center for Hair and Skin Science, Department of Dermatology and Allergy, Charitéplatz 1, 10117 Berlin, Germany. Email: [email protected]
Ulrike Alexiev, Department of Physics, Freie Universität Berlin, Arnimallee 14, 14195 Berlin, Germany. Email: [email protected]
Search for more papers by this authorCorresponding Author
Annika Vogt
Charité—Universitätsmedizin Berlin, Corporate Member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Clinical Research Center for Hair and Skin Science, Department of Dermatology and Allergy, Berlin, Germany
Correspondence
Annika Vogt, Charité—Universitätsmedizin Berlin, Corporate Member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Clinical Research Center for Hair and Skin Science, Department of Dermatology and Allergy, Charitéplatz 1, 10117 Berlin, Germany. Email: [email protected]
Ulrike Alexiev, Department of Physics, Freie Universität Berlin, Arnimallee 14, 14195 Berlin, Germany. Email: [email protected]
Search for more papers by this authorNadine Döge
Charité—Universitätsmedizin Berlin, Corporate Member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Clinical Research Center for Hair and Skin Science, Department of Dermatology and Allergy, Berlin, Germany
Institute for Pharmacy, Pharmacology and Toxicology, Freie Universität Berlin, Berlin, Germany
Search for more papers by this authorSabrina Hadam
Charité—Universitätsmedizin Berlin, Corporate Member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Clinical Research Center for Hair and Skin Science, Department of Dermatology and Allergy, Berlin, Germany
Search for more papers by this authorPierre Volz
Department of Physics, Freie Universität Berlin, Berlin, Germany
Search for more papers by this authorAlexander Wolf
Department of Physics, Freie Universität Berlin, Berlin, Germany
Search for more papers by this authorKarl-Heinz Schönborn
W.O.M. World of Medicine GmbH, Berlin, Germany
Search for more papers by this authorUlrike Blume-Peytavi
Charité—Universitätsmedizin Berlin, Corporate Member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Clinical Research Center for Hair and Skin Science, Department of Dermatology and Allergy, Berlin, Germany
Search for more papers by this authorCorresponding Author
Ulrike Alexiev
Department of Physics, Freie Universität Berlin, Berlin, Germany
Correspondence
Annika Vogt, Charité—Universitätsmedizin Berlin, Corporate Member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Clinical Research Center for Hair and Skin Science, Department of Dermatology and Allergy, Charitéplatz 1, 10117 Berlin, Germany. Email: [email protected]
Ulrike Alexiev, Department of Physics, Freie Universität Berlin, Arnimallee 14, 14195 Berlin, Germany. Email: [email protected]
Search for more papers by this authorCorresponding Author
Annika Vogt
Charité—Universitätsmedizin Berlin, Corporate Member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Clinical Research Center for Hair and Skin Science, Department of Dermatology and Allergy, Berlin, Germany
Correspondence
Annika Vogt, Charité—Universitätsmedizin Berlin, Corporate Member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Clinical Research Center for Hair and Skin Science, Department of Dermatology and Allergy, Charitéplatz 1, 10117 Berlin, Germany. Email: [email protected]
Ulrike Alexiev, Department of Physics, Freie Universität Berlin, Arnimallee 14, 14195 Berlin, Germany. Email: [email protected]
Search for more papers by this authorAbstract
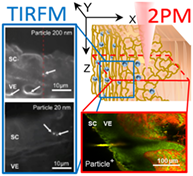
The question whether nanoparticles can cross the skin barrier is highly debated. Even in intact skin rare events of deeper penetration have been reported, but technical limitations and possible artifacts require careful interpretation. In this study, horizontal scanning by 2-photon microscopy (2 PM) of full-thickness human skin samples placed in a lateral position yielded highly informative images for skin penetration studies of fluorescently tagged nanoparticles. Scanning of large fields of view allowed for detailed information on interfollicular and follicular penetration in tissue blocks without damaging the sample. Images in histomorphological correlation showed that 2P-excited fluorescence signals of fluorescently tagged 20 and 200 nm polystyrene nanoparticles preferentially accumulated in the stratum corneum (SC) and in the upper part of vellus hair follicles (HFs). Rare events of deeper penetration in the SC and in the infundibulum of vellus HFs were observed at sites of high focal particle aggregations. Wide-field 2 PM allows for imaging of nanoparticle penetration in large tissue blocks, whereas total internal reflection microscopy (TIRFM) enables selective detection of individual nanoparticles as well as clusters of nanoparticles in the SC and within the epidermal layer directly beneath the SC, thus confirming barrier crossing with high sensitivity.
REFERENCES
- 1A.Chiang, E.Tudela, H. I.Maibach, J. Appl. Toxicol. 2012, 32, 537.
- 2P. H.Hoet, I.Brüske-Hohlfeld, O. V.Salata, J. Nanobiotechnol. 2004, 2, 12.
- 3K.Thomas, P.Sayre, Toxicol. Sci. 2005, 87, 316.
- 4G. J.Nohynek, J.Lademann, C.Ribaud, M. S.Roberts, Crit. Rev. Toxicol. 2007, 37, 251.
- 5J.Lademann, H.Weigmann, C.Rickmeyer, H.Barthelmes, H.Schaefer, G.Mueller, W.Sterry Skin Pharmacol. Appl. Skin Physiol. 1999, 12, 247.
- 6A. V.Zvyagin, X.Zhao, A.Gierden, W.Sanchez, J. A.Ross, M. S.Roberts, J. Biomed. Opt. 2008, 13, 064031.
- 7L. L.Lin, J. E.Grice, M. K.Butler, A. V.Zvyagin, W.Becker, T. A.Robertson, H. P.Soyer, M. S.Roberts, T. W.Prow, Pharm. Res. 2011, 28, 2920.
- 8M. E.Darvin, K.Konig, M.Kellner-Hoefer, H. G.Breunig, W.Werncke, M. C.Meinke, A.Patzelt, W.Sterry, J.Lademann Skin Pharmacol. Physiol. 2012, 25, 219.
- 9D.Papakostas, F.Rancan, W.Sterry, U.Blume-Peytavi, A.Vogt, Arch. Dermatol. Res. 2011, 303, 533.
- 10B. W.Barry, Eur. J. Pharm. Sci. 2001, 14, 101.
- 11C.Scheicher, M.Mehlig, H.-P.Dienes, K.Reske, Adv. Exp. Med. Biol. 1995, 378, 253.
- 12A. F.Charest, J.McDougall, M. B.Goldstein, Am. J. Kidney Dis. 2000, 36, 976.
- 13A.Egemen, S.Aksit, Z.Kurugöl, S.Erensoy, A.Bilgiç, M.Akilli, Vaccine 1998, 16, 1511.
- 14A.Sabchareon, P.Chantavanich, S.Pasuralertsakul, C.Pojjaroen-Anant, V.Prarinyanupharb, P.Attanath, V.Singhasivanon, W.Buppodom, J.Lang, Pediatr. Infect. Dis. J. 1998, 17, 1001.
- 15M.Schneider, F.Stracke, S.Hansen, U. F.Schaefer, Dermatoendocrinology 2009, 1, 197.
- 16B.Godin, E.Touitou, Adv. Drug Deliv. Rev. 2007, 59, 1152.
- 17A.Patzelt, H.Richter, R.Buettemeyer, H. J.Huber, U.Blume-Peytavi, W.Sterry, J.Lademann Eur. J. Pharm. Biopharm. 2008, 70, 234.
- 18N.Döge, S.Hönzke, F.Schumacher, B.Balzus, M.Colombo, S.Hadam, F.Rancan, U.Blume-Peytavi, M.Schäfer-Korting, A.Schindler, E.Rühl, P. S.Skov, M. K.Church, S.Hedtrich, B.Kleuser, R.Bodmeier, A.Vogt, J. Control. Release 2016, 242, 25.
- 19 Organisation for economic co-operation and development (OECD), OECD Guideline for Testing of Chemicals. Skin Absorption: in vitro Method No. 428, Section 4: Health Effects.; Adopted 13 April 2004, Paris, France 2004, pp. 1–8.
- 20A.Vogt, C.Wischke, A. T.Neffe, N.Ma, U.Alexiev, A.Lendlein, J. Control. Release 2016, 242, 3.
- 21S. L.Jacques, Phys. Med. Biol. 2013, 58, R37.
- 22M.Ulrich, S.Lange-Asschenfeldt, J. Biomed. Opt. 2013, 18, 061212.
- 23K.König, J. Biophotonics 2008, 1, 13.
- 24S.Seidenari, F.Arginelli, S.Bassoli, J.Cautela, P. M.French, M.Guanti, D.Guardoli, K.König, C.Talbot, C.Dunsby, Dermatol Res Prac 2011, 2012, 8.
- 25K.König, I.Riemann, J. Biomed. Opt. 2003, 8, 432.
- 26E.Yew, C.Rowlands, J. Innov. Opt. Health Sci. 2014, 7, 1330010.
- 27F.Helmchen, W.Denk, Nat. Methods 2005, 2, 932.
- 28W. R.Zipfel, R. M.Williams, W. W.Webb, Nat. Biotech. 2003, 21, 1369.
- 29W.Denk, J. H.Strickler, W. W.Webb, Science 1990, 248, 73.
- 30N.Döge, E.Thiel, G.Seewald, S.Albrecht, A.Vogt, J.Liebscher, K. H.Schönborn, Optik Photonik 2015, 10, 39.
10.1002/opph.201500035 Google Scholar
- 31L. H.Laiho, S.Pelet, T. M.Hancewicz, P. D.Kaplan, J. Biomed. Opt. 2005, 10, 024016.
- 32D. C.Carrer, C.Vermehren, L. A.Bagatolli, J. Control. Release 2008, 132, 12.
- 33M. E.Darvin, H.Richter, Y.Zhu, M. C.Meinke, F.Knorr, S. A.Gonchukov, K.König, J.Lademann, Quantum Electron. 2014, 44, 646.
- 34M. S.Roberts, Y.Dancik, T. W.Prow, C. A.Thorling, L. L.Lin, J. E.Grice, T. A.Robertson, K.König, W.Becker, Eur. J. Pharm. Biopharm. 2011, 77, 469.
- 35V. R.Leite-Silva, M.Le Lamer, W. Y.Sanchez, D. C.Liu, W. H.Sanchez, I.Morrow, D.Martin, H. D. T.Silva, T. W.Prow, J. E.Grice, Eur. J. Pharm. Biopharm. 2013, 84, 297.
- 36E.Decencière, E.Tancrède-Bohin, P.Dokládal, S.Koudoro, A. M.Pena, T.Baldeweck, Skin Res. Technol. 2013, 19, 115.
- 37P.Volz, A.Boreham, A.Wolf, T. Y.Kim, J.Balke, J.Frombach, S.Hadam, Z.Afraz, F.Rancan, U.Blume-Peytavi, A.Vogt, U.Alexiev, Int. J. Mol. Sci. 2015, 16, 6960.
- 38A.Boreham, P.Volz, D.Peters, C. M.Keck, U.Alexiev, Eur. J. Pharm. Biopharm. 2017, 110, 31.
- 39G.Zoubari, S.Staufenbiel, P.Volz, U.Alexiev, R.Bodmeier, Eur. J. Pharm. Biopharm. 2017, 110, 39.
- 40J.Lademann, U.Jacobi, C.Surber, H.-J.Weigmann, J.Fluhr, Eur. J. Pharm. Biopharm. 2009, 72, 317.
- 41A.Vogt, S.Hadam, I.Deckert, J.Schmidt, A.Stroux, Z.Afraz, F.Rancan, J.Lademann, B.Combadiere, U.Blume-Peytavi, Exp. Dermatol. 2015, 24, 73.
- 42A.Vogt, B.Combadiere, S.Hadam, K. M.Stieler, J.Lademann, H.Schaefer, B.Autran, W.Sterry, U.Blume-Peytavi, J. Invest. Dermatol. 2006, 126, 1316.
- 43F.Rancan, Q.Gao, C.Graf, S.Troppens, S.Hadam, S.Hackbarth, C.Kembuan, U.Blume-Peytavi, E.Rühl, J.Lademann, A.Vogt, ACS Nano 2012, 6, 6829.
- 44K.Kirchberg, T.-Y.Kim, S.Haase, U.Alexiev, Photochem. Photobiol. Sci. 2010, 9, 226.
- 45T.-Y.Kim, H.Uji-i, M.Möller, B.Muls, J.Hofkens, U.Alexiev, Biochemistry 2009, 48, 3801.
- 46F.Rancan, M.Giulbudagian, J.Jurisch, U.Blume-Peytavi, M.Calderon, A.Vogt Eur. J. Pharm. Biopharm. 2017, 116, 4.
- 47H.Trommer, R.Neubert, Skin Pharmacol. Physiol. 2006, 19, 106.
- 48C.Mathes, A.Melero, P.Conrad, T.Vogt, L.Rigo, D.Selzer, W. A.Prado, C. de Rossi, T. M.Garrigues, S.Hansen, S. S.Guterres, A. R.Pohlmann, R. C. R.Beck, C. M.Lehr, U. F.Schaefer, J. Control. Release 2016, 223, 207.
- 49J.Lademann, H.Richter, A.Teichmann, N.Otberg, U.Blume-Peytavi, J.Luengo, B.Weiss, U. F.Schaefer, C. M.Lehr, R.Wepf, W.Sterry, Eur. J. Pharm. Biopharm. 2007, 66, 159.
- 50A.Teichmann, U.Jacobi, H.-J.Weigmann, W.Sterry, J.Lademann, Skin Pharmacol. Physiol. 2005, 18, 75.
- 51J.Lademann, N.Otberg, H.Richter, H.-J.Weigmann, U.Lindemann, H.Schaefer, W.Sterry, Skin Pharmacol. Physiol. 2001, 14, 17.
- 52B.Mahe, A.Vogt, C.Liard, D.Duffy, V.Abadie, O.Bonduelle, A.Boissonnas, W.Sterry, B.Verrier, U.Blume-Peytavi, B.Combadiere, J. Invest. Dermatol. 2009, 129, 1156.
- 53A.Vogt, S.Hadam, M.Heiderhoff, H.Audring, J.Lademann, W.Sterry, U.Blume-Peytavi, Exp. Dermatol. 2007, 16, 946.
- 54R.Alvarez-Roman, A.Naik, Y. N.Kalia, R. H.Guy, H.Fessi, J. Control. Release 2004, 99, 53.
- 55C. S.Campbell, L. R.Contreras-Rojas, M. B.Delgado-Charro, R. H.Guy, J. Control. Release 2012, 162, 201.
- 56R.Alvarez-Román, A.Naik, Y.Kalia, H.Fessi, R. H.Guy, Eur. J. Pharm. Biopharm. 2004, 58, 301.
- 57F.Du, S.Hönzke, F.Neumann, J.Keilitz, W.Chen, N.Ma, S.Hedtrich, R.Haag, J. Control. Release 2016, 242, 42.